
Glaucoma
Glaucoma
Last Section Update: 12/2022
Contributor(s): Shayna Sandhaus, PhD; Maureen Williams, ND
1 Overview
Summary and Quick Facts for Glaucoma
- Glaucoma refers to a group of similar conditions that damage the retina and optic nerve and can cause vision loss and blindness. The two major forms of glaucoma are primary open-angle glaucoma, which accounts for about 90% of glaucoma cases, and acute angle-closure glaucoma.
- After reading this protocol, you will understand how glaucoma emerges and discover that making lifestyle changes to control risk factors can lessen the risk of glaucoma development and progression. You will also learn about exciting findings related to a number of natural compounds with the ability to target multiple mechanisms underlying the progression of glaucoma.
- The best approach to glaucoma is regular eye check-ups to make sure intraocular pressure (IOP) is not rising, and if it is, to take aggressive measures to lower IOP. The goal for both conventional and natural therapies is to lower IOP as much as possible and slow or halt the progression of symptoms.
What is Glaucoma?
Glaucoma refers to a group of similar conditions that damage the retina and optic nerve and can cause vision loss and blindness. The two major forms of glaucoma are primary open-angle glaucoma, which accounts for about 90% of glaucoma cases, and acute angle-closure glaucoma.
Elevated intraocular pressure (IOP), which is caused by poor drainage of aqueous humor from the front of the eye, is common in glaucoma. Oxidative stress and mitochondrial dysfunction may play an additional role in retinal ganglion cell death. Many people may not be aware they have glaucoma, as the condition is usually asymptomatic until irreversible damage has already been done.
Natural interventions such as French maritime pine bark and bilberry extract may help slow progression of the disease.
What are the Risk Factors for Glaucoma?
- Family history
- Elevated IOP
- Advanced age
- Ethnicity—African-Americans have the highest risk
- Other medical conditions such as diabetes, hypertension, and thyroid problems
- Other eye conditions
- Prolonged corticosteroid use
- Lack of physical activity
What are the Signs and Symptoms of Glaucoma?
Note: The symptoms listed below generally do not occur until the disease has progressed significantly. Routine eye exams are necessary to catch the disease in earlier stages.
- Primary open-angle glaucoma ‒ Loss of peripheral vision
- Acute angle-closure glaucoma ‒ Symptoms may include extreme eye pain, headaches, blurred vision, red eyes, halos around lights, tender and firm eyes, nausea, and vomiting. The acute form of glaucoma requires emergency medical treatment to prevent vision loss.
What are Conventional Medical Treatments for Glaucoma?
- Alpha agonists (eg, apraclonidine HCl [Iopidine] and brimonidine tartrate [Alphagan])
- Beta blockers (eg, timolol maleate [Istalol, Timoptic XE] and betaxolol [Betoptic])
- Carbonic anhydrase inhibitors (eg, brinzolamide [Azopt] and dorzolamide HCl [Trusopt])
- Cholinergic medications (eg, pilocarpine HCl [Isopto Carpine, Pilopine HS])
- Prostaglandin analogs (eg, travoprost [Travatan] and bimatoprost [Lumigan])
- Surgery (laser or filtering)
What Dietary and Lifestyle Changes Can Be Beneficial for Glaucoma?
- Engage in regular physical activity
- Quit smoking
What Natural Interventions May Be Beneficial for Glaucoma?
- French maritime pine bark and bilberry extract. The combination of these two antioxidant-rich extracts, when combined with a standard glaucoma drug, was shown to reduce IOP.
- Antioxidants. Several other antioxidants such as epigallocatechin-gallate (EGCG) from green tea, vitamin C, and vitamin A may also counter the oxidative damage caused by glaucoma.
- Coenzyme Q10 (CoQ10) and pyrroloquinoline quinone (PQQ). Both CoQ10 and PQQ have been shown to support mitochondrial health. As mitochondrial dysfunction may play an important role in glaucoma progression, supplementation with these natural compounds could be beneficial.
- Coleus forskohlii. A special preparation of Coleus forskohlii has been shown to reduce IOP and may be useful for thyroid problems, a risk factor for glaucoma.
- Essential minerals. A number of minerals have been linked to eye health and glaucoma, including magnesium, chromium, selenium, and zinc.
- Other natural interventions that may be beneficial for eye health include melatonin, rutin, ginkgo biloba, and green coffee extract.
2 Introduction
Glaucoma is the second leading cause of irreversible blindness worldwide. The disease affects about 5 million Americans, mostly over age 40. Distressingly, many of these individuals are unaware of their affliction until long after the optic nerve has already been permanently damaged.
The term “glaucoma” refers to a group of similar conditions that damage the retina and optic nerve, leading to visual impairment. Glaucoma is sometimes called a “silent thief” because it slowly robs its victims of peripheral vision, which can go unnoticed until the loss becomes significant enough to interfere with everyday life.
Although glaucoma-related vision loss is not reversible, the progression of the disease can nearly always be slowed or halted. When diagnosed and treated early, it seldom leads to blindness.
Prescription medications and surgery can control the clinical manifestations, but the most commonly prescribed drugs carry unpleasant side effects, while there are risks associated with surgery.
Fortunately, recent scientific studies have illuminated natural strategies to help attenuate the progression of glaucoma. Investigations have shown that a combination of plant-based interventions derived from French maritime pine bark and bilberry target one of the most common underlying problems with glaucoma: increased pressure in the front of the eye, a condition known as elevated intraocular pressure (IOP).
Human studies reveal that these natural compounds complement conventional glaucoma medications as well, acting synergistically to optimize IOP.1
Moreover, conventional therapies do little to address a major contributor to visual impairment in glaucoma—mitochondrial dysfunction.2,3 Coenzyme Q10 and pyrroloquinoline quinone (PQQ) are two powerful mitochondrial protectants that may play a considerable, yet unappreciated role in maintaining visual acuity for glaucoma patients.
After reading this Life Extension protocol, you will understand how glaucoma emerges and discover that making lifestyle changes to control risk factors can lessen the risk of glaucoma development and progression. You also will learn about exciting findings related to a number of natural compounds with the ability to target multiple mechanisms underlying the progression of glaucoma.
3 Structures of the Eye
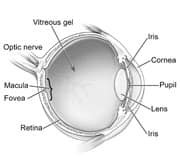 |
| Figure 1: Structures of the eye |
Back of the Eye
The eye is a spherical structure. It is connected at its rear pole to the brain via the optic nerve. The optic nerve is a fibrous tube containing over one million horizontally running nerve fibers (axons), each one originating from a type of retinal cell called a ganglion cell. The retina and optic nerve are pictured in Figure 1.
The retina is composed of a thin sheet of cells (and related structures) that form the back wall of the eye. Its primary role is to capture light and transform it into electrical signals. The signals are transmitted to the brain by the optic nerve, where they are interpreted as the objects we “see.”
Ganglion cell axons are responsible for transmitting these electrical signals. The axons spread out across the retina to converge at the optic disc, the point of origin of the optic nerve. The optic disc is where damage from glaucoma is typically detected by an eye exam.
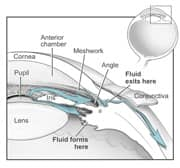 |
| Figure 2: Front of the eye |
Front of the Eye
When we look at our eyes in the mirror, we see four main features of the front of the eyeball: the white sclera, the black pupil, the colored iris, and the dome-shaped cornea overlaying the iris and pupil. In most cases of glaucoma, the trouble lies immediately behind the cornea in the outflow of a fluid called aqueous humor (or aqueous fluid). Normally, aqueous humor flows from behind the iris (posterior chamber), where it is formed, to the front of the iris (anterior chamber) where it drains through the trabecular meshwork into Schlemm's canal and ultimately into the blood circulation (Figure 2). Aqueous humor should not be confused with tears, which are formed outside the eye.
4 Types of Glaucoma
There are two major forms of glaucoma: open angle glaucoma and angle-closure glaucoma. About 90% of cases of glaucoma are primary open angle glaucoma (POAG). The majority of others are angle-closure glaucoma.
Less common forms of glaucoma include congenital glaucoma, which tends to run in families and is present at birth; normal tension glaucoma; pigmentary glaucoma; pseudoexfoliative glaucoma; traumatic glaucoma; neovascular glaucoma; and iridocorneal endothelial syndrome.
In the last several years, glaucoma has come to be described as a “neurodegenerative disease,” because it shares features with several brain disorders including Alzheimer's disease, amyotrophic lateral sclerosis (Lou Gehrig's disease), and Parkinson's disease.4
5 Signs and Symptoms of Glaucoma
Signs and symptoms of glaucoma differ depending upon the type of glaucoma. Most patients do not have any symptoms in the disease's early stages. Others may experience severe pain and rapidly compromised vision. Even without symptoms, people with glaucoma are vulnerable to a loss of peripheral vision, followed by reductions in central vision and blindness. (Symptoms should always be reported to the examining ophthalmologist or optometrist.)
- Most people with open angle glaucoma have no symptoms until they notice a loss of peripheral vision. The vision loss can be slowly progressive, leading to tunnel vision and finally blindness.
- Closed angle glaucoma is an acute condition (meaning it comes on quickly with a rapid rise in IOP) and is often associated with severe eye pain. Angle closure glaucoma is a medical emergency and must be treated quickly to prevent vision loss. Symptoms may include extreme eye pain, headaches, blurred vision, red eyes, halos around lights, tender and firm eyes, and nausea and vomiting. An eye exam usually shows a shallow anterior chamber and mildly dilated pupils, sometimes drug related.
- Congenital glaucoma can be marked by excess tearing of the eyes (usually associated with a malformed tear duct drainage system), iris abnormalities, extreme sensitivity to light, and a large and hazy cornea.
6 Risk Factors for Glaucoma
There are many risk factors for glaucoma, ranging from factors you cannot control (genetic abnormalities and age) to lifestyle factors. Some of the known risk factors for glaucoma include:
Intraocular Pressure (IOP)
Normal IOP is between 10 millimeters mercury (mm HG) and 20 mm Hg. Higher-than-normal IOP is perhaps the most significant risk factor for glaucoma. It is important to distinguish between “intraocular pressure” and “blood pressure”; they are not synonymous. Intraocular pressure (IOP) refers to the pressure caused by the aqueous humor secreted by the ciliary body, while blood pressure refers to the pressure exerted by the blood on the blood vessel (artery) wall.
Age
People older than 60 are more likely to develop glaucoma. For some ethnic groups, risk begins at an earlier age.
Ethnicity
African-Americans have the highest risk for glaucoma in the United States (12%). Among Asian Americans and U.S. Hispanics, the glaucoma risk is 6.5%.5
Medical Conditions
Glaucoma is especially closely related to diabetes and high blood pressure. In a 2011 study, researchers examined medical records of over 2 million people older than 40 who were enrolled in a U.S. managed care network. The records revealed a 35% increased risk in people with diabetes of developing open angle glaucoma and a 17% increased risk in those with hypertension. Both conditions together raised the risk to 48%.6
An association has also been drawn between thyroid disease and glaucoma, according to a study of 12,376 participants from the CDC's 2002 National Health Interview Survey. Researchers found that the prevalence of glaucoma was almost double in people with thyroid problems versus those without thyroid problems.7
Other Eye Conditions
Based on a study of 2,650 patients where 579 patients (21.84%) had secondary glaucoma, eye-related causes, in order of frequency, were post vitrectomy surgery, eye trauma, corneal pathology, aphakia, neovascular glaucoma, pseudophakia, and uveitis. There were also cases secondary to tumor, myopia, pseudoexfoliation syndrome, retinopathy of prematurity, aniridia, iridocorneal endothelial syndrome, and chemical injury to the eye.8
Corticosteroids
Glaucoma risk is raised by prolonged use of corticosteroids, including corticosteroid-containing eye drops for reducing eye inflammation and inhalers for treating asthma.
Lack of Physical Activity
Research suggests an association between low levels of physical activity and low ocular perfusion pressure (OPP), a risk factor for glaucoma. OPP is a calculation derived from IOP and blood pressure.9
Genetics
Having a family history of glaucoma is a well-established risk factor, and the role of genetics in glaucoma continues to receive scientific scrutiny. In the late 1990s, researchers began identifying glaucoma gene mutations, including several in a gene that encodes for the protein TIGR found in the trabecular meshwork. They discovered the mutations by studying families with POAG. The mutation occurred in 4.4% of these patients, compared to 0.3% of the general population.10
Since then, several other genes (eg, MYOC, OPTN) have been associated with POAG. Among the most recently noted is ADAMTS10, which was discovered in a unique population of beagles with glaucoma.11
Another gene linked to glaucoma is CYP1B1. In some populations, CYP1B1 mutations are found in over half of all cases of congenital glaucoma.12
Researchers expect to find many more genes and other events involved in the initiation and progression of glaucoma. An important aspect will be to identify people with genetic and other risk factors and to counsel them about ways to reduce the likelihood of developing glaucoma.
Lastly, other drugs besides steroids can raise the risk of glaucoma, especially of angle-closure glaucoma. Categories of drugs known as anticholinergics, and adrenergics are the most common. Sulfa drugs, antihistamines, and decongestants can also cause problems. And several anti-cancer drugs can increase the risk of open angle glaucoma. Because the cause of the glaucoma is linked to the particular drug usage, the first line of treatment is to discontinue the drug. Note: If you have glaucoma, make sure to inform your healthcare provider and pharmacist. They should know what drugs to avoid.
7 Pathophysiology of Glaucoma
In the past, doctors thought of glaucoma as a disease with only one major feature: increased IOP, or essentially raised pressure in the eye. Although we now know that glaucoma can occur even in people with normal IOP, this is still the most common underlying symptom of the disease. In the most common form of glaucoma—open angle glaucoma—IOP can be subtly raised long before symptoms become discernible to the patient. This provides a critical window of time during which aggressive measures can be taken to reduce IOP and head off symptoms before they develop; hence the importance of regular eye check-ups, even if you do not have any symptoms. Once symptoms manifest and glaucoma is detected, it is important to take immediate and aggressive action to protect your eyesight.
Elevated IOP is caused by abnormal drainage of aqueous humor from the front chamber of the eye. In a healthy eye, fluid from the front chamber drains into a region known as the trabecular meshwork through an acute angle formed by the intersection of the cornea and iris. If the fluid cannot drain through this angle, it backs up into the eye itself, causing elevated IOP. There are two main reasons for a blockage at this angle: either the angle remains open and the fluid has complete access to the trabecular meshwork but for some reason its outflow is impeded (open angle glaucoma), or there is a physical barrier in the angle, sometimes caused by deformity in the iris, that causes reduced flow (closed angle glaucoma).
Open Angle Glaucoma
Open angle glaucoma occurs even though there is no obstruction to the flow of aqueous humor. It may be caused by a mutation in the GLC1A gene, which is responsible for the production of a protein called myocilin that is normally present in the trabecular network. This condition is known as primary open angle glaucoma (POAG). Secondary open angle glaucoma can be caused when particulate matter, such as clumps of protein and shedded portions of surrounding cells and fibers, clog the outflow channels.
Importantly, the events leading to impaired trabecular meshwork drainage in non-genetic open-angle glaucoma share several pathological characteristics with atherosclerosis, such as endothelial dysfunction.13-15 Therefore, individuals who wish to preserve the integrity of their trabecular meshwork should consider the suggestions in Life Extension's “Atherosclerosis and Cardiovascular Disease” protocol as well.
Closed Angle Glaucoma
Primary angle-closure glaucoma is most common in eyes with a shallow (flatter) front chamber. Secondary angle closure glaucoma is usually related to abnormal biological events in the eye, such as displacement of the iris against the cornea, which inhibits the aqueous humor from reaching the trabecular meshwork. Surgery or trauma to the eye can also lead to scar tissue that interferes with drainage. Tumors, too, can grow in the aqueous production and outflow system and interfere with the trabecular meshwork.
Congenital Glaucoma
Congenital glaucoma is present at birth. It is related to improper formation of the aqueous fluid outflow system during fetal development. Several gene mutations have been associated with congenital glaucoma. Congenital glaucoma is usually diagnosed at birth or within the first year of life.16
Normal Tension Glaucoma
Not all people with glaucoma have elevated IOP. When IOP is normal but the person still has typical symptoms of glaucoma, the condition is called normal tension glaucoma.
Pseudoexfoliative Glaucoma
Pseudoexfoliative glaucoma (PEX) is distinguished by clumps of amyloid protein that accumulate in the eye and ultimately end up blocking the outflow of aqueous humor by clogging the trabecular network. The cause is unknown, although a mutation in the LOXL1 gene may play a role. PEX is more common in women and in people of Northern European dissent.
Anatomic Changes in Glaucoma
In glaucoma, the retina and optic nerve at the back of the eye grow thinner as ganglion cells and axons die. This thinning can be seen by a doctor during an ophthalmic exam. Eye doctors refer to the visible changes as “cupping.”
A relatively new technology called optical coherence tomography (OCT) allows physicians to measure the progressive thinning of the retina and the cupping of the optic nerve and correlate the changes with visual field loss. Researchers have proposed that OCT be used for studying the effect of new drugs and devices being developed for treating glaucoma.17 Other common tests, which provide similar diagnostic information, include Heidelberg Retina Tomography (HRT) and the GDx Nerve Fiber Analyzer (GDx).
Beyond the visual symptoms of cupping, glaucoma's damage extends deep into the cells of the eye. Like all human cells, the cells of your eyes are full of structures called mitochondria that produce energy for the cell to function. In glaucoma, researchers are learning that the mitochondria in the retinal ganglion cell become damaged. Without healthy mitochondria, cells are unable to engage in a natural repair processes following normal wear and tear, oxidative stress, and injury. As a result, the retinal ganglion cells become susceptible to a process called apoptosis, or cell death.18
In the eye, it appears that oxidative stress is a major feature of mitochondrial damage. Free radical attack in the sensitive retinal ganglion cells causes mitochondrial damage, which in turn causes cell death. Excessive calcium within the cells is also implicated in retinal ganglion cell death.19 Life Extension has long been at the forefront of identifying novel, natural ways to boost mitochondrial health.
Researchers have also uncovered another possible contributor to retinal ganglion cell death: excessive glutamate. Glutamate is the body's main excitatory neurotransmitter. Although it is vitally important to a healthy brain and nervous system, too much glutamate is toxic because it causes overstimulation of nerve cells. Normally, glutamate is cleared quickly. In glaucoma, however, it appears that high levels of glutamate in the retinal ganglion over-stimulate cells, resulting in cell death. Researchers are looking at ways to reduce glutamate signaling in the eye, thus protecting the retinal ganglion cells from its toxic effects. This could be especially important for patients whose glaucoma does not respond to IOP-lowering treatment.20
8 Tests and Diagnosis
The simplest and most common test for glaucoma is an IOP reading, though increased IOP does not necessarily mean glaucoma is the cause, and no one test alone can be used to establish a diagnosis of glaucoma. To get an IOP reading, a doctor or technician typically touches the front of the eye with a small instrument called a tonometer. Many doctors will also do a dilated eye exam. The purpose of dilating the pupil is to look directly at the inside back of the eye to check for retinal and optic nerve damage. The doctor will probably take pictures and measurements to establish a baseline for comparing to future eye exams. A patient with glaucoma is also likely to have a visual field test to measure losses in peripheral vision and a visual acuity test to check for visual sharpness (acuity). The doctor may also use a technique called gonioscopy to study the angle of the drainage system and tonography to study the rate of fluid drainage.
Additional testing to establish a diagnosis of glaucoma often includes pachymetry (measures central cornea thickness), visual field testing, gonioscopy and possibly NFL scanning technology.
9 Treatments
Acute closed angle glaucoma is a medical emergency that must be treated immediately. However, the more common type of glaucoma—open angle glaucoma—can be an insidious disease with no symptoms, even as serious damage is being done to your retina and optic disk. The best approach to glaucoma is regular eye check-ups to make sure IOP is not rising, and if it is, to take aggressive measures to lower IOP.
A combination of natural therapies and conventional treatments can be used. The goal for both conventional and natural therapies is to lower IOP as much as possible and slow or halt the progression of symptoms.
Conventional Treatment
Medications for glaucoma work by decreasing production and/or increasing drainage of aqueous fluid. Most glaucoma medications are topical—that is, in the form of eye drops. Categories of glaucoma medications are alpha agonists, beta blockers, carbonic anhydrase inhibitors, and prostaglandin analogs. A doctor may recommend a combination of glaucoma medications.
Alpha agonists. Alpha agonists decrease fluid production and increase drainage. Two such drugs are apraclonidine HCl (Iopidine) and brimonidine tartrate (Alphagan). Dryness of mucous membranes is among the side effects caused by alpha antagonists.
Beta blockers. Beta blockers decrease production of aqueous fluid. They include timolol maleate (Istalol; Timoptic XE), betaxolol (Betoptic), levobunolol HCl (Betagan), metipranolol (OptiPranolol), and timolol hemihydrate (Betimol). Side effects of beta blockers include lowering of blood pressure and decreased heart rate.
Carbonic anhydrase inhibitors. Carbonic anhydrase inhibitors work by decreasing aqueous fluid production. They include brinzolamide (Azopt), dorzolamide HCl (Trusopt), and acetazolamide (Diamox; Sequels). These are available in pill and eye drop form. Systemic carbonic anhydrase inhibitor therapy may cause kidney dysfunction.
Cholinergic medications. Cholinergic medications lower IOP by constricting the pupil. This increases the volume of the eye's anterior chamber and improves access of aqueous fluid to the trabecular meshwork drainage system. Cholinergic medications are sometimes prescribed in combination with other glaucoma medications to help balance fluid production and drainage. Several cholinergic medications are pilocarpine HCl (Isopto Carpine; Pilopine HS) and carbachol (Isopto Carbachol). Constriction of the pupil can cause poor night vision.
Prostaglandin analogs. Prostaglandin analogs increase aqueous fluid drainage. Due to their ability to efficiently reduce IOP, prostaglandin analogs are becoming widely employed in clinical settings. Available medications are travoprost (Travatan), bimatoprost (Lumigan), and latanoprost (Xalatan). Side effects of these compounds are a change in eye color and lengthening of eyelashes; the skin surrounding the eye may darken as well.
Note: Some medications contain several active ingredients. One called Combigan is a beta blocker and alpha agonist. It combines brimonidine tartrate and timolol maleate. Another, Cosopt, is a beta blocker and carbonic anhydrase inhibitor. It combines dorzolamide HCl and timolol maleate. Both decrease production of aqueous fluid. An advantage is that patients get the benefit of both types of compounds in a single eye drop. A downside is the risk of side effects unique to each medicine.
Surgery
Most eye doctors in the United States start glaucoma treatment by prescribing a regimen of medicinal eye drops. As glaucoma progresses, patients are instructed to use higher doses or a combination of different types of eye drops. Surgery may be recommended for patients whose IOP is not responsive to medicines, whose glaucoma continues to worsen, or who experience uncomfortable side effects from glaucoma medications.
Laser surgery and filtering surgery are two common forms of surgery for POAG. Most are performed in a medical office or outpatient facility. The eye is temporarily numbed to keep the patient comfortable.
Laser surgery uses a high-energy laser beam to open obstructed trabecular drainage channels and to allow aqueous fluid to flow more freely from the anterior chamber of the eye. Many people who have this surgery, called laser trabeculoplasty, continue with glaucoma medication, although usually at a lower dose. Types of laser surgery for open angle glaucoma include argon laser trabeculoplasty (ALT) and selective laser trabeculoplasty (SLT). SLT is a newer and more selective procedure that targets individual cells of the trabecular meshwork.
Another laser treatment called laser cyclophotocoagulation works differently from ALT and SLT. Instead of increasing drainage, it reduces fluid production. It does so by destroying part of the ciliary body of the eye where aqueous fluid is formed.
For patients in whom laser surgery is not ideal, there is also filtering surgery. Here the eye surgeon manually makes a small opening in the white of the eye (sclera) and removes a small part of the trabecular meshwork and nearby structures. This procedure, called a trabeculectomy, gives the aqueous fluid an additional outflow route. The surgeon covers the scleral opening with a natural membrane to protect the inner eye and to capture the fluid against the sclera, where it is absorbed.
An alternative to natural drainage through the opening in a trabeculectomy is drainage through a surgically implanted valve. The valve allows aqueous fluid to bypass the trabecular meshwork altogether. Aqueous fluid drains through a small tube from the anterior chamber onto the outside surface of the eye. A drainage valve is sometimes used when a trabeculectomy fails. It can also be used for treating juvenile glaucoma or glaucoma that is caused by trauma or severe eye inflammation.
Some patients may be better candidates for one or the other type of treatment (trabeculectomy or ALT/SLT). For example, ALT is generally preferred for patients older than 50. Further, a major study, called the Advanced Glaucoma Intervention Study (AGIS), supported by the National Eye Institute of the National Institutes of Health, showed a difference in treatment outcomes based on race. Caucasians had better outcomes than African Americans when medical therapy was followed initially by trabeculectomy, for unknown reasons.23 Research comparing outcomes in Latinos and Caucasians showed no differences.24
Emergency glaucoma surgery for acute angle-closure glaucoma takes a different approach. A surgeon might create holes in the iris rather than the sclera. This treatment, performed using laser or conventional surgical techniques, rapidly decreases IOP by opening up the angle formed by the iris and drainage channels. Fortunately, the risk that a patient will develop angle-closure glaucoma is predictable based on the results of routine eye exams. Therefore, regular checkups can help avoid an acute angle closure crisis altogether because, upon detection of ocular anatomy favoring development of angle-closure glaucoma, a clinician can employ preventive procedures.
10 Nutrients
The key with glaucoma is to detect increased IOP as soon as possible and immediately act to counter it, before stronger prescription drugs or invasive surgery become necessary. Researchers have recently discovered a pair of nutrients that target underlying mechanisms of glaucoma.
If clinical signs like increased IOP have already developed, but there are still no noticeable symptoms, it is even more important to act quickly to prevent disease progression. In this case, natural therapies, when combined with standard glaucoma medicines, may act synergisticallyto lower IOP; natural ingredients may also counteract the underlying damage caused by glaucoma.
French Maritime Pine Bark and Bilberry
Human studies have shown a powerful effect of French maritime pine bark and bilberry extract on the underlying symptoms of glaucoma. These two nutrients are rich in proanthocyanidins, powerful antioxidants known for their ability to neutralize harmful free radicals. Proanthocyanidins have also been shown to support cardiovascular health.25
In a 2010 study combining treatment with French maritime pine bark and bilberry with the traditional glaucoma drug Latanoprost—a prostaglandin analog that increases aqueous fluid drainage—researchers found a clear benefit of the combination treatment.1
Pine bark and bilberry may act on a molecular level to decrease the production of aqueous humor, improve blood vessels structure and function, and decrease the resistance to fluid drainage.
Latanoprost causes smooth muscle cells, such as those in blood vessels and the eyes, to relax or contract. However, in part due to risk of side effects associated with its use, latanoprost eye drops may not be ideal for people with elevated IOP without symptoms. By contrast, the natural intervention of French maritime pine bark and bilberry is not associated with the side effects of latanoprost, which include ocular cysts, swelling, and inflammation.26
In this encouraging study, researchers studied 79 patients who had elevated IOP but no signs of glaucoma. Patients were randomized to receive either (1) an oral nutrient compound containing standardized French maritime pine bark extract and a phenolic bilberry (Vaccinium myrtillus) extract, (2) standard medical therapy with latanoprost eye drops alone, or (3) the nutrient compound and latanoprost drops, for 24 weeks.
IOP improved in patients in all treatment groups. The most rapid drop in pressure (28%) was seen in the latanoprost-only group, beginning four weeks after treatment began. In group 1, significant improvement began at six weeks. IOP reduction was 24% at week 16 and was maintained throughout the study. The most exciting results, however, were in the group receiving latanoprost in combination with pine bark and bilberry, those receiving the combination therapy. Patients in this group showed a 28% reduction in pressure at four weeks. Their reduction soared to 40% at 24 weeks. These results show the natural intervention amplifying the effect of the conventional intervention.
Antioxidants
Pine bark and bilberry are among the newest nutrients used to fight glaucoma, but research has long supported the use of antioxidants to counter the oxidative damage caused by glaucoma. Dietary antioxidants have been shown to protect retinal ganglion cells against damage. Antioxidants include glutathione, lutein, zeaxanthin, zinc, vitamin A, vitamin C, vitamin E, beta-carotene, bioflavonoids, EGCG from green tea, and curcumin, among others.
Laboratory studies show that antioxidant treatment helps mitigate risk factors for glaucoma.27 Research into epigallocatechin-gallate (EGCG), a powerful antioxidant found in green tea, for example, shows a potential impact on the physiology of retinal cells in patients with glaucoma.28 The researchers used electrical measurements of retinal activity to show the effect.
Carotenoids. Carotenoids are yellow, orange, and red pigments found in many plants including a wide range of fruits and vegetables. They have free radical-scavenging and anti-inflammatory effects, and some are precursors of vitamin A.63,64 Within the eye, a region of the retina called the macula is pigmented by the carotenoids lutein, zeaxanthin, and meso-zeaxanthin. These carotenoids help protect the eyes against inflammation and oxidative stress.65
A 2021 systematic review of 20 studies concluded that higher consumption of carotenoids is associated with lower risk of glaucoma. This analysis also found that higher levels of carotenoids in the eyes of people with glaucoma are generally associated with better visual performance. The authors suggest carotenoid treatment is neuroprotective and a potential adjunct therapy for glaucoma.66,67
One 18-month randomized controlled trial found supplementation with 10 mg lutein, 2 mg zeaxanthin, and 10 mg meso-zeaxanthin enriched macular pigment content in 42 people with open-angle glaucoma. Among participants in this study who received placebo, macular pigment did not change during the trial period. Similarly, by the end of the trial, visual function in twilight conditions had improved in the carotenoid group but was unchanged in those who received placebo.68 In another randomized controlled trial, 18 months of the same combination of carotenoids administered to 37 participants with open-angle glaucoma increased macular pigment concentrations. This increase in pigment concentration was associated with improvement in performance on a test of visual function (contrast sensitivity) and reduction in time needed for visual recovery after exposure to bright light (photostress recovery time).69 A combination supplement providing 10 mg lutein and 2 mg zeaxanthin, along with zinc, copper, alpha-lipoic acid, taurine, docosahexaenoic acid (DHA), and vitamins C and E, taken for six months reduced oxidative stress and symptoms of dry eye in a preliminary study in 30 subjects with primary open-angle glaucoma.70
Saffron extract is rich in the carotenoid derivatives crocin and crocetin.71,72 In a randomized controlled trial that included 34 people with primary open-angle glaucoma, adding 30 mg aqueous saffron extract daily to standard treatments for one month reduced intraocular pressure more than placebo. This effect was evident after three weeks.71
Because some earlier studies did not find an association between carotenoid intake and glaucoma risk, further rigorous controlled trials are needed to confirm the benefits that have been observed.73
Vitamin C. Vitamin C is also used in the formation of collagen, which gives strength and structure to tissues in the body. In the eye, collagen helps maintain the integrity of blood vessels and the trabecular meshwork. A recent study found that vitamin C serum levels were significantly lower in normal-tension glaucoma patients than in healthy controls.29
Vitamin A. Vitamin A is necessary for the formation of rhodopsin, a pigmented compound in specialized retinal cells the retina which allow the eye to see in low light. The eyes are strong indicators of vitamin A deficiency, becoming dry, itchy, or inflamed, and experiencing night blindness when levels are insufficient.30 Most anyone taking a multivitamin supplement will not be deficient in vitamin A.
Vitamin A can be obtained through food (green leafy vegetables, liver, kidney, egg yolks, butter, fortified dairy products, cold-liver oil, and orange-colored foods, for example) or supplemental beta-carotene. Beta carotene is a pro-vitamin of vitamin A. It is converted, as needed, into vitamin A in the liver or during intestinal absorption.
Ginkgo biloba
Ginkgo biloba extract has been studied as a neuroprotector of retinal ganglion cells in glaucoma due to its ability to open (dilate) blood vessels and its antioxidant effect. Along with oxidative stress and high IOP, blood vessel inadequacy has also been proposed as a contributor to glaucoma, especially in normal tension glaucoma.
Ginkgo biloba has been shown to increase blood volume and velocity of blood flow in the eyes of healthy people.31 In patients with normal tension glaucoma, studies show that it improves visual field loss.32,33 These encouraging findings will hopefully lead to more research.
Coleus forskohlii
Coleus forskohlii is one of 200 varieties of the plant Coleus (Solenostemon) found around the world. The therapeutic ingredient in Coleus forskohlii is found in its root, which was used originally in a paste form for treating a variety of disorders including cardiovascular conditions because of its vasodilating effect. In vitro studies show its significant antioxidant properties.34 In clinical studies involving both animals and humans, a special preparation of Coleus forskohlii, applied directly to the eye, was shown to reduce IOP by increasing intraocular circulation and decreasing aqueous humor inflow into the posterior cavity.35,36 Benefits were observed about an hour after application and remained significant for at least five hours.
Coleus forskohlii has also been used in the treatment of hypothyroidism as well, a condition in which the thyroid gland underperforms. Interestingly, hypothyroidism is a proven risk factor for glaucoma.7
Magnesium
Magnesium has long been recognized as nature's calcium balancer. Previous studies have demonstrated that calcium channel-blocking drugs offer benefits for some glaucoma patients. Armed with this revelation, researchers at the University Eye Clinic in Basel, Switzerland, evaluated the effect of supplemental magnesium on glaucoma patients. Magnesium (121.5 mg twice daily) was administered to 10 glaucoma patients for one month. At the conclusion of the study, results substantiated that magnesium supplementation improved the peripheral circulation, with an accompanying beneficial effect on the visual field in patients with glaucoma.37
Magnesium also has the ability to suppress the sympathetic nervous system. This is a reputation that earned magnesium credit in cardiology,38 acting as an anti-adrenergic, meaning that it can block the “fight-or-flight” reaction, which causes the pupil to dilate and put added pressure on the drainage angle in the anterior chamber of the eye.
Chromium and Selenium
The trace mineral chromium has won credit beyond stabilization of blood glucose levels by improving focusing of the eye and lowering IOP.39 Selenium has also been associated with glaucoma40 and zinc with other vision disorders including age-related macular degeneration.41
Palmitoylethanolamide
Palmitoylethanolamide (PEA) is a lipid compound made in the body in response to tissue damage and inflammatory signaling. PEA is also found in egg yolks, soybeans, peanuts, and other foods.54 PEA binds cell surface receptors that have broad effects on cellular function, and has demonstrated anti-inflammatory, analgesic, neuroprotective, and retinoprotective effects through a synergistic response with endocannabinoids.54,55 PEA is said to have an “entourage” effect where it can indirectly increase endocannabinoid receptor activation through various mechanisms such as inhibition of enzymes responsible for degradation of endocannabinoid ligands.56 Some evidence suggests PEA levels in eye tissues, which are responsible for regulating IOP, are lower in glaucoma patients than those without glaucoma.55,57
In a randomized, double-blind, crossover trial in 42 patients with primary open-angle glaucoma or ocular hypertension being treated with timolol eye drops and whose IOP was between 19 and 24 mm Hg, the addition of 600 mg oral PEA per day (300 mg twice daily) for two months led to a significant reduction in IOP compared with placebo.58 A randomized controlled trial in 32 patients with normal-tension glaucoma found those given 600 mg oral PEA daily (300 mg twice daily) for six months had improved visual field parameters and reduced IOP compared with those who received no treatment.59 In a crossover trial in 40 patients with primary open-angle glaucoma or normal tension glaucoma being treated with a topical prostaglandin analog, IOP decreased and tests of retinal function improved after supplementation with 600 mg PEA daily in addition to topical treatment for four months. Also, quality of life scores increased during the time PEA was taken.60
PEA administered as eye drops appears to be beneficial as well. The use of eye drops containing 0.05% PEA twice daily along with standard anti-glaucoma medication for 30 days was found to improve the health of ocular surface tissue in a controlled trial in 30 glaucoma patients (15 receiving PEA and 15 receiving placebo), indicating PEA may help prevent and treat negative side effects related to chronic glaucoma treatment.61
Iridotomy, a surgical intervention to treat closed-angle glaucoma, may cause a temporary rise in IOP due to an inflammatory response. A placebo-controlled trial in 15 patients undergoing iridotomy to prevent closed-angle glaucoma found taking 300 mg oral PEA twice daily for 15 days prior to the procedure resulted in no significant increase in IOP following surgery.62
Melatonin
Small amounts of the pineal hormone melatonin are synthesized in the retina of humans and most other animals. Melatonin is a powerful antioxidant that may help reduce oxidative damage in the eye. In studies of animals with induced glaucoma, researchers found that placing melatonin in the anterior chamber of the eye reversed the negative effect of ocular hypertension on retinal function and diminished the impact of ocular hypertension on retinal ganglion cells. These results indicate that melatonin could be a valuable resource for treating glaucoma.42
Rutin
Rutin, a bioflavonoid from the citrus family, has demonstrated the ability to lower IOP when used in conjunction with standard drugs. Moreover, experiments have revealed that orally ingested rutin is capable of reaching the eyes.43
11 Lifestyle Tips for Controlling IOP
Exercise
Research findings show that physical activity can have a long-term beneficial effect on ocular perfusion pressure (OPP) (OPP is a measurement derived from IOP and blood pressure), which reflects the status of blood vessels at the optic disc.9 This is an important finding given that low OPP is a risk factor for glaucoma.
The benefit appears to be related to cardiovascular fitness. In a study of over 5,500 men and women, researchers questioned participants about rates of earlier physical activity (15 years previously) and then tested them for IOP and blood pressure. They found an association between higher levels of activity and a 25% reduced risk of low OPP.
Caffeine and Glaucoma
Older research shows that caffeine can cause a temporary increase of IOP.45 The increase lasts for about two hours. Researchers looked at dietary histories of nearly 80,000 women in the Nurses' Health Study (NHS) and over 42,000 men in the Health Professionals Follow-up Study (HPFS) to determine whether repeated caffeine intake throughout the day would sustain IOP elevation and increase the risk of developing primary open angle glaucoma. They found no such association between overall caffeine intake and increased risk of primary open-angle glaucoma.46
Emotional Stress and Glaucoma
As described earlier, closed-angle glaucoma can be related to a structural abnormality in the eye where a narrow angle between the iris and cornea impedes the outflow of aqueous fluid. Acute stress (the fight-or-flight reaction) can narrow the angle even further (by dilating the pupil) and cause an acute glaucomatous event.
Smoking and Glaucoma
Cigarette smoking has been linked to several age-related eye diseases, including age-related macular degeneration, cataract, and severity of diabetic eye disease.51 Although some findings have suggested a role for smoking in glaucoma,52 others, including a systematic review of 11 earlier research studies, show little evidence that cigarette smoking causes primary open-angle glaucoma.53 However, the authors of the review article question the quality of several of the studies that showed no influence of cigarette smoking on glaucoma and, given the clear link between smoking and other eye diseases, believe that further research is needed to confirm their findings.
Sunglasses and Glaucoma
Although exposure to bright light and sun does not appear to be a risk factor for glaucoma, many people with glaucoma experience a sensitivity to light and glare. The problem can be solved by wearing sunglasses that block at least 99% of UVB rays and 95% of UVA rays.
12 Summary
Glaucoma is a common cause of blindness worldwide. It occurs more often in African Americans, Latinos, and Asians than in Caucasians. The most common event associated with glaucoma is an increase in IOP. Many therapies are designed to lower IOP in order to decrease pressure on the retina and optic nerve. Uncontrolled pressure damages retinal ganglion cells and their axons and causes the loss of peripheral vision and, if untreated, can lead to complete blindness. Glaucoma is a multifactorial condition. Genetic defects and nutritional deficiencies are risk factors for glaucoma. Certain behaviors influence IOP and may impact the development and/or progression of glaucoma.
New natural interventions, including a combination of pine bark and bilberry, show great promise in reducing the underlying symptoms of glaucoma, especially when used in combination with traditional glaucoma medications. Antioxidants are also valuable to reduce oxidative damage to the eyes, while minerals are important for general eye health.
In the future, more research is needed to understand the multiple underlying factors that contribute to glaucoma and develop conventional and natural interventions that will help prevent and reverse this major cause of blindness.
Disclaimer and Safety Information
This information (and any accompanying material) is not intended to replace the attention or advice of a physician or other qualified health care professional. Anyone who wishes to embark on any dietary, drug, exercise, or other lifestyle change intended to prevent or treat a specific disease or condition should first consult with and seek clearance from a physician or other qualified health care professional. Pregnant women in particular should seek the advice of a physician before using any protocol listed on this website. The protocols described on this website are for adults only, unless otherwise specified. Product labels may contain important safety information and the most recent product information provided by the product manufacturers should be carefully reviewed prior to use to verify the dose, administration, and contraindications. National, state, and local laws may vary regarding the use and application of many of the therapies discussed. The reader assumes the risk of any injuries. The authors and publishers, their affiliates and assigns are not liable for any injury and/or damage to persons arising from this protocol and expressly disclaim responsibility for any adverse effects resulting from the use of the information contained herein.
The protocols raise many issues that are subject to change as new data emerge. None of our suggested protocol regimens can guarantee health benefits. Life Extension has not performed independent verification of the data contained in the referenced materials, and expressly disclaims responsibility for any error in the literature.
- Steigerwalt, R.D., Belcaro, G., Morazzoni, P., Bombardelli, E., Burki, C., Schonlau, F. Mirtogenol potentiates latanoprost in lowering intraocular pressure and improves ocular blood flow in asymptomatic subjects. Clin Ophthalmol 14 (2010):471-6.
- Lascaratos G et al. Mitochondrial dysfunction in glaucoma: Understanding genetic influences. Mitochondrion. 2011 Nov 28. [Epub ahead of print]
- Lee S et al. drial dysfunction in glaucoma and emerging bioenergetic therapies. Exp Eye Res. 2011 Aug;93(2):204-12. Epub 2010 Aug 4.
- Gupta N et al. Glaucoma as a neurodegenerative disease. Curr Opin Ophthalmol. 2007 Mar;18(2):110-4.
- American Academy of Ophthalmology. www.geteyesmart.org/eyesmart/eye-health-news/press-releases/20110401.cfm, accessed 11-18-11.
- Newman-Casey, P.A., Talwar, N. Nan, B. Musch, D.C. Stein, J.D. The Relationship Between Components of Metabolic Syndrome and Open-Angle Glaucoma. Ophthalmology 118 (2011): 1318-26.
- Cross, J.M., Girkin, C.A., Owsley, C., McGwin, G. Jr. The association between thyroid problems and glaucoma. Br J Ophthalmol 92 (2008):1503-5.
- Gadia, R., Sihota, R., Dada, T., Gupta, V. Current profile of secondary glaucomas. Indian J Ophthalmol 56 (2008): 285-89.
- Yip, J.L., Broadway, D.C., Luben, R., Garway-Heath, D.F., et al. Physical Activity and Ocular Perfusion Pressure: The EPIC-Norfolk Eye Study. Invest Ophthalmol Vis Sci 52 (2011): 8186-92.
- Stone, E.M. Fingert, J.H., Alward, W.L.M., Nguyen, T.D., et al. Identification of a gene that causes primary open angle glaucoma. Science 275 (1997): 668-70.
- Kuchtey J, Olson LM, Rinkoski T, MacKay EO, Iverson TM, et al. Mapping of the Disease Locus and Identification of ADAMTS10 As a Candidate Gene in a Canine Model of Primary Open Angle Glaucoma. PLoS Genet 7(2011): e1001306.
- Patel, H. Y., Richards, A. J., De Karolyi, B., Best, S. J., Danesh-Meyer, H. V. and Vincent, A. L., Screening glaucoma genes in adult glaucoma suggests a multiallelic contribution of CYP1B1 to open-angle glaucoma phenotypes. Clinical & Experimental Ophthalmology 29 (2011): 4320-6.
- Bulboaca A et al. [Endothelial dysfunction in patients with open-angle glaucoma and atheromatous lesions]. logia. 2003;58(3):52-5.
- Resch H et al. helial dysfunction in glaucoma. Acta Ophthalmol. 2009 Feb;87(1):4-12. Epub 2008 May 27.
- Venkataraman ST et al. Vascular reactivity of optic nerve head and retinal blood vessels in glaucoma--a review. Microcirculation. 2010 Oct;17(7):568-81. doi: 10.1111/j.1549-8719.2010.00045.x.
- Mandal AK, Chakrabarti D. Update on congenital glaucoma. Indian J Ophthalmol 59 (2011) Suppl:S148-57.
- Weinreb, R.N., Kaufman, P.L. Glaucoma Research Community and FDA Look to the Future, II: NEI/FDA Glaucoma Clinical trial design and endpoints symposium: measures of structural change and visual function. Invest Ophthalmol Vis Sci 52 (2011): 7842-51.
- Kong, G.Y.X., et al. Hypothesis and Innovation: Mitochondrial Dysfunction and Glaucoma. J Glaucoma 18 (2009): 93-100.
- McElnea, E.M. et al. Oxidative stress, mitochondrial dysfunction and calcium overload in human lamina cribrosa cells from glaucoma donors. Mol Vis. 17 (2011): 1182-91.
- Fang, J.H., Wang, X.H,. Xu, Z.R., Jiang, .FG. Neuroprotective effects of bis(7)-tacrine against glutamate-induced retinal ganglion cells damage. BMC Neuroscience 11 (2010):31.
- Quinzii CM et al. Coenzyme Q and mitochondrial disease. Dev Disabil Res Rev. 2010 Jun;16(2):183-8.
- Rucker R et al. Potential physiological importance of pyrroloquinoline quinone. Altern Med Rev. 2009 Sep;14(3):268-77.
- AGIS Investigators. The Advanced Glaucoma Intervention Study (AGIS): 9. Comparison of glaucoma outcomes in black and white patients within treatment groups. Am J Ophthalmol. 2001 Sep;132(3):311-20.
- Nguyen D, Flynn WJ. Response to Laser Trabeculoplasty in the Latino and Caucasian Population. ARVO 2011. Abstract 2611.
- Nishioka, K., Hidaka, T., Nakamura, S., et al. Pycnogenol, French maritime pine bark extract, augments endothelium-dependent vasodilation in humans. Hypertens Res. 30 (2007): 775-80.
- Alm A., Grierson I., Shields, M.B.. Side Effects Associated with Prostaglandin Analog Therapy. Surv Ophthalmol 53 (2008) Suppl1:S93-105.
- Zhou. L., Higginbotham, E., Yue, B. Effects of ascorbic acid on levels of fibronectin, laminin and collagen type 1 in bovine trabecular meshwork in organ culture. Current Eye Research 17 (1998): 211-17.
- Falsini, B., Marangoni, D., Salgarello, T., Stifano, G., et al. Effect of epigallocatechin-gallate on inner retinal function in ocular hypertension and glaucoma: a short-term study by pattern electroretinogram. Graefes Arch Clin Exp Ophthalmol 247 (2009): 1223-33.
- Yuki K et al. Reduced-serum vitamin C and increased uric acid levels in normal-tension glaucoma. Graefes Arch Clin Exp Ophthalmol. 2010 Feb;248(2):243-8. Epub 2009 Sep 8.
- Zanon-Moreno V et al. Association between a SLC23A2 gene variation, plasma vitamin C levels, and risk of glaucoma in a Mediterranean population. Mol Vis. 2011;17:2997-3004. Epub 2011 Nov 17.
- Park, J.W., Kwon, H.J., Chung, W.S., Kim, C.Y., Seong, G.J. Short-Term Effects of Ginkgo Biloba Extract on Peripapillary Retinal Blood Flow in Normal Tension Glaucoma. Korean J Ophthalmol 25 (2011): 323-8.
- Park, K.H. Comparison of optic nerve head blood flow in normal-tension glaucoma with asymmetric visual field loss. J Korean Ophthalmol Soc 40 (1999):1318-23.
- Kim, S.W., Kim, C.Y., Seong, G.J. The short term effects of bimatoprost on optic nerve head and peripapillary retinal blood flow. J Korean Ophthalmol Soc. 45 (2004): 1322-1329.
- Khatun, S., Chatterjee, N.C., Cakilcioglu, U. Antioxidant activity of the medicinal plant Coleus forskohlii Briq. African Journal of Biotechnology 10 (2011):2530-35.
- Caprioli, J., Sears, M., Bausher, L., et al. Forskolin lowers intraocular pressure by reducing aqueous inflow. Invest Ophthalmol Vis Sci 25 (1984):268-77.
- Hartman, H.B., Roehr, J.E., Kotyuk, B.L., et al. HP 663: a novel compound for the treatment of glaucoma. Drug Dev Res. 12 (1988):197-209.
- Gaspar, A.Z., Gasser, P., Flammer, J. The influence of magnesium on visual field and peripheral vasospasm in glaucoma. Ophthalmologica 209 (1995):11-13.
- Altura, B.M., Altura, B.T. Magnesium and the cardiovascular system: experimental and clinical aspects updated. In Sigel H, Sigel A (eds): “Metal Ions in Biological Systems, Vol. 26, Compendium on Magnesium and Its Role in Biology, Nutrition, and Physiology.” New York: Marcel Dekker, pp359-416, 1990.
- Head, K. Natural Therapies for Ocular Disorders Part Two: Cataracts and Glaucoma. Alternative Medicine Review 6 (2001): 141-66.
- Bruhn, R.L., Stamer, W.D., Herrygers, L.A., Levine, J.A., Noecker, R.J. Relationship Between Glaucoma and Selenium Levels in Plasma and Aqueous Humour. Br J Ophthalmol 93 (2009): 1155-58.
- National Eye Institute. http://www.nei.nih.gov/amd/summary.asp. Accessed December 10, 2011.
- Belforte, N.A., Moreno, M.C., de Zavalía, N., Sande, P.H., et al. Melatonin: A Novel Neuroprotectant for the Treatment of Glaucoma. J Pineal Res 48 (2010): 353-64.
- Pescosolido N et al. Oral administration of an association of forskolin, rutin and vitamins B1 and B2 potentiates the hypotonising effects of pharmacological treatments in POAG patients. Clin Ter. 2010;161(3):e81-5.
- Merritt, J,C,, Crawford, W.J., Alexander, P.C., et al. Effect of marijuana on intraocular and blood pressure in glaucoma. Ophthalmology 87 (1980):222-8.
- Peczon, J.D., Grant, W.M. Sedatives, stimulants, and intraocular pressure in glaucoma. Arch Ophthalmol. 72 (1964): 178-88.
- Kang, J.H., Willett, W.C., Rosner, B.A., Hankinson, S.E., Pasquale, L.R. Caffeine Consumption and the Risk of Primary Open-Angle Glaucoma: A Prospective Cohort Study. Invest Ophthalmol Vis Sci. 49 (2008): 1924-1931.
- Wen, X., Takenaka, M., Murata, M., Homma, S. Antioxidative activity of a zinc-chelating substance in coffee. Biosci Biotechnol Biochem 68 (2004): 2313-8.
- Clifford, M.N. Chlorogenic acids: Their complex nature and routine determination in coffee beans. Food Chem 4 (1979): 63-71.
- Stich, H.F. The beneficial and hazardous effects of simple phenolic compounds. Mutat Res 259 (1991): 307-24.
- Nakajima, Y. Shimazawa, M., Mishima, S., Hara, H. Water extract of propolis and its main constituents, caffeoylquinic acid derivatives, exert neuroprotective effects via antioxidant actions. Life Sciences 80 (2007): 370-7.
- Zhang, X., Kahende, J., Fan, A.Z., Barker, L., et al. Smoking and visual impairment among older adults with age-related eye diseases. Prev Chronic Dis 8 (2011): A1-8.
- Klein, B.E., Klein, R., Ritter, L.L. Relationship of drinking alcohol and smoking to prevalence of open-angle glaucoma. The Beaver Dam Eye Study. Ophthalmology. 100 (1993): 1609-1613.
- Edwards, R., Thornton, J., Ajit, R., Harrison, R.A., Kelly, S.P. Cigarette smoking and primary open angle glaucoma: a systematic review. J Glaucoma. 17 (2008): 558-566.
- Adornetto A, Rombolà L, Morrone LA, et al. Natural Products: Evidence for Neuroprotection to Be Exploited in Glaucoma. Nutrients. Oct 16 2020;12(10)doi:10.3390/nu12103158
- Keppel Hesselink JM, Costagliola C, Fakhry J, Kopsky DJ. Palmitoylethanolamide, a Natural Retinoprotectant: Its Putative Relevance for the Treatment of Glaucoma and Diabetic Retinopathy. Journal of ophthalmology. 2015;2015:430596. doi:10.1155/2015/430596
- Petrosino S, Di Marzo V. The pharmacology of palmitoylethanolamide and first data on the therapeutic efficacy of some of its new formulations. Br J Pharmacol. Jun 2017;174(11):1349-1365. doi:10.1111/bph.13580
- Chen J, Matias I, Dinh T, et al. Finding of endocannabinoids in human eye tissues: implications for glaucoma. Biochemical and biophysical research communications. May 20 2005;330(4):1062-7. doi:10.1016/j.bbrc.2005.03.095
- Gagliano C, Ortisi E, Pulvirenti L, et al. Ocular hypotensive effect of oral palmitoyl-ethanolamide: a clinical trial. Invest Ophthalmol Vis Sci. Aug 3 2011;52(9):6096-100. doi:10.1167/iovs.10-7057
- Costagliola C, Romano MR, dell'Omo R, Russo A, Mastropasqua R, Semeraro F. Effect of palmitoylethanolamide on visual field damage progression in normal tension glaucoma patients: results of an open-label six-month follow-up. Journal of medicinal food. Sep 2014;17(9):949-54. doi:10.1089/jmf.2013.0165
- Rossi GCM, Scudeller L, Lumini C, et al. Effect of palmitoylethanolamide on inner retinal function in glaucoma: a randomized, single blind, crossover, clinical trial by pattern-electroretinogram. Sci Rep. Jun 26 2020;10(1):10468. doi:10.1038/s41598-020-67527-z
- Di Zazzo A, Roberti G, Mashaghi A, Abud TB, Pavese D, Bonini S. Use of Topical Cannabinomimetic Palmitoylethanolamide in Ocular Surface Disease Associated with Antiglaucoma Medications. Journal of ocular pharmacology and therapeutics : the official journal of the Association for Ocular Pharmacology and Therapeutics. Nov 2017;33(9):670-677. doi:10.1089/jop.2016.0117
- Pescosolido N, Librando A, Puzzono M, Nebbioso M. Palmitoylethanolamide effects on intraocular pressure after Nd:YAG laser iridotomy: an experimental clinical study. Journal of ocular pharmacology and therapeutics : the official journal of the Association for Ocular Pharmacology and Therapeutics. Dec 2011;27(6):629-35. doi:10.1089/jop.2010.0191
- Scanlon G, Loughman J, Farrell D, McCartney D. A review of the putative causal mechanisms associated with lower macular pigment in diabetes mellitus. Nutrition Research Reviews. 2019;32(2):247-264. doi:10.1017/S095442241900012X
- Higdon J. Oregon State University. Data on file.
- Loskutova E, Nolan J, Howard A, Beatty S. Macular pigment and its contribution to vision. Nutrients. May 29 2013;5(6):1962-9. doi:10.3390/nu5061962
- Lem DW, Gierhart DL, Davey PG. Carotenoids in the Management of Glaucoma: A Systematic Review of the Evidence. Nutrients. Jun 6 2021;13(6)doi:10.3390/nu13061949
- Daga FB, Ogata NG, Medeiros FA, et al. Macular Pigment and Visual Function in Patients With Glaucoma: The San Diego Macular Pigment Study. Invest Ophthalmol Vis Sci. Sep 4 2018;59(11):4471-4476. doi:10.1167/iovs.18-24170
- Loughman J, Loskutova E, Butler JS, Siah WF, O'Brien C. Macular Pigment Response to Lutein, Zeaxanthin, and Meso-zeaxanthin Supplementation in Open-Angle Glaucoma: A Randomized Controlled Trial. Ophthalmol Sci. Sep 2021;1(3):100039. doi:10.1016/j.xops.2021.100039
- Hunter AML, Loskutova E, Lingham G, O'Brien CJ, Butler JS, Loughman J. Higher Macular Pigment Levels are Associated with Better Contrast Sensitivity and Photostress Recovery Time in Patients with Open-Angle Glaucoma Supplemented with Carotenoids. Investigative Ophthalmology & Visual Science. 2022;63(7):2699 – A0063-2699 – A0063.
- Sanz-Gonzalez SM, Raga-Cervera J, Aguirre Lipperheide M, et al. Effect of an oral supplementation with a formula containing R-lipoic acid in glaucoma patients. Arch Soc Esp Oftalmol (Engl Ed). Mar 2020;95(3):120-129. Efecto de la suplementacion oral con una formula que contiene acido R-lipoico en pacientes con glaucoma. doi:10.1016/j.oftal.2019.11.009
- Jabbarpoor Bonyadi MH, Yazdani S, Saadat S. The ocular hypotensive effect of saffron extract in primary open angle glaucoma: a pilot study. BMC Complement Altern Med. Oct 15 2014;14:399. doi:10.1186/1472-6882-14-399
- Heitmar R, Brown J, Kyrou I. Saffron (Crocus sativus L.) in Ocular Diseases: A Narrative Review of the Existing Evidence from Clinical Studies. Nutrients. Mar 18 2019;11(3)doi:10.3390/nu11030649
- Ramdas WD. The relation between dietary intake and glaucoma: a systematic review. Acta Ophthalmol. Sep 2018;96(6):550-556. doi:10.1111/aos.13662
