
Atherosclerosis and Cardiovascular Disease
Atherosclerosis and Cardiovascular Disease
Last Section Update: 03/2025
Contributor(s): Maureen Williams, ND; Shayna Sandhaus, PhD; Chancellor Faloon, Health & Wellness Author; Franco Melis; Stephen Tapanes, PhD
1 Overview
Summary and Quick Facts for Atherosclerosis and Cardiovascular Disease
- Atherosclerosis can occur anywhere in the body, but it is particularly dangerous when it affects the arteries that supply oxygenated blood to essential organs such as the brain and heart. These conditions are called atherosclerotic cerebrovascular and cardiovascular diseases. Coronary artery disease is the most common type of heart disease, affecting over 20 million Americans.
- Two of the most feared complications of atherosclerosis are heart attack and stroke.
- A healthy diet and lifestyle are the cornerstone of atherosclerosis prevention and treatment.
- Research has revealed that several targeted nutrients can help protect vascular health and may reduce cardiovascular risk. A comprehensive nutritional regimen can target all the risk factors that contribute to atherosclerosis.
- Comprehensive blood testing helps identify and target specific risk factors, facilitating a personalized, targeted treatment regimen that can be used to preserve and improve cardiovascular health.
What is Atherosclerosis?
Atherosclerosis is a chronic progressive condition of the arteries marked by characteristic lesions known as atheromas or atherosclerotic plaques. It is the main cause of heart attack, stroke, serious peripheral artery disease events, and cardiovascular death.1,2 Atherosclerosis is generally caused by a combination of endothelial dysfunction, inflammation, and oxidative stress, which may be induced by a range of conditions such as smoking, unhealthy lifestyle, abnormal lipid levels, insulin resistance, obesity, and high blood pressure.3
As atherosclerosis progresses, plaques can expand within arteries, obstructing blood flow to tissues and sometimes causing site-specific symptoms. A plaque can also become highly inflamed and prone to rupture. Plaque rupture leads to formation of a blood clot that can block blood flow or break off into circulation and obstruct smaller vessels, sometimes resulting in major, life-threatening, catastrophic cardiovascular events.2 Blood clot formation can also be triggered by erosion of the cells covering a plaque. Plaque erosion is a less common but important cause of major cardiovascular events, frequently in people without classic cardiovascular risk factors, but is associated with a better prognosis than plaque rupture.4
What are Risk Factors for Atherosclerosis and Cardiovascular Disease?
There are many conditions associated with atherosclerosis and cardiovascular risk. Some important risk factors include5,6:
- Older age
- Family history
- Unhealthy diet and sedentary lifestyle
- Smoking
- Dyslipidemia (imbalanced levels of cholesterol and triglycerides)
- Hypertension
- Elevated glucose and insulin levels
- Chronic kidney disease
- Obesity
- High homocysteine levels
- A range of chronic inflammatory conditions
- Chronic infections such as human immunodeficiency virus/acquired immunodeficiency syndrome (HIV/AIDS)
It is important to note that a growing proportion of cardiovascular events, including heart attacks, are now occurring in people without well-established risk factors.7,8
What are the Signs and Symptoms of Atherosclerosis?
Atherosclerosis is generally asymptomatic until very late stages. It is therefore critical for all susceptible individuals to take preventative measures and monitor their cardiovascular health.
What are the Treatments for Atherosclerosis?
- Cholesterol-lowering drugs
- Anti-platelet drugs, including aspirin
- Blood pressure-lowering drugs
- Blood glucose-lowering drugs
- Surgeries, such as coronary artery bypass and percutaneous coronary intervention (angioplasty)
What Dietary and Lifestyle Changes Can Benefit Atherosclerosis?
- Eat a balanced, plant-based diet rich in fruits and vegetables (eg, a Mediterranean-style diet)
- Include heart-healthy foods like extra virgin olive oil, cold water fish, and fiber-rich whole grains
- Exercise regularly
- Get adequate sleep (duration and quality)
- Build healthy social networks
- Do not smoke
- Limit or avoid alcohol intake
What Nutrients May Counteract Atherosclerosis?
- Omega-3 fatty acids. Omega-3 fatty acids help prevent the development and progression of atherosclerosis through multiple mechanisms, including reducing inflammation, lowering triglyceride levels, improving endothelial function, and inhibiting blood clot formation.9
- Coenzyme Q10 (CoQ10). Treatment with CoQ10 can improve vascular endothelial function and lipid profiles in patients with atherosclerosis, in part through decreasing oxidative stress and inflammation.10,11 In combination with selenium, CoQ10 decreased cardiovascular mortality in a long-term clinical trial.12
- B vitamins. Folate and other B vitamins have been found to lower the risk of stroke, largely by reducing homocysteine levels.13
- Curcumin. Clinical trials have shown curcumin can promote metabolic health, support weight loss, improve lipid levels, and lower high blood pressure, oxidative stress, and inflammation.14
- Lipoic acid. Lipoic acid has been found to improve endothelial function by reducing oxidative stress and inflammation and increasing endothelial nitric oxide synthesis.15
- Lycopene. In clinical trials, lycopene has demonstrated anti-atherogenic effects such as improvement in lipid profiles, blood pressure, and endothelial function, and reduction of inflammation and oxidative stress.16
- Garlic extracts. Garlic and its extracts, in various preparations, have been shown to exert broad anti-atherogenic effects through lipid- and blood pressure-lowering effects, as well as lowering inflammation, homocysteine, and protecting against coronary artery calcification.17
- Polyphenols. This broad class of plant-derived molecules includes an array of anti-atherogenic compounds including resveratrol, quercetin, hesperidin from citrus peel, catechins from tea, flavanols from cocoa, proanthocyanidins from grape seeds, French maritime pine bark, arjuna bark, aronia and hawthorn berries, and more. Their beneficial effects are attributable in large part to combatting oxidative stress and inflammation, reducing blood pressure and atherogenic lipids, and promoting healthy endothelial function.18,19
- Other natural interventions that may help counteract some of the processes that contribute to atherosclerosis and its progression include ginkgo biloba, lutein, magnesium, L-arginine, vitamins K and E, and more.
2 Introduction
Atherosclerosis is a chronic inflammatory disease process characterized by plaque lesions in the arterial walls.20 Plaque is made up of fatty substances, immune cells, smooth muscle cells, and dead and dying cell debris, enclosed under a fibrous cap. A growing plaque may restrict blood flow and cause symptoms of ischemia (reduced blood flow). As atherosclerosis progresses, it may trigger the formation of a blood clot, which can lead to serious complications such as a heart attack or stroke.21
Atherosclerosis is often categorized by the area affected. Some of the more clinically important types of atherosclerosis are22-24:
- Coronary artery disease, which affects the arteries of the heart
- Peripheral artery disease, which usually affects the arteries in the legs, but may involve arteries in the arms or pelvis
- Cerebrovascular disease, which involves the arteries that supply blood to the brain
- Aortic atherosclerosis, affecting the large artery (aorta) leading away from the heart
- Renal artery stenosis, which involves narrowing of the arteries that bring blood to the kidneys
- Mesenteric artery ischemia, which is a lack of blood flow to the intestines due to atherosclerosis
Coronary artery disease is the most common type of heart disease, affecting over 20 million American adults, and is the leading cause of death in the United States.25,26 The prevalence of coronary artery disease increases with age: almost 11% of U.S. adults aged 45 years and older and 17% of those aged 65 years and older have coronary artery disease.25 Nevertheless, it is important to note that the atherosclerotic process often begins in childhood, particularly in children with overweight and obesity and those with high blood glucose levels.27
The consequences of other types of atherosclerosis are also tremendously significant. For example, cerebrovascular disease is a cause of stroke, and peripheral artery disease can restrict activities and result in loss of limbs.20
Many factors contribute to the development of atherosclerosis, some of which are modifiable. These include smoking, poor diet, physical inactivity, and excessive alcohol consumption, as well as conditions such as high blood pressure, high cholesterol and/or triglycerides, diabetes and insulin resistance, obesity, sleep disorders, air pollution exposure, imbalanced gut microbiome, and certain bacterial and viral infections.21,25 In addition, elevated blood levels of homocysteine and high-sensitivity C-reactive protein (hs-CRP) have consistently been found to be correlated with increased atherosclerosis risk.28,29 Tracking risk factors through periodic blood testing can help monitor overall vascular health and plan diet, lifestyle, and nutrient supplementation regimens accordingly.
In this protocol, you will learn how atherosclerosis develops and contributes to potentially deadly cardiovascular events. You will also learn how you can use blood testing and other strategies to assess your cardiovascular risk. This protocol will also help you understand the different medical options available to treat vascular disease, and how dietary and lifestyle changes, along with targeted nutritional supplements, can help support overall cardiovascular health.
3 Atherosclerosis Development & Progression
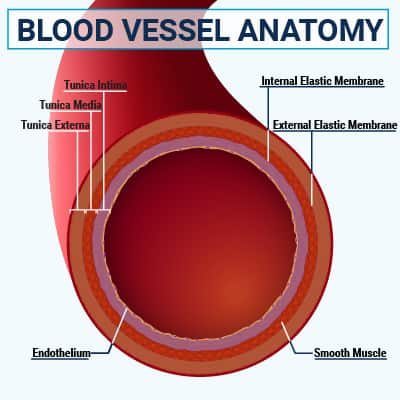
Endothelial cells form a single-cell layer on the inside of blood vessels that facilitates blood flow, communicates with various blood cells, and acts as a barrier between the blood and the vessel wall. Endothelial cells are connected by tight junctions; however, in the initial stage of atherosclerosis, turbulent blood flow interrupts these connections, making them more permeable, or leaky, and allowing lipoproteins to penetrate into the space under the endothelial cells, known as the intima. Sites of turbulent blood flow—generally at branch points in the major arteries—are where the atherosclerotic process usually begins and are the most common sites of arterial plaques.2,21
Nitric oxide (NO) affects vascular function and health by promoting blood vessel relaxation, regulating endothelial permeability, inhibiting blood clotting, and controlling vascular smooth muscle cell proliferation.3 NO is produced via the action of an enzyme made by endothelial cells, called endothelial nitric oxide synthase (eNOS), on the amino acid L-arginine.3,30 It can also be synthesized from dietary nitrates, such as those found in beets and green leafy vegetables.31 Healthy endothelial cells regulate vascular tone in response to a complex network of signals by modulating their production of eNOS.32 Malfunction of the eNOS pathway, such as due to aging, inflammation, oxidative stress, and nutrient deficiencies, results in a cycle of endothelial NO deficiency, dysregulated vascular tone, and endothelial damage.3,30 Endothelial dysfunction is characterized by reduced endothelial NO synthesis and is a defining feature of atherosclerosis.3,32
Endothelial dysfunction, inflammation, and oxidative stress are key contributors to atherosclerosis initiation, as well as its progression. When endothelial cell functions are compromised, lipid and immune cells infiltrate the deeper layers of the vessel wall, triggering inflammation and free radical production. In a vicious cycle, this leads to more endothelial damage.2 Factors such as aging, dyslipidemia, high blood pressure, diabetes, obesity, unhealthy diet, lack of exercise, and smoking help initiate atherosclerosis by promoting endothelial dysfunction, oxidative stress, and inflammatory pathways.3
Once trapped in the intima, lipoproteins undergo various changes, including aggregation and oxidation. It is thought that signals from oxidized lipids and other factors in the vessel wall trigger activation of endothelial cells, resulting in expression of cell surface proteins that attract and adhere to circulating immune cells called monocytes. These monocytes enter the intima of the blood vessel wall and undergo transformation into macrophages, specialized cells whose role is to engulf and destroy infectious agents, cancer cells, or other unhealthy substances.33 Within the vessel wall, macrophages take up the excess aggregated and oxidized lipoproteins. In their lipid-laden state, they are known as foam cells. Accumulating foam cells frequently die and break down, releasing pro-inflammatory molecules, pro-clotting factors, enzymes, cholesterol, and cellular debris, forming the necrotic core of an atherosclerotic plaque.2,21 Interestingly, other macrophages in atherosclerotic plaques take on the role of resolving inflammation. Pro-resolving macrophages are believed to contribute to plaque and atherosclerosis regression.33
Vascular smooth muscle cells normally make up a layer of the blood vessel wall known as the media, and are responsible for contraction and relaxation of blood vessels.2 As plaques develop and grow, smooth muscle cells migrate into the intima, where they can undergo transformation to become more like macrophages and, ultimately, foam cells. Other migratory smooth muscle cells proliferate and secrete collagen and other structural molecules that form a fibrous cap under the endothelial cell layer, which helps stabilize the plaque against rupture.21
Immune cells known as T cells and B cells play a nuanced role in atherosclerotic plaques. T cells within plaques release cellular messengers known as cytokines that regulate inflammatory processes. B cells produce antibodies to low-density lipoprotein (LDL), oxidized LDL, apolipoprotein B (ApoB), cellular debris, and even certain pathogens. These antibodies can promote or inhibit atherosclerosis by modulating the immune response.21,34,35
Atherosclerosis Progression
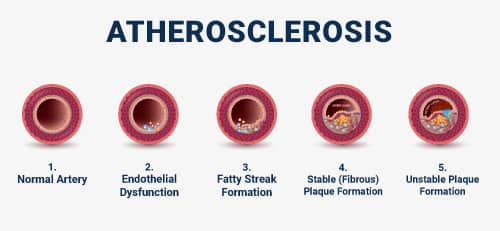
Atherosclerosis develops over many years. During the course of the disease, affected arteries undergo changes to their composition and structure.36 Classic rupture-prone plaques progress in recognizable ways and are the leading cause of fatal heart attacks and sudden death. However, plaques without characteristic high-risk features are responsible for a significant proportion of major coronary events due to plaque erosion or, rarely, eruptive calcified nodules.1
Fatty streak formation. Atherosclerosis begins with accumulation of modified lipoproteins, smooth muscle cells, and extracellular matrix (non-cell components of body tissue) in the intimal layer of the vessel wall due to endothelial dysfunction. Immune cells then enter the vessel wall, leading to increased inflammatory signaling and the accumulation of foam cells. As foam cells coalesce, a visible fatty streak forms. Fatty streak formation can begin as early as childhood.2,21
Advanced atherosclerosis. The progression of atherosclerotic plaque is defined by three key features: the formation of a fibrous cap that covers the lesion under the endothelial cell layer, development of a necrotic core, and calcification.2
The fibrous cap is made by smooth muscle cells that migrate to the endothelial region and secrete collagen and other extracellular materials. The cap can be thought of as a structural support that protects against rupture. Its ability to stabilize the plaque depends on its thickness and collagen content.2,21
Foam cells, lipids and lipoproteins, and immune cells can continue to accumulate under the fibrous cap. As the foam cells die, a necrotic core filled with cholesterol and cellular debris may form. A growing necrotic core can cause a plaque to expand into the lumen (the opening of the vessel where blood flows) and impede blood flow.2,21
Vascular calcification is another important feature of advanced atherosclerosis. Calcium is released from dying smooth muscle cells and macrophages in the deeper layers of the vessel wall, forming microcalcifications under the necrotic core that can evolve into larger calcifications.2,21 Calcified plaque is generally more stable than non-calcified plaque; nevertheless, coronary artery calcification has been closely correlated with the risk of cardiovascular events and mortality, probably because it reflects overall atherosclerosis burden.37,38
Plaque stability. A highly calcified plaque with a small core and a thick fibrous cap tends to be more stable, while one with microcalcifications and a large inflamed necrotic core is more prone to rupture. Macrophages contribute to vulnerability by ramping up inflammation and releasing enzymes called matrix metalloproteinases (MMPs) that break down the fibrous cap. T-cell infiltration of the cap has also been implicated in destabilizing plaque.21,39
Importantly, the size of a plaque and its obstruction of a vessel opening are unrelated to its stability.38 For instance, when a plaque obstructs 70–80% of a coronary vessel, shortness of breath and chest pain (angina), especially with exertion, may occur. Over time, restricted coronary blood flow can weaken the heart muscle. On the other hand, rupture of an unstable plaque can give rise to an acute catastrophic cardiovascular event.23
Plaque destabilization and thrombosis. Plaque destabilization results in thrombosis (the formation of a blood clot) in the blood vessel. Plaques can become destabilized through rupture, erosion, or eruptive calcified nodules. Plaques that rupture and plaques that erode have very different compositions and characteristics, tend to be located in different regions of arteries, and tend to occur in different populations.4,7
- Plaque rupture. The fibrous cap of a highly inflamed lipid-rich plaque is vulnerable to rupture, allowing blood to come in contact with the necrotic core, triggering thrombosis. Plaque rupture is the cause in the majority of serious cardiovascular events, including heart attacks, strokes, critical peripheral artery disease events, and cardiovascular deaths.1,2
- Plaque erosion. A very different type of plaque is susceptible to erosion rather than rupture.40 Plaque erosion is implicated in roughly 30–40% of acute coronary events.4,7 Plaque erosion-related blood clots and acute coronary events result from a breach in the endothelial barrier covering an erosive-type plaque, exposing the intact fibrous cap to blood flow.1 Circulating platelets become activated by exposed smooth muscle cells and the non-cellular matrix of the cap, triggering thrombosis. Cytotoxic T cells and white blood cells called neutrophils are attracted to the site of endothelial erosion and play a role in this process.1,4
- Eruptive calcified nodules. A third mechanism of plaque destabilization known to cause cardiovascular events involves eruption of calcified nodules through the vessel lining and into the lumen, leading to blood clot initiation.43 Although the cause of calcified nodule formation is not fully understood, mechanical stress on the endothelium is thought to contribute. Eruptive calcified nodules are a relatively rare cause of acute coronary thrombosis often associated with extensive coronary artery calcification and advanced age.1
Compared to rupture-prone plaques, plaque erosion tends to occur in younger individuals, in women more than men, and in people with a milder extent and severity of atherosclerosis.40,41 In addition, eroded plaques are less inflamed, with less or absent lipid-laden and necrotic core, and have a higher concentration of vascular smooth muscle cells in a thick intact fibrous cap compared with plaques that rupture.1 People with plaque erosion have better outcomes than those with plaque rupture, possibly because they have a lower overall atherosclerosis burden and fewer traditional cardiovascular risk factors.4,42
Plaque destabilization and thrombosis can lead to life-threatening events.39 A blood clot can grow and block blood flow where it forms or may break off and travel in the bloodstream as an embolism. An embolism poses the threat of becoming trapped in a small vessel and obstructing blood flow elsewhere in the body. Blood clots that block heart or brain blood flow are especially dangerous because they result in heart attack or stroke, respectively.2
Plaque healing. associated with catastrophic outcomes, the eventual outcome in most cases is repair. As healing occurs, the thrombus is stabilized by a structural protein called fibrin. Vascular smooth muscle cells infiltrate and further stabilize the site, and a new endothelium is eventually generated. Some healed plaques do not cause any clinical events, but others undergo structural changes over a period of days to weeks that eventually occlude the vessel, resulting in an acute ischemic event (eg, a heart attack) long after the initial plaque destabilization.4 Another possible outcome is multiple cycles of asymptomatic plaque rupture and healing, with continued atherosclerosis progression and narrowing of arteries.23,39
Refer to Life Extension’s protocol on Blood Clot Prevention for more information.
4 Signs & Symptoms
Atherosclerosis often causes no symptoms until there is severe and potentially deadly arterial blockage. This is why early monitoring of cardiovascular risk factors through preventive blood testing and adhering to a healthy diet and lifestyle are important. When symptoms do occur, they vary depending on the location of the blockage and whether the obstruction is chronic or acute.
Coronary artery disease can cause a type of chest pain or discomfort known as angina. Angina usually occurs when blood flow to the heart muscle is chronically restricted due to narrowing of one or more coronary arteries. It may be described as heaviness, pressure, or a squeezing sensation on the left side or center of the chest and may be accompanied by shortness of breath, fatigue, nausea, a heartburn-like sensation, a lump in the throat, or other symptoms. In stable angina, symptoms are triggered by exertion or stress and resolve with rest, whereas in unstable angina—an acute medical emergency—symptoms come on unpredictably and do not respond to rest. Sweating, fatigue, breathlessness, and nausea even in the absence of chest pain are considered angina-equivalent when they occur in people with a high cardiovascular risk.44,45
Acute coronary syndrome is a life-threatening event that occurs when blood flow to the heart muscle is suddenly severely decreased. This includes unstable angina and heart attack. Acute coronary syndrome is often accompanied or preceded by chest pain, or pain or discomfort in the arm, jaw, or back.46 Other symptoms that may herald acute coronary syndrome include shortness of breath, unusual fatigue and weakness, sleep disturbance, nausea, digestive upset, lightheadedness, sweating, headache, and anxiety. Such symptoms have been reported anywhere from three months to two days before acute coronary syndrome.47
Peripheral artery disease may cause pain in the extremities (usually the legs) due to chronic blood flow restriction. This pain, known as claudication and frequently felt in the calf, is typically triggered by exertion and relieved with rest; however, in more advanced disease with greater blood flow restriction, symptoms are more persistent.47
Cerebrovascular atherosclerosis affecting the intracranial, vertebral, and carotid artery systems in the neck and skull can give rise to clots that cause sudden obstructive events such as strokes and transient ischemic attacks (TIAs).24 Neurological symptoms such as weakness, numbness, confusion, speech problems, dizziness, loss of coordination and balance, and vision problems mark these events.47,48 In addition, it is now thought that early-stage carotid artery disease, once referred to as asymptomatic, may be marked by “age-related” cognitive decline and impairment resulting from chronic restriction in brain blood flow.48
Renal artery stenosis can restrict blood flow to one or both kidneys, which, over time, contributes to high blood pressure and chronic kidney disease (CKD).49,50
Atherosclerosis tends to affect multiple locations within an individual. For instance, people with peripheral artery disease often have carotid and coronary artery diseases.51,52
Diagnosis
Atherosclerosis can be diagnosed in the absence of symptoms, with non-urgent symptoms, or in an acute or emergency setting. Exam and diagnostic testing will vary, depending on the context.
In a non-emergency setting, diagnosis of atherosclerosis begins with a complete history and physical exam. Risk factors related to lifestyle, personal medical history, and family history can be assessed and blood pressure can be measured.22 This should be followed by laboratory assessment of the lipid profile and glucose metabolism. Laboratory tests include a full lipid panel with total cholesterol, LDL-cholesterol, high-density lipoprotein (HDL) -cholesterol, and triglyceride levels. Test results may also provide calculated values for non–HDL-cholesterol and a total-to-HDL-cholesterol ratio, which appear to be more closely correlated with cardiovascular risk than total or LDL-cholesterol levels.68 Testing for additional important cholesterol fractions, including oxidized LDL and LDL particle size and count, should also be considered. Basic glucose metabolism testing would begin with fasting blood glucose and hemoglobin A1C (HbA1c).
In some cases, such as in individuals at higher risk and those presenting with symptoms, additional diagnostics may be ordered, including:
- Electrocardiogram (EKG or ECG) detects abnormalities in the heart’s electrical activity and can identify a current or past heart attack22
- Coronary artery calcium (CAC) scoring (see below)69-71
- Other heart and vessel imaging tests, such as22:
- angiography, a type of X-ray that uses a dye to visualize arteries
- cardiac magnetic resonance imaging (MRI) to detect tissue damage or altered coronary blood flow
- cardiac positron emission tomography (PET) to help visualize blood flow in the small arteries of the heart
- ankle-brachial index (ABI) test to diagnose peripheral artery disease
- stress test to assess the heart’s ability to respond to and recover from physical stress, such as exercise
5 Cardiovascular Risk Assessment
Cardiovascular risk assessment is a crucial part of cardiovascular health maintenance. Established cardiovascular risk factors like high blood pressure and obesity can provide substantial insight into your cardiovascular health and can be diagnosed in a basic physical exam. Accessible inexpensive blood tests, including a standard lipid (cholesterol) panel and glucose level, provide information about other traditional risk factors. Additional blood tests, such as hs-CRP and ApoB, may add predictive value to an overall assessment. These biomarkers can also be helpful in monitoring the effects of lifestyle, nutritional, and medical interventions.
The following section explores many factors that can be part of a comprehensive cardiovascular risk assessment. Everyone interested in maximizing their healthspan should be sure to regularly monitor their risk with periodic lab testing, biomarker assessment, and overall health evaluations with their health care provider.
Understanding Cardiovascular Risk
Many decades of medical research have clarified the associations between various risk factors and cardiovascular disease. Some of these risk factors, like age and family history, are beyond our control. These are said to be “non-modifiable” risk factors. On the other hand, risk factors like high blood pressure and elevated LDL-cholesterol are “modifiable,” since therapies or diet and behavior adjustments can modulate the risk associated with these factors.
The level of certainty that specific factors influence cardiovascular risk and atherosclerosis development varies. Some risk factors are very clearly linked to cardiovascular disease and have been demonstrated to be important in rigorous research. These are described in this Protocol as “established cardiovascular risk factors.”
On the other hand, some risk factors appear to influence cardiovascular risk, but the research is not as robust and conclusions about the influence of these risk factors are not as certain. We describe these as “emerging cardiovascular risk factors.”
Generally, a prudent approach for most people who want to maximize their healthspan is to first ensure that they are aware of risk conferred by non-modifiable factors like age, race, and family history. Then, established modifiable cardiovascular risk factors should be assessed and optimized to the extent possible. Lastly, attention should be given to emerging modifiable risk factors.
Estimating Cardiovascular Risk
Over the years, the American Heart Association has developed risk calculators to estimate an individual’s chance of a cardiovascular event. PREVENT (Predicting Risk of Cardiovascular Disease EVENTs), launched in 2023, is an American Heart Association tool for cardiovascular disease risk assessment. The PREVENT tool takes into account markers of cardiovascular, metabolic, and kidney health, and can be used by those 30‒79 years of age without known cardiovascular disease to quantify their 10- and 30-year risks of heart attack, stroke, and heart failure. The calculations are based on age, gender, cholesterol levels, systolic blood pressure, body mass index (BMI), estimated glomerular filtration rate (eGFR), smoker versus non-smoker status, and current use of blood glucose-lowering, blood pressure-lowering, and cholesterol-lowering drugs. Optional information about one’s urine albumin-to-creatinine ratio (a marker of kidney function), hemoglobin A1c (HbA1c, a marker of blood glucose control), and zip code (a marker used to estimate social determinants of health) can be added to improve accuracy when these numbers are relevant and available.72
The risk calculator can be accessed here: PREVENT Online Calculator.
PREVENT has been found to be accurate across a large population of U.S. adults.72 Nevertheless, it does not assess known cardiometabolic risk factors such as stress, social isolation, and physical inactivity, and does not incorporate information about family history, mental health and sleep disorders, and other non-metabolic/non-renal chronic inflammatory conditions. This can lead to inaccuracies in estimating an individual’s risk. Furthermore, while PREVENT has demonstrated usefulness in the United States, it may not be an accurate or precise tool for assessing risk in populations in other world regions.73 Also, it is intended for estimating risk in those without a history of heart disease, stroke, or heart failure.
Importantly, as of early-2025, the PREVENT calculator is still a relatively new tool. As such, the implications of particular risk estimates based on PREVENT are still being ironed out by researchers. For example, the threshold risk estimate at which to initiate statin therapy is still being refined in the medical literature.74 Therefore, it is necessary to view the risk estimates generated by the PREVENT calculator as an informative tool in shaping discussions about whether specific therapies are appropriate, and the PREVENT risk estimates should not be used in isolation to make intervention decisions.
Non-Modifiable Risk Factors
Age & Genetics
Atherosclerosis mainly afflicts older individuals, in part due to cumulative molecular and epigenetic effects from modifiable risk factors that result in changes in vascular structure and function.75 Most heart attacks and strokes, especially ones involving plaque rupture, occur in those over 55 years of age.7,21,25 A phenomenon known as inflammaging, chronic low-level inflammation that often accompanies aging, is an important driver of atherosclerosis.76
Family History
Family history is an important risk factor for atherosclerosis. Evidence points to both genetics and shared environmental conditions as explanations for the link between an individual’s risk and the vascular disease history in their immediate family.81
Apolipoprotein E (ApoE) Variants
Apolipoprotein E (ApoE) is a type of lipoprotein fraction that plays a role in lipid transport and distribution to tissues, as well as in modulating inflammation.82 ApoE plays a critical role in the removal of triglyceride- and cholesterol-laden lipoproteins such as LDL from circulation.83 There are three known genetic variants of ApoE, and they influence atherosclerosis risk differently. ApoE3 is the most common variant, occurring in approximately 70–80% of people, and is considered neutral with no strong influence on atherosclerosis or dementia risk. ApoE4, occurring in about 14% of people, is associated with a higher risk of cardiovascular disease. ApoE2 occurs in roughly 5–10% of people and is generally associated with a lower risk of coronary artery disease—although it may increase the risk of atherosclerosis in certain cases.82,83 In addition, ApoE2 and ApoE4 are closely associated with decreased and increased risk, respectively, of Alzheimer disease, and may play a role in cancer progression.82,84 Genetic testing for ApoE variants may help an individual better understand the genetic contribution of their ApoE status to their risk for atherosclerosis.
Gender
Coronary atherosclerosis develops approximately seven to 10 years later in women than men, and men have a three-fold higher risk of acute coronary syndrome (heart attack or unstable angina) than women before the age of 60. The gap in atherosclerosis risk between genders narrows after 60 years and disappears at around 75 years of age. This can be explained in part by the cardio-protective effects of pre-menopausal estrogen levels, since estrogen has positive effects on lipid profiles and inhibits blood clotting mechanisms.85
Race
The relationship between race and atherosclerosis risk is complex and not fully understood. While non-Hispanic White individuals have been reported to have a higher incidence of coronary artery disease than other racial groups, Black individuals have higher risks of fatal heart attack or stroke than White individuals. Hispanic individuals, particularly those of Mexican descent, have a lower incidence of coronary artery disease than other racial groups, despite being more likely to have adverse risk profiles. Asian people overall have lower risks of coronary artery disease and cardiovascular events; however, Asian-Indian and Filipino individuals have been found to have higher risks than other racial groups. The reasons for these differences are multifactorial, including genetic differences that affect susceptibility and disparities in social and economic conditions.81,86
Established Modifiable Risk Factors
Atherosclerosis and the early phases of cardiovascular disease often begin early in life and progress slowly over decades. Developing healthy habits early and maintaining them throughout life can go a long way toward preserving cardiovascular health.
The following table includes established risk factors that can be influenced, for example, by adopting a healthy diet and lifestyle, intervening with targeted nutrients, and using medications when appropriate.87 The importance of being aware of your risk profile as it relates to established, modifiable risk factors cannot be overstated. In fact, modifiable risk factors account for the majority of all cardiovascular events and deaths due to cardiovascular causes.88
|
Risk Factor |
Opportunity for Optimization |
Where to Learn More |
|---|---|---|
|
Unhealthy Diet |
Adopt a healthy, plant-rich, minimally processed diet like the Mediterranean diet. |
Refer to the “Diet & Lifestyle Considerations” section of this Protocol |
|
Not Enough Exercise/Physical Activity |
Most adults should engage in:
AND
|
Refer to the “Be Physically Active” section of this Protocol and Life Extension’s Exercise Enhancement Protocol |
|
Inadequate or Unhealthy Sleep |
Most adults should get 7–9 hours of quality sleep per night. |
Refer to the “Maintain Healthy Sleep” section of this Protocol |
|
Smoking Tobacco |
Stop smoking |
Refer to the “Stop Smoking and Avoid Second-Hand Smoke Exposure” section of this Protocol |
|
High Cholesterol/Unhealthy Lipid Profile |
The normal range for LDL-cholesterol is <99 mg/dL, although people at high risk for cardiovascular disease should target an LDL below 70 mg/dL. Life Extension considers an LDL level of 40–80 mg/dL to be optimal for many adults. |
Life Extension’s Cholesterol Management Protocol |
|
High Blood Pressure |
Most adults should strive to achieve blood pressure ≤120/80 mm Hg. Life Extension considers a target of about 115/75 mm Hg to be optimal for many adults. |
Life Extension’s High Blood Pressure (Hypertension) Protocol |
|
High Blood Sugar (Insulin Resistance/Elevated Glucose) |
Most adults should target fasting glucose level in the range of 70–99 mg/dL. Life Extension considers a target of about 80–86 mg/dL to be optimal for many adults. |
Life Extension’s Diabetes and Glucose Control Protocol |
|
Being Overweight |
Achieve and maintain a BMI of 18.5–24.9 |
Refer to the “Maintain a Healthy Body Weight” section of this Protocol and Life Extension’s Weight Management Protocol |
Unhealthy Diet
An unhealthy diet contributes to the development and progression of atherosclerosis and is the most significant modifiable risk factor for coronary artery disease.89 On the other hand, adopting healthy dietary practices reduces the risk of atherosclerosis.89-91
Refer to the “Adopt a Healthy Diet” section later in this protocol for more information.
Lack of Physical Activity
Physical activity improves metabolic health and reduces inflammation,92 while sedentary time has the opposite effects.93
Refer to the “Be Physically Active” section later in this protocol for more information.
Inadequate or Unhealthy Sleep
Healthy sleep—defined by appropriate circadian timing, adequate duration, regularity, and continuity—is associated with lower risk of cardiovascular and metabolic diseases, whereas chronic sleep deprivation, variability, and fragmentation increase these risks.94-96 The presence of obstructive sleep apnea is also associated with increased cardiovascular risk.63
Refer to the “Maintain Healthy Sleep” section later in this protocol for more information.
Tobacco Smoking
Tobacco use and second-hand smoke exposure dramatically increase the risks of coronary artery disease, stroke, peripheral vascular disease, congestive heart failure, and other chronic diseases.97,98
Refer to the “Stop Smoking and Avoid Second-Hand Smoke Exposure” section later in this protocol for more information.
Unhealthy Blood Lipid Levels
High cholesterol, especially LDL-cholesterol, and triglyceride levels (known as dyslipidemia) are key risk factors for atherosclerosis and cardiovascular events. Even among people whose lipid levels are within the normal range, a less-favorable lipid profile correlates with an increased risk of atherosclerosis and future cardiovascular events.99
Refer to Life Extension’s Cholesterol Management protocol for more information.
High Blood Pressure
High blood pressure is an established risk factor for cardiovascular disease, atherosclerosis, and cardiovascular events. High blood pressure induces endothelial dysfunction, oxidative stress, and inflammation, and increases plaque and lipid deposition.3,100,101 Even in young people without high blood pressure, those with more optimal blood pressure may have a lower risk of future cardiovascular events.102
Refer to Life Extension’s High Blood Pressure (Hypertension) protocol for more information.
Insulin Resistance and High Blood Glucose
Insulin resistance and high blood glucose levels (hyperglycemia) are independent contributors to atherosclerosis.103 Insulin resistance, even in the context of normal blood glucose levels, promotes unhealthy lipid metabolism and obesity, activates inflammatory pathways, and reduces NO production, promoting endothelial dysfunction and accelerated atherosclerosis.103,104 Hyperglycemia also promotes oxidative stress, inflammation, and endothelial dysfunction, and leads to increased glycation, a spontaneous chemical reaction that abnormally links glucose to other molecules, disrupting normal cellular function.103
Refer to Life Extension’s Diabetes and Glucose Control protocol for more information.
Obesity
Obesity is a chronic disease characterized by the accumulation of visceral and subcutaneous fat. Excess body fat (especially abdominal fat) contributes to atherosclerosis by disrupting the balance of fat-derived cytokines (adipokines); increasing oxidative stress, inflammation, and endothelial dysfunction; and impairing autophagy, or cellular cleanup.105-107 When abdominal obesity is present alongside low HDL-cholesterol levels, high triglyceride levels, high blood pressure, and impaired glucose regulation, it constitutes a condition called metabolic syndrome. Metabolic syndrome is associated with increased risk of type 2 diabetes, atherosclerosis and cardiovascular disease, and death.108,109
Refer to Life Extension’s Weight Management protocol for more information.
Emerging Cardiovascular Risk Factors & Biomarkers
A significant percentage of cases of acute cardiovascular events are not completely attributable to established risk factors like high blood pressure, high cholesterol levels, diabetes, and smoking. Decades of public health and medical prevention and treatment efforts have successfully decreased the role of these traditional risk factors, yet coronary artery disease remains a major cause of death worldwide.110 In fact, coronary artery disease in people without standard modifiable risk factors has been estimated to be responsible for 1.4 million deaths globally each year.7,40,111
Greater attention is now being given to the roles of emerging risk factors, sometimes called “risk-enhancing factors,” in atherosclerosis and possible mitigation strategies.
|
Risk Factor |
Opportunity for Optimization |
|---|---|
|
Coronary Artery Calcium (CAC) Score |
Optimal score is as close to zero as possible. Less than 100 may be acceptable depending on other risk factors. |
|
Apolipoprotein B100 (ApoB) and ApoB to Apolipoprotein A1 (ApoA1) ratio |
A normal range for ApoB is roughly 50–150 mg/dL but varies somewhat between labs. Emerging evidence suggests an ApoB:ApoA1 ratio of ≤ 0.60 is associated with the lowest risk of cardiovascular events, while a ratio of >0.90 is linked to the highest risk.112 |
|
Lipoprotein (a) |
This marker provides information about your cardiovascular risk based mainly on genetics.113 A normal level is <75 nmol/L |
|
Oxidized Low-Density Lipoprotein (ox-LDL) |
Currently there is no standard unit or reference range for ox-LDL, but low levels have been associated with low cardiovascular risk.114 |
|
Ceramide Score (CERT1 and CERT2) |
Cert1 is based on levels of three ceramides and their ratios, while CERT2 also incorporates phosphatidylcholine levels. Ceramide scores range from 0 to 12. An optimal score for CERT1 is 0–2 and CERT2 is 0–3.115 |
Coronary Artery Calcium Scoring and Carotid Artery Plaque Burden
Coronary artery calcium (CAC) is a highly characteristic feature of subclinical atherosclerosis.71 Calcium buildup in coronary artery walls can be measured with a simplified computed tomography (CT) scan. The CT scan generates a number, called the CAC score, that corresponds with the amount of calcium detected and the risk of cardiovascular events.116 The results are reported in risk categories, ranging from zero to greater than 400. A score of zero suggests no atherosclerosis, and a score of 100 or more suggests treatment should be initiated.71
The CAC score is a strong cardiovascular risk predictor in individuals with no symptoms and no diagnosis of heart disease.69,117 It is useful for guiding primary prevention strategies and decisions regarding medications and other therapies.69,71
Because the CAC test uses computed tomography, the person undergoing the test is exposed to a low dose of radiation, comparable to the amount of radiation exposure associated with a mammogram or to background radiation exposure present in most cities over a 3–4 month period.118
Carotid artery ultrasound is a non-invasive method for assessing the presence and extent of atherosclerotic plaque in the arteries of the neck. It uses mobile equipment; exposes the patient to no radiation; and can be used in individuals with no symptoms or diagnosis of cardiovascular disease to assess risk of cardiovascular events as accurately as CAC.119,120 Carotid artery plaque burden, assessed by ultrasound, has been shown to predict risk of stroke, major adverse cardiovascular events, and death from any cause.119-121
For example, in one observational study, 5,716 asymptomatic individuals were evaluated using carotid artery ultrasound and CT of the coronary arteries; after a median of 12.4 years of monitoring, those in the highest quartiles of baseline CAC scores and carotid artery plaque burden had 15% and 23% higher risk of death for any reason, respectively, compared with those in the lowest quartiles. In addition, among those who underwent a follow-up carotid artery ultrasound during the study, an increase in plaque burden was associated with a 5% increase in risk of death.120
This growing body of research indicates CAC score and carotid artery plaque burden represent accurate non-invasive measures of cardiovascular risk. They may be used in conjunction with cardiovascular risk estimators and other risk factors and biomarkers to improve preventive care for those without known heart disease.117
Moving Beyond the Standard Lipid Panel
Apolipoprotein B-100 (ApoB) and Apolipoprotein A1 (ApoA1)
Apolipoprotein B is the main structural protein in atherogenic lipoproteins, including LDL and lipoprotein (a), and is a reliable measure of the number of those lipoproteins in circulation.122 Apolipoprotein A1 (ApoA1) is a major component of HDL particles and plays a vital role in lipid transport.123 A growing body of evidence indicates that measurement of ApoB is a more accurate marker of cardiovascular risk than even LDL-cholesterol.124,125 In fact, the retention of ApoB-containing lipoproteins in the arterial wall is widely considered to be the primary initiating factor in the development of atherosclerotic plaque.126
Elevated ApoB levels and elevated ApoB-to-ApoA1 ratios have been associated with increased risk of several cardiovascular problems, including heart attack, coronary heart disease, and stroke, as well as cardiovascular mortality.112,127-130
Lipoprotein (a)
Lipoprotein (a), also called Lp(a), is an independently synthesized and secreted molecule with a lipid core and a shell composed of phospholipids, cholesterol, and Apo B-100. Lp(a) differs from LDL in that it is bound to another particle, called apolipoprotein(a).113 Elevated Lp(a) levels appear to play a causal role in the development of atherosclerotic cardiovascular disease.131 Lp(a) levels are mostly determined by genetics (as opposed to diet and lifestyle as with other blood lipid markers) and are not responsive to standard lipid-lowering therapies.113
One study published in 2024 aimed to investigate the association between Lp(a) levels and long-term coronary artery plaque progression using data from 267 patients with a median follow-up of 10.2 years. Patients with Lp(a) levels of 125 nmol/L or higher had a significantly higher percent plaque volume (6.9% vs. 3.0%) compared to those with lower Lp(a) levels. The study found that every doubling of Lp(a) was associated with a 0.32% increase in plaque volume over 10 years. Higher Lp(a) levels were also linked to increased presence of low-density plaques and greater inflammatory signaling in fat tissue surrounding the heart. These findings suggest elevated Lp(a) levels are a strong predictor of high-risk plaque progression and inflammation.132
Generally, Lp(a) levels below 75 nmol/L are considered low risk, levels between 75 and 125 nmol/L are intermediate risk, and levels above 125 nmol/L are high risk.133 Importantly, Black Americans have been found to have nearly three-fold higher Lp(a) levels compared with White Americans.134
Because it is genetically-determined and does not respond to current medical therapies or lifestyle modifications, Lp(a) should be interpreted in the context of family history and other cardiovascular risk markers, and serves primarily as a marker to identify people who might benefit from a more intensive overall cardiovascular risk reduction strategy.131,135
Oxidized Low-Density Lipoprotein (oxLDL)
Oxidized low-density lipoprotein (oxLDL) is an important contributor to atherosclerosis as a result of its damaging effects on endothelial cells, platelets, smooth muscle cells, and immune cells. These effects occur through activation of the oxLDL receptor, lectin-like oxidized low-density lipoprotein receptor 1 (or LOX-1).142 OxLDL is involved in the formation of foam cells, fatty streaks, and atherosclerotic plaques. It induces endothelial dysfunction, platelet activation, proliferation and migration of smooth muscle cells, and inflammation.143 A large body of evidence demonstrates that oxLDL is closely associated with coronary and peripheral artery disease, hypertension, ischemic stroke and acute coronary syndrome, as well as cardiovascular risk factors including diabetes, obesity, chronic inflammation, and metabolic syndrome.114,144,145
Ceramides
Ceramides, a type of lipid found in cell membranes, are emerging as contributors to cardiovascular disease. Elevated levels of ceramides are linked to poor lipid metabolism and are found in atherosclerotic plaques. Certain types of ceramides have been identified as predictors of major cardiovascular events. These lipids contribute to cardiovascular disease by promoting inflammation, oxidative stress, endothelial dysfunction, and the transport of LDL into blood vessel wall cells, contributing to atherosclerosis and a host of cardiometabolic problems such as hypertension, obesity, and type 2 diabetes.146,147
Figure 3: Ceramides and cardiovascular disease risk
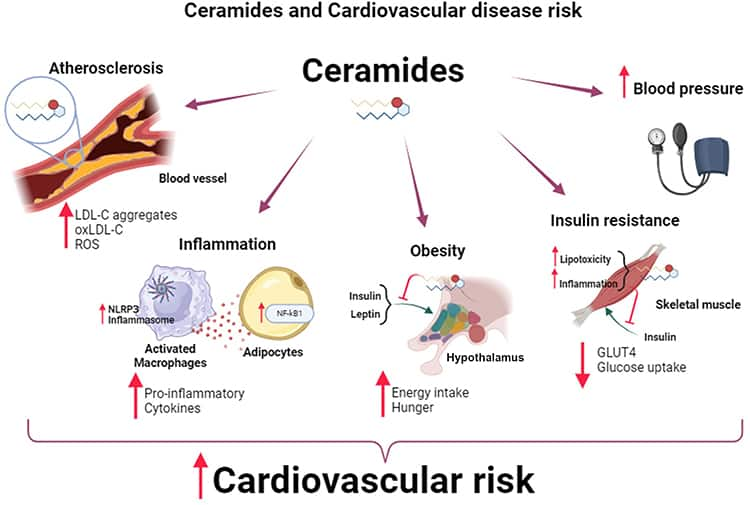
Diet plays a crucial role in regulating ceramide levels. Diets high in long-chain saturated fatty acids, typical of the Western diet, can increase ceramide production, while diets rich in unsaturated fats, such as the Mediterranean diet, can reduce ceramide levels. Emerging evidence suggests managing ceramide levels through diet may help lower the risk of cardiovascular disease, although more research is needed to confirm these findings.146-148 Dietary changes discussed elsewhere in this Protocol are also likely to improve ceramide levels.146
Inflammatory Factors & Biomarkers
High Sensitivity/Cardiac C-reactive Protein (hs-CRP)
C-reactive protein (CRP) is a circulating protein produced mainly in the liver and is a biomarker of inflammation.152 High sensitivity (hs)-CRP is a test that detects the very small rises in CRP associated with vascular inflammation and atherosclerosis. High sensitivity-CRP has consistently demonstrated a robust correlation with cardiovascular outcomes and is a strong predictor of cardiovascular risk.29,36,153 Even after successful lipid-lowering therapy, persistently elevated hs-CRP has been associated with increased risk for adverse cardiovascular outcomes.154
Generally, hs-CRP levels below 1 mg/L are considered low-risk. However, it has also been suggested that “lower is better” when it comes to hs-CRP levels in the context of chronic disease risk. More clinical trial data are needed to determine whether people who already have relatively low hs-CRP levels (eg, around 1 mg/L) can benefit from treatment to further lower their hs-CRP levels.
Myeloperoxidase
Myeloperoxidase (MPO) is an enzyme produced mainly by white blood cells to generate free radicals needed to combat microbial pathogens. Excess MPO appears to play a role in atherosclerosis by accumulating in the lining of arteries, where it promotes oxidative stress and inflammation, impairs nitric oxide, and contributes to endothelial dysfunction.155 Elevated blood MPO levels have been correlated with coronary and peripheral artery disease, high blood pressure, heart failure, stroke, overall and cardiovascular mortality, and other cardiovascular and related conditions, and are a marker of poor prognosis.156
Elevated Fibrinogen
Fibrinogen is a protein found in the blood that plays a critical role in blood clotting. Fibrinogen promotes atherosclerosis by increasing inflammation, inducing expression of immune cell adhesion molecules on blood vessel surfaces, and stimulating vascular smooth muscle cell migration and proliferation.157 Observational studies indicate higher fibrinogen levels are correlated with increased risks of coronary artery and peripheral artery disease.157,158
Nutrient Biomarkers
Omega-3 Index
Circulating fats from food and supplements have a powerful effect on risks of many diseases, including atherosclerotic cardiovascular disease. Levels of beneficial long-chain omega-3 polyunsaturated fats eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), atherogenic trans-fats, and the ratio of omega-6 to omega-3 fatty acids can be measured in red blood cells to reflect cell and tissue levels throughout the body.
The omega-3 index measures the concentration of EPA and DHA relative to all other fats in red blood cell membranes and is a validated method of assessing tissue omega-3 fatty acid concentrations.159,160 An analysis of data from 2,500 participants in the Framingham Heart Study found higher omega-3 index values correlated with substantially lower risks of total cardiovascular events and overall mortality: those with the highest omega-3 index (>6.8%) had a 34% lower risk for death from any cause and 39% lower risk of having cardiovascular disease relative to those with the lowest (<4.2%).160 Life Extension considers an omega-3 index of 8% or higher to be optimal.
Vitamin D
Vitamin D has anti-inflammatory effects that may promote cardiovascular health, and vitamin D deficiency has been associated with increased risk of atherosclerosis and cardiovascular disease.161,162 The risk of vitamin D deficiency is greater in people with darker skin, overweight/obesity, poor overall health, and those residing at a greater distance from the equator.163,164
Homocysteine
Homocysteine is an amino acid made in the body from dietary methionine. High homocysteine levels are associated with atherosclerosis, cardiovascular events (especially stroke), and cardiovascular and all-cause mortality. Current evidence suggests excess homocysteine may contribute to atherosclerosis by inducing inflammation and oxidative stress, disrupting NO production and methylation pathways, interfering with cell protein function and lipid metabolism, contributing to vascular smooth muscle and endothelial cell dysfunction, and promoting endothelial cell death.165,166 Furthermore, high homocysteine levels enhance clot formation, which may be one of the pathways contributing to a higher risk of major cardiovascular events.167,168 Elevated homocysteine levels can be caused by certain genetic variations, aging, insufficient intake of several B vitamins, some medications, and disease states including hypothyroidism, diabetes, and kidney disease.166 Life Extension recommends that homocysteine blood levels be kept below 8 µmol/L.
Refer to Life Extension’s Homocysteine Reduction protocol for more information.
Environmental Pollution
Air Pollution
Air pollution can contain a complex mix of airborne particles of various sizes (coarse, fine, and ultrafine) and gases.169,170 Exposure to high levels of air pollution has been linked to increased likelihood of atherosclerosis, heart attack, stroke, and heart failure, as well as higher rates of coronary artery disease-related and all-cause mortality.169,171 Increasing evidence shows fine particle air pollution triggers inflammation and endothelial dysfunction and is a major contributing factor in atherosclerosis onset and progression.172,173 Adverse health effects from short- and long-term fine particle pollution exposure have been shown to occur in urban centers worldwide, even at levels below those deemed safe by the World Health Organization.110,171,174 In fact, it is estimated that air pollution is responsible for 7 million avoidable deaths around the world every year.172
In recent decades, wildfire smoke has become a major source of air pollution around the globe.175 Current evidence suggests exposure to fine particle pollution from wildfire smoke is associated with increased cardiovascular disease mortality.176,177 Other components of wildfire smoke, including oxide gases, volatile organic compounds, polycyclic aromatic hydrocarbons, and metals, may also contribute to its toxic effects.175
Noise Pollution
Noise pollution, often due to road traffic, aircraft, or railway sounds, increases stress-related inflammation, oxidative stress, and endothelial dysfunction, and promotes other cardiovascular risk factors, especially high blood pressure. Multiple studies have shown chronic noise exposure, particularly at night, at intensities above 50 decibels (such as noise generated by a refrigerator, a moderate rain, or a quiet conversation) increases the risk of coronary artery disease and major cardiovascular events, and the risk increases with rising noise levels.110,178,179
Light Pollution
Light pollution, defined as artificial nighttime sky illumination, is thought to increase atherosclerotic mechanisms by interrupting sleep health and circadian rhythms and enhancing stress hormone imbalance. Observational evidence has linked light pollution with increased risks of high blood pressure, high blood glucose levels, obesity, atherosclerosis progression, and coronary artery disease-related hospitalization and death.110
Microplastic and Nanoplastic Pollution
Microplastics and nanoplastics are ubiquitous in the environment, and have been found in drinking water, a wide range of foods, cosmetics, and even the air.180,181 Preclinical evidence has implicated micro- and nanoplastic pollution in cardiovascular disease.182 In an interesting observational study that analyzed plaque samples from 257 patients who had undergone surgery to remove carotid artery plaque, micro- and nanoplastics were detected in plaque samples from more than half of the participants. After an average follow-up of almost 34 months, patients with these contaminants in their plaque had a 4.5-fold increased risk of a combined outcome of stroke, heart attack, or death from any cause.183
Perfluoroalkyl and Polyfluoroalkyl Substances (PFAS)
Perfluoroalkyl (per)- and polyfluoroalkyl substances, or PFAS as they are commonly known, are a group of chemicals used to create heat-, water-, and oil-resistant coatings in a large variety of consumer products. Virtually every person has been exposed to PFAS, and blood levels can build up over time. Exposure to certain PFAS has been linked to several adverse health outcomes, including increased risk of altered metabolism, certain cancers, and dampened immune function.184 High blood levels of PFAS have been compellingly associated with dyslipidemia, and may potentially be associated with vascular disease and atherosclerosis.185 However, while the observational link between PFAS exposure and adverse cardiovascular outcomes is intriguing, additional research is required to establish causation.
Metals
Exposure to contaminant metals in the environment, such as arsenic, cadmium, and lead, is a recognized contributor to atherosclerosis and cardiovascular deaths. These pollutants, which are widely found in air, water, soil, and food, can damage vasculature by triggering oxidative stress and chronic inflammation, leading to a range of pathogenic processes including endothelial dysfunction and hypertension.186 One study conducted a baseline measurement of urinary contaminant metals, including cadmium, tungsten, uranium, cobalt, copper, and zinc, and found that high levels of urinary metals were associated with higher baseline coronary artery calcium (CAC) scores. During 10 years of follow up, it was found that high baseline urinary metals were associated with greater progression of CAC.187 Edetate disodium (EDTA)-based infusion is an approved treatment for lead poisoning, and since the 1950s has been used by some physicians with the belief that it has a beneficial effect on atherosclerosis, based largely on anecdotal evidence.188 However, multiple clinical trials have failed to show a clear, consistent benefit of EDTA chelation for atherosclerosis, in part due to wide variability in studied infusion protocols, dosages, number of infusions, and other variables.189,190 Two large randomized controlled trials meant to clarify whether this therapy improves cardiovascular outcomes failed to demonstrate a robust beneficial effect.191,192 Still, some evidence suggests chelation may hold some cardiovascular benefit for those with diabetes or peripheral vascular disease.189 Given the absence of compelling evidence for improved cardiovascular outcomes in controlled clinical trials, EDTA chelation is not approved by the Food and Drug Administration for the prevention or treatment of cardiovascular disease.188
Chronic Infections
Infectious diseases appear to be another potential risk factor for cardiovascular events. Respiratory tract infections (eg, influenza, pneumonia, and COVID-19), gastric Helicobacter pylori, periodontal disease, and other infections have been associated with increased cardiovascular risk. Infections can disturb gut microbial balance, increase gut permeability, promote thrombosis, and have direct toxic effects on the endothelium, all of which can contribute to increased inflammation, oxidative stress, and endothelial dysfunction.110 Furthermore, some evidence indicates that chronic viral infections, such as with human immunodeficiency virus (HIV), cytomegalovirus (CMV), and hepatitis C virus (HCV), can induce age-related immune dysfunction (senescence) and contribute to atherosclerosis progression.200
Refer to Life Extension’s Immune Senescence protocol for more information.
Mental Health
Mental health disorders, including depression, are closely associated with cardiovascular events. In addition, psycho-emotional distress due to loneliness, social isolation, or chronic psychosocial stress may play an important role in atherosclerosis development and progression. These types of stressors can dysregulate the physiologic stress response and metabolic pathways, promoting traditional cardiovascular risk factors and triggering low-grade chronic inflammation in the arteries.110
Refer to the “Build Psycho-Social Health and Well-Being” section later in this protocol for more information.
Hormonal Influences on Cardiovascular Risk
Sex Hormone Deficiency
In both men and women, sex hormone levels decline with age.201
In men, low testosterone (hypogonadism) has been associated with greater risk of cardiovascular disease and death. Men who use testosterone replacement therapy to correct low testosterone levels may have a reduced risk of death and do not have an increase in cardiovascular risk.202-207 More information is available in Life Extension’s Male Hormone Restoration Protocol.
Women have generally been found to have lower risk of atherosclerosis than men—until after menopause when the risk gradually equalizes. It is widely accepted that this reflects the protective effects of estrogen on cardiovascular tissues during the reproductive years.85,208,209 Although the risks and benefits of menopausal hormone therapy have been extensively debated, current evidence suggests initiating hormone replacement therapy before 60 years of age or within 10 years of menopause offers cardiovascular benefits and minimal risks.210,211 More information is available in Life Extension’s Menopause Protocol.
Dehydroepiandrosterone (DHEA) is an androgenic steroid hormone that is metabolized into testosterone and estrogen. It also has independent functions that affect immune, metabolic, and cardiovascular health.212,213 DHEA levels peak in early adulthood and decline with aging.214 Blood levels of DHEA are typically assessed as DHEA-sulfate (DHEA-S). Among people with existing cardiovascular disease, low DHEA-S levels have been associated with greater risks of death from any cause, death from cardiovascular causes specifically, and non-fatal cardiovascular events.215 In the general population of older people, low DHEA-S has been associated with greater risk of death in men.216 A meta-analysis of 14 case-control studies found that lower DHEA-S levels were associated with greater risk of coronary heart disease.217 However, it is not yet clear if DHEA supplementation protects cardiovascular health because the available evidence is mixed.218-222 More information is available in Life Extension’s DHEA Restoration Therapy Protocol.
Thyroid Hormone Imbalance
The thyroid gland produces two hormones: thyroxine (T4) and triiodothyronine (T3). T4 circulates in greater amounts and functions as a precursor to T3 (the physiologically active form) in tissues throughout the body. Thyroid hormones play an important role in regulating energy metabolism and modulating body weight, temperature, growth, and nervous system signaling.223 In the cardiovascular system, thyroid hormones increase the activity of adrenaline, raising blood pressure, heart rate, and cardiac output.223,224
Abnormal thyroid hormone levels (hyperthyroidism [high thyroid hormone levels] and hypothyroidism [low thyroid hormone levels]) can contribute to dyslipidemia, arrhythmias, heart failure, and atherosclerosis, and increase the risk for cardiovascular illness and death.225 The metabolic and cardiovascular effects of thyroid conditions can also be seen in individuals with subclinical hyper- and hypothyroidism, in which thyroid hormone levels are maintained in the normal range but thyroid stimulating hormone (TSH, a pituitary hormone that regulates thyroid function through negative feedback) is outside of the normal range.226,227
More rigorous randomized controlled trials are necessary to fully understand the cardiovascular effects of treating subclinical thyroid conditions. Until more is known, treatment should be considered on a case-by-case basis, taking into account a patient’s overall risk profile.228
Refer to Life Extension’s Hyperthyroidism and Hypothyroidism Protocols for more information.
6 Diet & Lifestyle Considerations
Atherosclerosis prevention relies on addressing modifiable risk factors. Assessing your risk and making appropriate dietary and lifestyle changes are key. This section outlines several considerations that can help you understand your risk of cardiovascular disease and the steps you can take to reduce it.
Adopt a Healthy Diet
A heart-healthy diet can be achieved through the following strategies:
Eat more plant foods and move towards a Mediterranean eating pattern. Healthy plant-based dietary patterns have been associated with lower cardiovascular risk and mortality and may protect against atherosclerosis by reducing inflammatory signaling, lowering associated risk factors like diabetes and hypertension, and supporting a healthy gut microbiome.89 The Mediterranean diet in particular has the strongest evidence of benefit in terms of reducing risks of heart attack, stroke, cardiovascular death, and all-cause death.229 It is characterized by a high intake of olive oil, vegetables, fruits, whole grains, legumes (beans and lentils), and nuts and seeds, and may include modest amounts of seafood, dairy products, eggs, and lean poultry and meats. In clinical trials, Mediterranean diet interventions have consistently been shown to reduce plaque and improve biomarkers of atherosclerosis.230
Avoid processed foods. Processed meats and other highly processed foods, many of which are high in added salt, sugars (including sweetened beverages), and trans fats, have been linked to increased coronary artery disease risk and are generally not part of a healthy diet.231-234
Reduce sodium and increase potassium intake. While sodium intake has been correlated with increased risk of coronary and carotid artery atherosclerosis,235 potassium (found naturally in fruits, vegetables, legumes, and potatoes) improves vascular health and function.236 Increasing potassium intake and reducing sodium intake, such as by replacing table salt with a salt substitute containing 25% potassium chloride, has been associated with reduced cardiovascular risk.237
Increase fiber intake. Numerous observational studies have linked increased dietary fiber with reduced risks of atherosclerosis, stroke, and peripheral vascular disease.238 Dietary fibers are indigestible or partially-digestible carbohydrates and lignins (structural components of plants) in plant foods. Soluble fibers are characterized by their ability to interact with water to form a thick solution or a gel. Many soluble fibers are readily fermented by intestinal bacteria and converted into anti-inflammatory compounds called short-chain fatty acids. Both soluble and insoluble fibers are important for health, and plant foods generally contain both, in varying proportions. High-fiber foods include legumes, whole grains, brans, nuts and seeds, vegetables, and fruits.239
Eat healthy fats. Saturated fat has long been considered unhealthy, and indeed it directly contributes to the formation of LDL-cholesterol. Replacement of dietary saturated fat with polyunsaturated fats (eg, from fish, safflower, and sunflower oils) and monounsaturated fats (eg, from olive oil, canola oil, avocados, and some nuts) has been found to reduce both LDL-cholesterol levels and cardiovascular events.240 It is important to note that when carbohydrates and sugars have been used as fat replacements, no cardiovascular benefit has been observed, and overall mortality may have increased.240,241 Perspectives on dietary fats are evolving, as evidence suggests the context may partly determine its quality. For example, saturated fat from whole foods may be less harmful than those in ultra-processed foods.241,242 Nevertheless, most sources continue to recommend restricting saturated fat intake and replacing those calories with unsaturated fats.242
Eat cold water fish. A meta-analysis of 25 observational studies with a total of more than 2 million subjects found that higher fish consumption and greater intake of omega-3 fatty acids (found in high concentrations in cold water fish [eg, salmon, herring, and trout]) were each associated with lower risk of death due to cardiovascular causes. Based on the data, the risk reduction was calculated to be 4% per 20 grams of fish eaten per day or 80 mg of omega-3 polyunsaturated fatty acids consumed per day.243
Use extra-virgin olive oil. Extra virgin olive oil has been intensively studied for its health benefits both on its own and as a component of the Mediterranean diet. Olive polyphenols, such as hydroxytyrosol and oleuropein, have demonstrated anti-inflammatory, antioxidant, anti-hypertensive, anti-diabetic, anti-thrombotic, HDL-raising, and anti-atherosclerotic effects in preclinical research.244 Meta-analyses of observational studies and randomized controlled trials have confirmed a link between olive oil consumption and reduced risks of cardiovascular disease and all-cause mortality. These studies suggest 20 grams (about 1.5 tablespoons) of olive oil daily may confer the maximum benefit.245,246
Reduce calorie intake. Another essential feature of a healthy diet is balanced calorie intake and energy output, since overeating can contribute to metabolic disturbance and increased cardiovascular risk.233,247 Calorie restriction increases endothelial NO synthesis and improves vascular function, and clinical trials have shown that a 25–30% reduction in calorie intake can lower blood pressure and reduce cardiovascular and metabolic disease risk in people with and without obesity.248-250 Intermittent fasting reduces calorie intake by restricting eating to a limited time period each day or through alternate day fasting. Clinical evidence indicates intermittent fasting can promote improvements in cardiovascular and metabolic health parameters, but is not more effective than continuous calorie restriction.251-253 Observational studies have found that not eating breakfast is associated with greater cardiovascular risk and cognitive decline,254-256 which is consistent with other lines of evidence that show a benefit to cardiometabolic risk factors when energy intake takes place earlier in the day.257
Enjoy filtered coffee. Coffee may be best known as a source of caffeine, a nervous system stimulant, but coffee beans are also rich in free radical-scavenging and anti-inflammatory compounds, especially chlorogenic acids. Preclinical and clinical evidence indicate chlorogenic acids may improve lipid and glucose metabolism, endothelial function, and blood pressure.258-260 Roasting reduces the chlorogenic acid content of coffee beans, such that dark roasted coffee beans have the least and green (unroasted) coffee beans have the most chlorogenic acids and other polyphenols.258
Numerous observational studies have reported a link between moderate coffee consumption (possibly including decaffeinated coffee) and lower risks of cardiovascular disease, type 2 diabetes, and some cancers, with the greatest benefits seen in those drinking 3–4 cups (at about 4 ounces per cup) daily.261,262 Even in patients with existing cardiovascular disease, drinking four or more cups of coffee daily has been associated with lower mortality.263 Some research suggests only coffee brewed with a filter has cardio-protective effects, whereas unfiltered coffee (such as boiled coffee or espresso) can worsen lipid profiles and has been correlated with increased mortality risk.264,265 Notably, occasional coffee drinking may increase the risk of atrial fibrillation and temporarily raise blood pressure, but habitual moderate coffee consumption has not been correlated with hypertension or atrial fibrillation risk.266,267
Be Physically Active
The Physical Activity Guidelines for Americans recommends at least 150 minutes per week of moderate-intensity or 75 minutes of vigorous-intensity aerobic activity spread out across two or more days, plus muscle strengthening activity on at least two days, every week. Importantly, the document acknowledges any amount of physical activity is better than none.268 Regular physical activity has been shown to suppress inflammatory processes that promote atherosclerosis and reduce cardiovascular risk.269,270 Exercise is well known to improve lipid profiles, and emerging research suggests it may indirectly lower Lp(a) levels as well.271,272
Moderately intense aerobic activities include brisk walking, biking on level ground or slightly hilly terrain, water aerobics, yard work, or playing doubles tennis. As a general rule, you will be able to talk, but not sing the words to a song, when engaged in moderate-intensity activity. Examples of vigorous activities are jogging, swimming laps, biking fast or on hilly terrain, vigorous dancing, and playing basketball. Muscle-strengthening activities like heavy gardening, some types of yoga, and exercises that involve weightlifting, the use of elastic bands, and using one’s own body weight (eg, push-ups or sit-ups) are also important.268 One observational study that included data from 216,339 older adults (average age 69.9 years) participating the in the National Health and Nutrition Examination Survey found only 25% engaged in any amount of weight training, and those who did had lower risks of cardiovascular and all-cause death than those who did not. Interestingly, the benefit of weight training was only seen in those who also engaged in aerobic activity.273 Other research has shown exercise programs that include both aerobic and strength training may result in greater cardiovascular and mortality benefits than aerobic exercise alone.269,274
Although prolonged sedentary time is independently associated with cardiovascular harm, getting 60–75 minutes per day of moderate- to vigorous-intensity activity appears to overcome this effect.275 Furthermore, engaging in light-intensity activities has been associated with reduced arterial stiffness and can also reduce sedentary time and its negative effects.276
People with existing atherosclerosis can benefit from increasing their physical activity. Formal protocols called “cardiac rehabilitation” have been shown to improve cardio-respiratory fitness and increase walking distance, duration, and pace in patients with coronary artery disease, peripheral artery disease, or both.277
Maintain a Healthy Body Weight
Body weight and waist circumference are often recorded as vital signs, along with blood pressure and pulse rate, during health evaluations. The goals from the American Heart Association are a BMI (calculated using body weight and height) between 18.5 and 24.9 kg/m2 and a waist circumference less than 40 inches in men and 35 inches in women.278
An interesting phenomenon called the obesity paradox has emerged in some observational research showing reduced mortality in individuals whose BMIs indicate overweight or mild obesity compared with those whose BMIs indicate normal weight.279-281 While the reason for this paradox is uncertain, one proposed explanation is that body composition and fitness are not measurable using BMI.279,281 Sarcopenic obesity, in which age-related muscle loss is combined with excess fat accumulation, is associated with increased risks of a range of health problems including metabolic disorders, cardiovascular disease, and cardiovascular and all-cause mortality.282
It is important to emphasize that a largely conclusive body of evidence supports a link between overweight/obesity and increased risks of coronary artery and other cardiometabolic diseases.283-285 Weight loss has been shown to reduce cardiovascular risk and is an important objective in obese patients.286,287 Because even modest weight loss improves risk factors for atherosclerosis in those with overweight or obesity, an initial goal of 5‒10% loss of body weight is appropriate.288
Maintain Healthy Sleep
Seven to nine hours of sleep per night is generally recommended as optimal for adults.289-291 Shorter sleep duration and poorer sleep quality, including sleep fragmentation, have been linked to greater atherosclerotic plaque burden as well as worse cardiovascular and metabolic health.94 Furthermore, irregular sleep schedules that result in variable sleep duration and timing can disrupt circadian rhythms and have been associated with increased cardiovascular risk.94,292,293
Obstructive sleep apnea is a major cause of sleep fragmentation and is increasingly recognized as an important contributor to coronary artery disease.63 Treating sleep apnea with continuous positive airway pressure (CPAP) has been shown to reduce carotid artery thickening, especially in patients with severe sleep apnea.294 While adherence to CPAP is challenging for many sleep apnea patients, it is critical to heart disease prevention.295,296 In a systematic review and meta-analysis that included findings from 10 randomized controlled trials and three observational studies, the risk of major adverse cardiovascular events and cardiovascular mortality was reduced in a subgroup of sleep apnea patients who used CPAP four or more hours per night, although no effect was found when analyzing data from all CPAP users.297
Sleep hygiene is an umbrella term for healthy sleep habits that encourage proper and restful sleep. Habits such as avoiding caffeine, alcohol, and light exposure late in the day, having a consistent bedtime routine, and maintaining a regular sleep/wake schedule can help improve sleep duration and quality and reduce sleep fragmentation.289 Although there have not been controlled sleep hygiene intervention trials in people with cardiovascular diseases, sleep and lifestyle habits that encourage restful, restorative sleep are important for everyone.
Build Psycho-Social Health and Well-Being
Mental health and cardiovascular health are fundamentally connected, such that depression, anxiety, and chronic stress are closely associated with coronary artery disease and its risk factors.57,298,299 A positive outlook may also be important for heart health: pessimism has been associated with a 30% increase in coronary artery disease mortality, 41% increase in cardiovascular mortality, and 42% increase in stroke risk.300
Observational studies have linked loneliness and poor social health to increased risk of coronary artery disease and stroke, more health care visits and hospitalizations, and worse outcomes from cardiovascular events.301 Even in adolescents and young adults, loneliness has been associated with hypertension and other health problems.302 On the other hand, strong social relationships have been associated with a 50% decrease in mortality rate.301
Although the mechanisms linking loneliness to heart disease and mortality are not completely understood, it appears to involve increased stress signaling, leading to increased inflammation and oxidative stress, impaired lipid and glucose metabolism, and atherosclerosis.303-305 In addition, loneliness can contribute to poor self-care (eg, smoking, drinking alcohol, lack of exercise, unhealthy eating, and irregular sleep habits).303
While interventions to treat mental health disorders are well described, less is known about how to combat social isolation and loneliness. Building mental resilience through self-awareness, relaxation, and stress management practices, working to increase positive interactions with other people and pets, and engaging in self-care as much as possible may help reduce the impact of loneliness and social isolation on cardiovascular and overall health. Community programs and policy initiatives that support healthy connections may further contribute to better health outcomes.305
Stop Smoking and Avoid Second-Hand Smoke Exposure
Tobacco smoke exposure is a well-known risk factor for atherosclerosis. It is known to damage the heart and blood vessels, elevate cholesterol levels and blood pressure, and prevent oxygen from adequately reaching all of the body’s organs and tissues. Research suggests smoking, passive exposure to smoke, vaping, and waterpipe use can all cause oxidative stress, vascular dysfunction and inflammation, platelet coagulation, and dyslipidemia.97,98,306
Quitting smoking, though difficult, can lead to reversal of some of its cardiovascular harms, reducing the risk of developing and dying from heart disease.307 Successful quitting is tailored to the individual and may involve individual or group counseling and/or nicotine replacement.308 Avoiding places where people are smoking and asking others not to smoke in your house or car may help reduce exposure to second-hand smoke.307
Limit Alcohol Intake
The relationship between alcohol and cardiovascular disease is complex and controversial. Among other harms, alcohol abuse increases the risks of stroke, hypertension, arrhythmias, and heart failure.309 Furthermore, drinking more than one to two drinks per day or binge drinking can increase arterial stiffness and impair endothelial function.310,311 While some research indicates a possible cardiovascular benefit from low-to-moderate alcohol consumption, other findings suggest it may increase all-cause mortality.312 In the absence of conclusive evidence of benefit, the U.S. 2020–2025 Dietary Guidelines recommend avoiding or reducing alcohol intake as a health measure.309,313
Consider an Anti-Platelet Drug
Aspirin has long been used as a preventive measure against heart attacks. This approach is based on meta-analyses showing a clear benefit in patients who had already experienced a heart attack, ischemic stroke, or a coronary intervention.314 This type of usage, referred to as “secondary prevention,” continues as part of current medical practice.315 Even low doses of aspirin can reduce platelet aggregation, which is the immediate cause of thrombosis leading to heart attack and ischemic stroke.316
In 2022, the U.S. Preventive Services Task Force updated its recommendations to advise that daily low-dose aspirin not be initiated as “primary prevention” (ie, to prevent heart disease in those with no cardiovascular event history) in individuals aged 60 years and older. The task force found aspirin-related harms from serious bleeding events outweighed cardiovascular benefits in this population.317 Aspirin should also be avoided by anyone with an increased risk of bleeding due to health conditions or medications.318,319 Thus, low-dose aspirin is only recommended for primary prevention in 40–59-year-olds with a 10% or higher 10-year risk of a cardiovascular event.317 Consult your health care provider before initiating daily low-dose aspirin, as the risks and benefits vary between individuals.
Another class of antiplatelet drugs, known as P2Y12 inhibitors, has emerged as a safer and more effective approach to cardiovascular risk reduction. This family of drugs includes clopidogrel (Plavix), ticlopidine (Ticlid), ticagrelor (Brilinta), and prasugrel (Effient). One meta-analysis found P2Y12-treated coronary artery disease patients had a 12% lower risk of cardiovascular events, with a 23% lower risk of heart attack in particular, than those treated with aspirin. Despite causing a similar number of major bleeding events, P2Y12 inhibitors caused fewer adverse side effects overall than aspirin.320
7 Medical Approaches to Prevention & Treatment of Atherosclerotic Cardiovascular Disease
The American Heart Association provides atherosclerosis treatment guidelines to health care providers and their patients.188 These guidelines are intended to lower the risk of cardiovascular disease, events, and mortality. The cornerstone of therapy for atherosclerosis is managing the underlying conditions that lead to its progression. This section summarizes several key conditions that should be targeted.
Managing Blood Lipids
Cholesterol-lowering Therapy
Intrusion of LDL-cholesterol (specifically ApoB) into the arterial wall is an essential step in initiating atherosclerosis.36,321 Therefore, lowering LDL-cholesterol levels is frontline prevention and therapy. Lifestyle interventions, including quitting smoking, adopting a healthy diet, being physically active, achieving a healthy weight, and getting healthy sleep, are universally beneficial, but are especially important for those with dyslipidemia (a plaque-promoting lipid profile).188,321
When lipid-lowering through diet, lifestyle, and nutrient supplements does not achieve target levels, prescription medication is recommended. Atorvastatin (Lipitor) and rosuvastatin (Crestor), both high-potency statins and the most widely prescribed drugs for preventing cardiovascular disease, are recommended by the American Heart Association as first-line treatment for cholesterol lowering.188 Statins inhibit the activity of HMG-CoA reductase, a key enzyme in cholesterol synthesis, and may also reduce coronary heart disease risk by other mechanisms, such as reducing inflammation. Ezetimibe, which inhibits cholesterol absorption, is often used in combination with statins to meet cholesterol-lowering goals.322 Other cholesterol-lowering drugs include bempedoic acid, bile acid sequestrants, and PCSK9 inhibitors.323
As risk of a heart attack or stroke increases, target LDL-cholesterol levels decrease. For individuals at high cardiovascular risk, Life Extension recommends LDL-cholesterol levels be kept below 70 mg/dL. Targets for fasting blood triglyceride levels are <80 mg/dL for individuals with any cardiovascular risk factors and <60 mg/dL for those with a history of cardiovascular disease. Life Extension further recommends targeting non-fasting triglyceride levels (which may be even more indicative of atherosclerosis risk) of <116 mg/dL.
Refer to Life Extension’s Cholesterol Management Protocol for more information.
Triglyceride-lowering Therapy
High triglyceride levels (hypertriglyceridemia) are associated with atherosclerotic cardiovascular disease, and tend to co-occur with other known risk factors, including various other lipid and cholesterol abnormalities, and with diabetes. Standard diet and lifestyle management of cardiometabolic risk factors applies to triglycerides as well; managing hypertension and diabetes, stopping smoking, being physically active, appropriate weight loss, and healthy diet are all indicated.324
In most people with hypertriglyceridemia, there are both genetic and acquired causes. Acquired causes can include the side effects of medications, other medical conditions, and inappropriate diet and alcohol consumption.324,325
Medical-grade omega-3 fatty acids have been tested for their ability to improve cardiovascular outcomes in patients with hypertriglyceridemia. In particular, a prescription-only, purified, ethyl ester of EPA called icosapent ethyl (Vascepa) has been shown to improve cardiovascular outcomes and is FDA approved to reduce the risk of atherosclerotic cardiovascular disease in patients with residual hypertriglyceridemia while on statin therapy.326,327 Interestingly, the cardiovascular benefits of icosapent ethyl are not adequately explained by a triglyceride-lowering effect alone. Other actions of EPA include favorable effects on other lipids, inflammation, oxidative stress, inflammatory signaling, platelet activation, and arterial stiffness, as well as increasing vasodilation, NO availability, and plaque stability. EPA also reduces gene expression related to cardiovascular disease.324,328
In the Reduction of Cardiovascular Events with Icosapent Ethyl-Intervention (REDUCE-IT) trial, 4 grams per day of icosapent ethyl reduced the risk of cardiovascular events compared with a mineral oil placebo in statin-treated patients with cardiovascular disease or diabetes plus at least one other risk factor, and with high triglyceride levels. The trial, which involved 8,179 participants, found treatment resulted in a 25% reduction in risk of a composite outcome that included cardiovascular death, nonfatal myocardial infarction, nonfatal stroke, coronary revascularization, or unstable angina, and a 31% reduction in heart attacks during a median of 4.9 years.329
An earlier trial, known as JELIS, was a randomized open-label trial that included 18,645 Japanese participants with total cholesterol of ≥250 mg/dL and LDL-cholesterol of ≥170 mg/dL, but without serious heart or other diseases. After 4–8 weeks of abstaining from all lipid-lowering medication, participants were randomized to receive either a low-dose statin drug or that drug plus 1.8 grams of highly purified EPA per day, and followed for an average of 4.6 years. Those who received treatment with EPA and statins had a 19% reduction in major coronary events compared with statin therapy alone. However the addition of EPA did not offer significant protection against any type of coronary fatality.330 A follow-up sub-analysis of the JELIS data found that in those with low HDL-cholesterol and high triglycerides, the addition of EPA resulted in a 53% lower rate of major coronary events compared with statins alone.331
In a third trial that enrolled 2,506 coronary artery disease patients with low ratios of EPA-to-arachidonic acid (an omega-6 fatty acid), the rate of a composite of major adverse cardiovascular events that included cardiovascular death, nonfatal heart attack, nonfatal ischemic stroke, unstable angina pectoris, and coronary revascularization was 9.1% in those who received 1,800 mg icosapent ethyl per day and 12.6% in those who did not. This result did not reach statistical significance; however, a reduction in strictly coronary events in the icosapent ethyl group was statistically significant.332
A trial called EVAPORATE that included 80 statin-treated subjects with coronary artery disease and high triglyceride levels showed icosapent ethyl may slow coronary artery disease progression. Participants were given 4 grams of icosapent ethyl or a mineral oil placebo daily for 18 months. In those treated with icosapent ethyl, unstable plaque volume was reduced by 17%, while in the placebo group, unstable plaque volumes more than doubled.333
On the other hand, a high-potency prescription combination of EPA plus DHA did not show cardiovascular benefits in a randomized controlled trial. The STRENGTH trial compared the effects of 4 grams per day of the carboxylic acid forms of EPA plus DHA to a corn oil placebo in 13,078 patients with high cardiovascular risk treated with statins, who also had high triglyceride and low HDL-cholesterol levels. After a maximum of five years of treatment, no difference in the rate of major cardiovascular events was found between the treatment and placebo groups.334
It is important to note that treatment with icosapent ethyl, despite its positive effects on cardiovascular events, including death, has been associated with an increased risk of developing atrial fibrillation.327
Managing Blood Pressure
Blood pressure control is essential in those who have atherosclerosis. Lifestyle strategies, including weight management, physical activity, restricted alcohol intake, and a healthy low-sodium/high-potassium diet, are the foundation of hypertension management; however, medication is often needed to optimally lower blood pressure.335
The American Heart Association defines normal blood pressure as <120/80 mm Hg and stage 1 hypertension as a blood pressure of ≥130/80 mm Hg.335 Based on evidence showing strict blood pressure management can substantially lower the risk of cardiovascular events and deaths, the American Heart Association currently recommends drug treatment for adults with hypertension plus a 10-year cardiovascular risk estimate of ≥10% or type 2 diabetes. In individuals with stage 2 hypertension (≥140/90 mm Hg) and/or CKD, the recommended target blood pressure is <130/80 mm Hg.188,336 Life Extension recommends an optimal target of 115/75 mm Hg for cardiovascular disease prevention in healthy individuals.
The most widely used drugs for first-line treatment of hypertension are thiazide diuretics (eg, hydrochlorothiazide [HCTZ, Microzide], chlorthalidone [Thalitone], and others), calcium channel blockers (eg, nifedipine [Procardia] and amlodipine [Norvasc]), angiotensin converting enzyme (ACE) inhibitors (eg, lisinopril [Zestril], captopril [Capoten], and others), and angiotensin II receptor blockers (ARBs) (eg, losartan [Cozaar], telmisartan [Micardis], and others). A large literature review concluded these classes of drugs were associated with similar cardiovascular benefits.335 Certain medications are preferred for patients with certain conditions, and sometimes combinations of two different classes of antihypertensives are needed to reach blood pressure goals.
Refer to Life Extension’s High Blood Pressure (Hypertension) Protocol for more information.
Managing Type 2 Diabetes
Appropriate management of type 2 diabetes is a fundamental part of atherosclerosis treatment. Glucose control and insulin signaling can be improved through adhering to healthy eating patterns such as the Mediterranean diet, increasing soluble fiber in the diet, increasing physical activity, taking targeted supplements, and the use of glucose-lowering medication.337,338
Hemoglobin A1c (HbA1c) is the main biomarker of long-term blood glucose control. An HbA1c below 5.7% is considered normal while ≥6.5% usually indicates type 2 diabetes. Intensive blood sugar control in diabetics, to a target HbA1c of below 7.0%, has been associated in some trials with better cardiovascular outcomes, but individualization of therapy to each patient is recommended by the American Diabetes Association, taking into account risk of hypoglycemic events, disease duration, life expectancy, and other factors.338,339
Metformin (Glucophage) is the most widely used oral anti-diabetes medication in the world. It not only lowers blood glucose levels, but also improves dyslipidemia.340 Multiple meta-analyses have concluded that metformin treatment reduces cardiovascular events, cardiovascular deaths, and all-cause mortality in type 2 diabetics with coronary artery disease.341-343
Refer to Life Extension’s Diabetes and Glucose Control Protocol for more information.
Targeting Residual Inflammation
Colchicine
In 2023, the FDA granted approval to low-dose colchicine (0.5–0.6 mg per day) to reduce cardiovascular risk in adults with established cardiovascular disease or with multiple risk factors. This approval represented a new approach to cardiovascular disease treatment and prevention—targeting inflammation as a driver of atherosclerosis.353
Colchicine, an anti-inflammatory compound originally derived from the autumn crocus (Colchicum autumnale) plant, is used to treat gout and for Mediterranean fever.354,355 It is also used off-label to treat pericarditis, a condition in which tissue encasing the heart becomes inflamed.356 Colchicine has demonstrated anti-atherogenic effects such as improving endothelial function, reducing clot formation, and reducing plaque progression in preclinical studies.357,358
In a meta-analysis of six randomized controlled trials involving a total of 13,165 subjects with coronary artery disease, those who received low-dose colchicine had lower risks of stroke, heart attack, and revascularization (surgery to restore coronary blood flow), as well as major cardiovascular events as a combined outcome, compared with those who received placebo; however, cardiovascular death and all-cause mortality risks were not significantly different, and colchicine increased the risk of gastrointestinal side effects. The analysis further showed colchicine had cardiovascular benefits only in subjects aged 65 years and younger.359 Another meta-analysis found low-dose colchicine reduced the risk of major cardiovascular events, without affecting mortality, in patients with acute coronary syndrome (sudden loss of coronary blood flow [ie, heart attack or unstable angina]) and when used perioperatively in patients who underwent percutaneous coronary intervention (PCI, a surgical procedure that opens coronary arteries).360,361
Other Therapeutic Strategies
Enhanced External Counterpulsation (EECP)
Enhanced external counterpulsation (EECP) is a procedure that involves sequential inflating and deflating of pressure cuffs on the calves, thighs, and buttocks in counter-synchrony with the heartbeat, in effect doubling the rate of blood pressure cycles. This increases lower extremity circulation and strengthens cardiac output in a way that is similar to exercise.362,363 EECP has been shown to increase NO production, improve endothelial function, and mitigate inflammatory signaling.362 Research also shows EECP stimulates the formation and growth of new coronary blood vessels, leading to better heart muscle oxygenation and function in patients with coronary artery disease.364,365 EECP has been reported to benefit patients with vascular diseases including angina, peripheral artery disease, heart failure, cerebrovascular disease, diabetes, and erectile dysfunction.362,363 EECP is typically administered in one-hour treatment sessions five days per week for seven weeks.366
EECP is currently FDA approved for the treatment of stable angina that has not responded to other treatments.366 In addition, research suggests it may be beneficial in patients with known coronary artery disease, class II–III heart failure, and peripheral artery disease, as well as those who have experienced a heart attack or ischemic stroke.363,367-370
It is important to note that, while EECP is non-invasive and safe for most people, it is contraindicated in a number of conditions, including uncontrolled hypertension, uncontrolled arrhythmias, clotting disorders, unstable angina, and severe heart failure, among others.366
Surgical Procedures and Interventions
Invasive procedures including surgical or catheter-based interventions are generally reserved for patients with severe coronary atherosclerosis. These techniques are used to restore coronary artery blood flow in acute or emergency circumstances, or when more conservative measures have not adequately relieved symptoms or improved quality of life.
Coronary artery bypass grafting (CABG) surgery, often referred to as heart bypass, involves using a section of heathy blood vessel taken from elsewhere in the body and attaching it to a coronary vessel above and below an area obstructed by plaque, creating an alternate pathway for blood flow to the heart muscle. As with any complicated, invasive, potentially life-saving surgical procedure, CABG carries considerable risks and potential complications.371,372
Percutaneous coronary intervention (PCI, also known as angioplasty) is a catheter-based intervention in which the heart is accessed through a puncture in a wrist or groin artery and a catheter is delivered to a coronary artery blocked with plaque. The inserted end of the catheter is equipped with a balloon, which is inflated, disrupting the atherosclerotic plaque and opening the narrowed artery, allowing greater blood flow. In many cases, a metallic stent is then permanently implanted at the site of the angioplasty to prop open the artery and maintain blood flow. Specialized drug-eluting stents are coated with slow-release drugs and sometimes used to prevent the artery from becoming blocked again.373,374
Atherectomy is a minimally invasive method of removing plaque from the insides of affected arteries to restore blood flow. It involves the use of a catheter equipped with a cutting tool, such as specialized blades or a laser.375 Atherectomy can be used to treat severe peripheral artery disease.376 It may help prevent ischemic tissue death and reduce the need for amputation; however, its benefits on outcomes, including mortality, are uncertain.377,378 Atherectomy is also an option for removing highly calcified plaque in coronary or carotid arteries.379,380 It has demonstrated comparable benefits to angioplasty in patients with total occlusion of a coronary artery, and may be used to prepare the artery for balloon dilatation or stent placement.381,382
In patients with stable coronary artery disease, invasive strategies are not necessarily superior to conservative medical management.383 The decision to opt for invasive therapies depends on patient preference, disease extent and complexity, and whether the procedure has a proven survival benefit in similar patients.384
Treatments Targeting Symptom Relief
Unstable angina, characterized by unpredictable, sudden onset chest pain that does not subside with rest, is a form of acute coronary syndrome and an emergency. Conversely, stable angina, which is predictable and episodic (typically aggravated by or occurring with exertion or stress), is a manageable condition.391-393 Several classes of medications are used to prevent or relieve stable angina.
Beta Blockers
Beta blockers (metoprolol [Lopressor, Toprol], atenolol [Tenormin], and others) reduce heart rate and strength of contraction, as well as blood pressure, and along with certain calcium channel blockers and nitrates are first-line therapy for relieving and preventing angina and increasing exercise tolerance in most cases. When a single one of these classes of medication does not provide adequate relief, another from among the three is often added.188 Beta blockers have the additional benefit of improving survival in those who have experienced a heart attack.394
Calcium Channel Blockers
Calcium channel blockers, such as diltiazem [Cardizem] and verapamil [Isoptin], are indicated for angina and can be used as an alternative to, or in combination with, beta blockers. Calcium channel blockers are used in patients intolerant of beta blockers due to severe obstructive airway disease or vasospastic disorders.188 Other calcium channel blockers used in the treatment of coronary artery disease and hypertension include amlodipine (Norvasc), felodipine (Plendil), and nifedipine (Procardia). Nifedipine is only used in combination with beta blockers. Combined therapy with beta blockers plus calcium channel blockers carries a risk of low blood pressure and slow heartrate.
Nitrates
Nitrates relax coronary and other blood vessel walls by relaxing smooth muscle, thereby reducing demand on the heart muscle.395 A long-acting nitrate (isosorbide mononitrate [Imdur]) may be used as an alternative to, or in combination with, beta blockers and/or calcium channel blockers to prevent angina, while short-acting nitrates (sublingual formulations of nitroglycerine [GoNitro, Nitrolingual, and others] and isosorbide dinitrate [Dilatrate or Isordil]) are used to provide immediate symptom relief or as short-term angina prevention, such as before exercise.44,188 Nitrates commonly cause side effects such as headache, low blood pressure, flushing, dizziness, and rapid heartbeat. In addition, some people develop nitrate tolerance, in which the medication becomes less effective with ongoing use.395
Ranolazine
Ranolazine (Ranexa) is a newer medication that may be used in combination with standard anti-angina medications to improve symptom relief. This medication relaxes the heart muscle without lowering blood pressure or heart rate.396 It is recommended as an add-on to treatment in those who continue to have angina symptoms despite therapy with tolerable doses of beta blockers, calcium channel blockers, and nitrates.44,188
8 Nutrients
There are many nutritional preparations and supplements that have been studied in the context of cardiovascular disease. Here, we summarize the evidence for many of these supplements. We have grouped these nutrients and supplements into the following categories:
- Amino Acids & Cofactors and Other Nutrients
- Carotenoids
- Fatty Acids & Fatty Acid Derivatives
- Gut-Health Support
- Herbal Extracts & Preparations
- Phytonutrients & Polyphenols
- Vitamins & Minerals
The order in which the categories are listed in this Protocol is alphabetical. However, the lists of nutrients within each category are generally organized with those nutrients or supplements with the best evidence at the top of each list.
Amino Acids & Cofactors and Other Nutrients
Coenzyme Q10
Coenzyme Q10 (CoQ10) is a naturally occurring lipid-soluble antioxidant that plays an important role in cellular energy metabolism. Because statin therapy significantly lowers blood CoQ10 levels, many health care providers recommend CoQ10 supplementation for those taking statins.397
CoQ10 may prevent atherosclerosis through several mechanisms, including reducing oxidative stress, inflammation, and cholesterol synthesis, as well as enhancing mitochondrial and cellular function.398-400 In a randomized placebo-controlled trial in 51 patients with untreated high LDL-cholesterol levels and moderate endothelial dysfunction, CoQ10, at 100 mg and 200 mg daily for eight weeks, improved endothelial function and increased NO production; in addition, LDL oxidation was reduced in those receiving 200 mg CoQ10 daily.11 A crossover trial in 11 subjects with peripheral artery disease found 80 mg per day of a highly bioavailable form of CoQ10 called MitoQ improved endothelial function, raised levels of an antioxidant enzyme, and increased walking capacity better than placebo after two weeks.401
A meta-analysis that included eight randomized controlled trials involving 526 coronary artery disease patients found 100–300 mg CoQ10 daily for 4–48 weeks decreased total cholesterol and increased HDL-cholesterol levels, but did not affect LDL-cholesterol levels.10 CoQ10 has also been found to improve exercise capacity and endothelial function and reduce mortality in randomized controlled trials in heart failure patients.402,403
A randomized placebo-controlled trial with 443 healthy, elderly, Swedish participants found 200 mg CoQ10 plus 200 mcg selenium daily for five years reduced cardiovascular mortality.12 The effect persisted at a 12-year follow-up, at which time 38.7% of those in the placebo group and 28.1% of those in the supplement group had died from cardiovascular causes. Analyses of the data showed patients with hypertension, diabetes, heart disease, and impaired functional capacity all benefited from CoQ10 plus selenium therapy.404 Several secondary studies based on data from this trial suggest mechanisms such as reduced oxidative stress, inflammation, and glycation405-407; improved cellular metabolism; and improvement in markers of aging408,409 may be partly responsible.
L-arginine
L-arginine, a conditionally essential amino acid found in protein-rich foods, has attracted attention for its ability to improve endothelial function and dilate blood vessels. L-arginine serves as a precursor for NO in the endothelium.410 It also enhances mitochondrial function, lowers oxidative stress, and reduces oxidized LDL levels.411,412 Multiple clinical trials have indicated L-arginine supplementation may have beneficial effects on blood pressure, endothelial and microvascular function, glucose metabolism, insulin sensitivity, lipid profiles, and body weight and composition.410,413 However, findings have been inconsistent.411,412 In a meta-analysis of 22 randomized controlled trials involving a total of more than 1,100 subjects, L-arginine supplementation, at doses of 4 grams per day or higher for at least 24 weeks, was found to reduce systolic and diastolic blood pressures, with no additional benefit from doses higher than 9 grams per day. The effect was consistently seen in men and women, healthy and unhealthy individuals, and those with and without high blood pressure.414 On the other hand, a meta-analysis that included 13 randomized controlled trials was unable to show L-arginine could improve measures of endothelial function,412 while another meta-analysis of two randomized controlled trials found a statistically non-significant 7% reduction in mortality in heart attack patients treated with L-arginine versus placebo.415 Findings from a pilot crossover trial suggest a sustained-release formulation of L-arginine may be more effective than standard immediate-release formulations for raising NO synthesis.416
Lipoic Acid
Lipoic acid, a naturally occurring antioxidant, serves as a coenzyme in metabolism of fats, carbohydrates, and proteins. It can restore radical-scavenging capacity to other antioxidants like thioredoxin (an antioxidant protein), vitamin C, and glutathione, which in turn can recycle vitamin E. Lipoic acid also helps manage proper serum glucose levels in diabetic patients. By decreasing oxidative stress and improving glucose and lipid metabolism, lipoic acid may promote cardiovascular health.417
A large systematic review of 11 clinical trials concluded alpha-lipoic acid improved endothelial function by reducing oxidative stress and inflammation and increasing endothelial NO synthesis.15 In addition, a meta-analysis of findings from five randomized controlled trials found lipoic acid improved flow-mediated dilation, a measure of endothelial function.418
Lipoic acid may be beneficial in individuals with conditions that increase cardiovascular risk. Multiple controlled trials have reported lipoic acid improved markers of blood glucose control and inflammation.419 Lipoic acid supplementation, at a dose of 600 mg daily for 12 weeks, was also found to reduce total cholesterol and triglyceride levels in a placebo-controlled trial in 46 patients with metabolic syndrome420 and reduce blood pressure in a small placebo-controlled trial conducted in 67 stroke patients.421
Propionyl-L-carnitine
Propionyl-L-carnitine is a naturally produced derivative of L-carnitine that rapidly passes into cells to supply free L-carnitine, a much-needed factor for mitochondrial energy production. L-carnitine and its derivatives are also free radical scavengers.422 Preclinical research suggests propionyl-L-carnitine may have a role in slowing atherosclerosis progression and improving glucose and lipid metabolism.423,424
Clinical trials indicate propionyl-L-carnitine may relieve symptoms in individuals with peripheral artery disease. A large review examined findings from 12 randomized controlled trials with a combined total of 1,423 participants. The accumulated evidence showed propionyl-L-carnitine, at doses of 1–2 grams per day, increased maximum and pain-free walking distances by 26% and 31%, respectively, in those with intermittent claudication (pain while walking due to atherosclerosis in the legs) relative to placebo. Propionyl-L-carnitine also improved endothelial function and quality of life.425
Glucosamine and Chondroitin
Known best as supplements for combating osteoarthritis and joint pain, glucosamine and chondroitin may have benefits beyond these indications. In one clinical trial, 3,000 mg glucosamine for four weeks improved vascular endothelial function in 20 healthy volunteers compared with 19 untreated individuals who served as controls.426
A growing body of observational evidence suggests glucosamine and chondroitin may have cardio-protective effects. Findings from a study that included data collected over a median of 8.9 years from 495,077 participants in the United Kingdom’s health resource databank (UK Biobank) suggested people taking glucosamine supplements for arthritis had a lower risk of death from cardiovascular disease, atherosclerosis, and heart attack and stroke.427 A previous study using the same database found glucosamine users were less likely to develop coronary artery disease or have a major cardiovascular event or stroke during seven years of monitoring.428 Chondroitin use was found in another study to be associated with 52% lower odds of heart attack during a 13-year period in people at high cardiovascular risk.429 Another observational study that used data from 38,021 adults in the United States who were followed for 15 years found that the use of glucosamine and chondroitin supplements was associated with a trend towards decreased cardiovascular mortality, but it did not reach statistical significance.430 Further rigorous investigations are necessary to elucidate the effects of glucosamine and chondroitin on cardiovascular health.
Carotenoids
Lycopene
Lycopene is an antioxidant carotenoid obtained mainly from cooked tomatoes and present in smaller amounts in watermelon, pink grapefruit, papaya, apricots, and guava.16,431 Lycopene not only reduces inflammation and oxidative stress, but has also demonstrated anti-atherogenic effects such as decreasing cholesterol levels, lowering blood pressure, inhibiting LDL oxidation, decreasing blood clot and foam cell formation, enhancing NO production and healthy endothelial function, and inhibiting smooth muscle proliferation.16
Observational studies have linked higher lycopene levels with lower atherosclerotic plaque burden in people with type 2 diabetes432 and decreased carotid arterial wall thickness, particularly in men.433-435 A meta-analysis of findings from 25 observational studies indicated individuals with the highest lycopene intake or blood levels had a 14% lower likelihood of cardiovascular disease, 26% lower likelihood of stroke, and 37% lower likelihood of death compared with individuals with the lowest lycopene intake or blood levels.436
A meta-analysis of data from 17 randomized controlled trials investigating the effects of tomato or lycopene interventions on cardiovascular risk factors showed supplementing with tomato lowered LDL-cholesterol levels and improved endothelial function (measured as flow-mediated dilation), and lycopene supplementation reduced systolic blood pressure.437 For example, in a randomized placebo-controlled trial involving 36 patients with stable cardiovascular disease, adding 7 mg per day of lycopene to statin therapy for two months led to improved endothelial function.438 In another randomized controlled trial, 144 middle-aged participants were assigned to receive 20 mg lutein (another carotenoid), 20 mg lutein plus 20 mg lycopene, or placebo daily for eight weeks. Carotid artery thickness was reduced in both treatment groups relative to the placebo group, but the combination of lutein and lycopene had a greater effect than lutein alone.439 In a randomized crossover trial in 28 volunteers with high cardiovascular risk, daily tomato juice consumption for four weeks reduced expression of adhesion molecules involved in the atherosclerotic process compared with water consumption, and the effect was correlated with a rise in lycopene levels.440
Lutein
Lutein, which belongs to the class of carotenoids known as xanthophylls, is found in high concentrations in many types of fruits and vegetables, and possesses strong anti-inflammatory and oxidative stress-reducing properties.441,442 Lutein, along with zeaxanthin (another xanthophyll), are best known for their impact on eye health; however, they may also affect cardiometabolic health.441 Lutein has demonstrated anti-atherosclerotic potential by improving endothelial function, arterial thickness, blood pressure, and lipid profiles in preclinical studies.443
In a randomized placebo-controlled trial in 65 individuals with early atherosclerosis, 20 mg lutein daily for three months resulted in decreased levels of the inflammatory cytokine interleukin (IL)-6, as well as LDL and triglyceride levels; it also decreased expression of an inflammatory protein.444 In another trial that included 117 healthy subjects, taking 10 or 20 mg of lutein per day for 12 weeks increased total antioxidant capacity compared with placebo; in addition, those receiving the 20 mg per day dose had reduced levels of CRP and malondialdehyde (a marker of oxidative stress).445
In observational studies, higher intake or blood levels of lutein, or lutein plus zeaxanthin, were associated with lower concentrations of IL-6, lower risk of coronary artery disease and stroke, and better cardiometabolic health.446-448 Lower lutein levels have also been correlated with increased carotid artery wall thickening.449
Fatty Acids & Fatty Acid Derivatives
Fish Oil
Fish oil is rich in omega-3 polyunsaturated fatty acids (PUFAs), including eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). These omega-3 PUFAs work in part by suppressing inflammation, the main driver of atherosclerosis.243 One way they do this is through the action of derivatives known as specialized pro-resolving mediators, or SPMs. SPMs mediate the resolution of inflammation, limiting tissue damage, clearing dead cells, and promoting repair without impairing immune defenses.450,451
A number of controlled trials and meta-analyses have examined the potential role of omega-3 PUFA supplementation in cardiovascular disease prevention. Although findings have been mixed, the overall evidence suggests omega-3 supplements, at doses of 2–4 grams per day, are most likely to reduce the risk of major adverse cardiovascular events in those who have already had one, individuals with very high triglyceride levels, and heart failure patients.452,453 One meta-analysis that included data from 19 randomized controlled trials with a total of more than 97,000 participants found supplementing with 2 grams of omega-3 fatty acids per day lowered the risk of cardiovascular mortality by 45%, but had no significant effects on other outcomes.454 Another meta-analysis of 28 randomized controlled trials with a total of 136,965 participants found omega-3 supplementation lowered the risk of cardiovascular events and deaths, but no other adverse outcomes.455 In a meta-analysis that examined findings from 17 randomized controlled trials, EPA supplementation was found to reduce cardiovascular events relative to a mineral oil placebo, but this benefit was not present when olive oil or corn oil was used as the placebo; importantly, the analysis also found that treatment with EPA plus DHA lowered the risk of cardiovascular disease-related death compared with no treatment (including no placebo).456
A comprehensive review of findings from 17 randomized controlled trials concluded that supplementing with 1.8–3.4 grams of omega-3 fatty acids per day for three to six months, or 4.4 grams per day for as little as one month, could effectively raise blood levels into a range that may help counteract some pathways that contribute to atherosclerosis.457
The FDA announced in mid-2019 that EPA/DHA supplements can make the qualified health claim that they may reduce the risk of hypertension and coronary artery disease, although the agency described the evidence available at the time as “inconclusive.”458
Although the use of fish oil has been associated with an increased risk of atrial fibrillation, there are important caveats and nuances regarding this concern, and the prodigious cardiovascular and other benefits of marine omega-3s overshadow this potential risk.
One of the strongest findings for an association with atrial fibrillation came from the REDUCE-IT trial that used 4 grams per day of a prescription-only, purified, ethyl ester of EPA called icosapent ethyl (Vascepa).329 Another trial, STRENGTH, used 4 grams of a prescription combination of EPA and DHA in a carboxylic acid form, and also found an increased risk.334 Most data linking atrial fibrillation and marine omega-3 fatty acids find that the risk is considerably greater in those with a previous history of atrial fibrillation, and with very high doses of omega-3s.
However, it is important to note that much remains to be learned about this association, and there is not any conclusive mechanistic explanation to suggest a causative role of omega-3s in atrial fibrillation. In fact, omega-3s have anti-arrhythmic activity.459 Furthermore, observational studies of circulating and adipose omega-3 levels have shown that higher levels were associated with a lower risk of atrial fibrillation.460 And an observational study in the large UK Biobank Cohort found that in those with existing atrial fibrillation, fish oil use was associated with a lower risk of major adverse cardiovascular events and heart attack.461
Cumulative evidence suggests individuals with existing atrial fibrillation should consider working with a health care practitioner regarding omega-3 supplementation, and doses below 4 grams per day may be advisable.
Specialized Pro-resolving Mediators (SPMs)
Polyunsaturated fatty acids stored in cell membranes can be metabolized into byproducts called eicosanoids that help regulate local inflammation. For example, prostaglandins made from the omega-3 PUFAs EPA and DHA downregulate inflammatory signaling, while those made from arachidonic acid, an omega-6 PUFA from animal fat, upregulate inflammation.462 A more recently discovered family of eicosanoids, called specialized pro-resolving mediators (SPMs), participate in actively terminating the inflammatory response through “stop” signals.462,463 Unlike anti-inflammatory therapies that suppress immune function, SPMs shape immune activity to promote tissue healing and limit pain.463 Inadequate resolution of inflammation is now recognized as an underlying feature of many chronic inflammatory conditions, including atherosclerosis.451,464
SPMs have been shown to promote anti-inflammatory activities of macrophages and reduce accumulation of necrotic macrophages and foam cells within atherosclerotic lesions.465 In one study, just five days of supplementing with 15 mL, 30 mL, or 60 mL of an SPM-enriched fish oil increased the expression of anti-inflammatory features in macrophages from subjects with peripheral artery disease and healthy volunteers, and this effect was strongest at the maximum dose.466 A study that used blood samples from subjects with atherosclerosis found SPM treatment reduced free radical production and reversed pro-thrombotic activity in neutrophils (a type of immune cell).467
Tissue and blood SPM concentrations are currently being investigated as possible biomarkers of cardiovascular risk. In addition, by addressing unresolved inflammation in atherosclerotic vessels, SPMs may have a therapeutic role in cardioprotection.463,468
Gut-Health Support
Supplemental Fiber
A meta-analysis of observational and clinical trials reported that increasing dietary fiber intake through food or supplements can reduce all-cause mortality and lower blood pressure, and may improve lipid profiles and glucose metabolism.469 One meta-analysis of findings from 28 randomized controlled trials found a median dose of 10.2 grams (about 2 teaspoons) of psyllium can reduce LDL-cholesterol, non‒HDL-cholesterol, and ApoB levels.470 A meta-analysis of data from 12 randomized controlled trials with 370 participants found supplementing with about 3 grams of glucomannan, a soluble fiber from konjac root, daily for three weeks or longer lowered LDL-cholesterol and non‒HDL-cholesterol levels, but did not affect ApoB levels.471 Other meta-analyses have shown supplementing with beta-glucan (a soluble fiber from oats and barley) can improve cardiovascular risk factors such as lipid levels, glucose control, and waist circumference.472-474
Probiotics
An imbalance in the composition of the gut microbiome can promote progression of atherosclerosis by up-regulating inflammation through decreased short-chain fatty acid production and increased inflammatory cytokine release. It may also lead to dysregulated cholesterol and bile acid metabolism.475 Probiotic supplements can be help counter these mechanisms, and have been found to shrink atherosclerotic plaques in animal research.476
A growing body of clinical research indicates probiotic supplements may improve atherosclerosis risk factors. For example, Lactobacillus plantarum 299v, at a dose of 50 million colony forming units (CFUs) daily for six weeks, decreased levels of fibrinogen and leptin (an inflammatory cytokine from fat cells), lowered blood pressure, reduced markers of oxidative stress and inflammation, and decreased monocyte activation in a randomized placebo-controlled trial with 36 healthy participants.477
Another probiotic of interest is L. reuteri NCIMB 30242. A randomized placebo-controlled trial with 127 participants found supplementing with 50 billion CFUs L. reuteri NCIMB 30242 twice daily for six weeks reduced levels of LDL-cholesterol by 11.6%, total cholesterol by 9.1%, non–HDL-cholesterol by 11.3%, and ApoB by 8.4% relative to placebo. In addition, ratios of LDL- to HDL-cholesterol and ApoB to ApoA were reduced by 13.4% and 9%, respectively, and hs-CRP and fibrinogen levels were reduced by 1.05 mg/L and 14.3%, respectively, compared with placebo.478
A randomized placebo-controlled trial in 128 non-diabetic individuals with high triglyceride levels found 2 grams of a probiotic supplement containing L. curvatus HY7601 and L. plantarum KY1032 (CFUs not specified) daily for 12 weeks reduced triglyceride levels and increased LDL size.479 (Larger and less dense LDL particles are less harmful than small dense LDL particles).480
Herbal Extracts & Preparations
Garlic
Garlic is a well-studied herbal medicine and food that has demonstrated antioxidant, anti-inflammatory, endothelial-protecting, lipid-lowering, blood pressure-lowering, blood clot-inhibiting, blood glucose-lowering, and anti-atherosclerotic effects in preclinical and clinical research.481,482 Its ability to decrease elevated CRP levels, reduce high blood pressure, lower high LDL-cholesterol levels, and improve poor blood glucose regulation has been demonstrated in multiple meta-analyses of randomized controlled trials.483-485 Together, these effects may result in improved cardiovascular health.
Garlic is rich in sulfur compounds that are believed to be largely responsible for its therapeutic actions, as well as its characteristic odor. Aging is a method of processing garlic that changes and stabilizes its sulfur compounds and increases its polyphenol content, possibly enhancing its therapeutic effects.486,487
A review of 10 randomized controlled trials concluded that, despite some inconsistent findings, garlic may improve vascular function, especially in people with high cardiovascular risk.488 Aged garlic extract, at a dose of 2,400 mg per day for one year, was found to increase measures of microcirculation and tissue blood flow in a randomized placebo-controlled trial in 93 patients with confirmed coronary artery atherosclerosis489 and a similar trial in 122 individuals with high cardiovascular risk scores.490 In another trial by the same research group that involved 104 subjects with coronary artery disease, 2,400 mg aged garlic extract daily for one year not only slowed progression of coronary artery calcification, but also reduced blood glucose levels and blood pressure.491 A randomized controlled trial in 55 participants with metabolic syndrome found 2,400 mg aged garlic extract per day reduced the volume of low-density plaque (the most unstable type of plaque) in the coronary arteries compared with placebo after one year.492 In a similar trial in 80 participants with type 2 diabetes, volume of a type of coronary artery plaque susceptible to rupture and triggering of a heart attack decreased by 29% in those taking 2,400 mg aged garlic extract daily versus a 57% increase in those taking placebo after one year.493
In a randomized controlled trial conducted in India, 56 healthy subjects received either a placebo or an aged black garlic extract called Garlzac for 12 weeks. The aged black garlic extract was standardized to contain at least 0.5% S-allyl-L-cysteine (SAC), and participants took 500 mg of the extract (providing 2.5 mg of SAC) or the placebo daily. Compared with those who took the placebo, study subjects who took the black garlic extract had significant reductions in triglycerides, LDL and total cholesterol, blood pressure, and fasting blood glucose, as well as an improvement in HDL cholesterol levels. Systolic blood pressure dropped by about 6.7 mm Hg in the garlic group compared with no significant change in the placebo group. A similarly meaningful difference was observed for total cholesterol, with a 24 mg/dL drop in the black garlic group and no significant change in the placebo group. LDL cholesterol fell by about 20 mg/dL in the black garlic group compared with just 5 mg/dL in the placebo group.494 This study provided intriguing preliminary evidence that aged black garlic extract improved several biomarkers relevant to cardiovascular health.
In a 12-week controlled clinical trial of aged black garlic consumption, improvement in markers of endothelial dysfunction was observed in 62 individuals with and without hypercholesterolemia. Subjects consumed four cloves of black garlic per day (12 g/day), after which there were significant reductions in endothelial adhesion molecules including monocyte chemoattractant protein-1 (MCP-1), intercellular adhesion molecule-1 (ICAM-1), and vascular cell adhesion molecule-1 (VCAM-1), suggesting benefits to endothelial function.495 Another 12-week trial using 6 g/day of aged black garlic supplementation in 60 participants led to a rise in HLD-cholesterol, reduction in ApoB, and reduced ApoB-to-LDL cholesterol ratio, without significant effects on triglycerides, LDL-cholesterol, or total cholesterol.496
Hawthorn
Hawthorn (Crataegus) species are flowering shrubs in the rose family that have traditionally been used to treat high blood pressure and heart disease.497 Extracts from hawthorn berries, leaves, and flowers are high in flavonoids and phenolic compounds with anti-inflammatory and free radical-scavenging effects, and have been found in preclinical research to lower blood pressure, improve lipid and glucose levels, and protect the heart muscle from injury due to oxidative stress and ischemia.497-499 Hawthorn may also inhibit foam cell formation, promote healthy endothelial function, and reduce atherosclerotic plaque growth.498 In an uncontrolled trial in 37 subjects with type 2 diabetes, hypertension, and overweight being medically managed to lower their cardiovascular risk, taking 20 mL of vinegar made from hawthorn berries after meals for four weeks resulted in reduced body weight and BMI, blood pressure, blood glucose levels, and HbA1c, and improved lipid profiles.500 Although several randomized controlled trials have suggested hawthorn extracts may have mild blood pressure-lowering effects,501-503 little is known about their possible benefits in patients with atherosclerosis or on long-term cardiovascular outcomes.
Ginkgo Biloba
Ginkgo (Ginkgo biloba) has been used for thousands of years to treat a wide range of conditions and promote healthy circulation, relax the airways, and support brain function.504,505 More recent evidence shows ginkgo may help dilate blood vessels, regulate blood glucose and lipid levels, protect endothelial cells, promote endothelial NO synthesis, reduce oxidized LDL levels, and slow the effects of aging on vascular function.505-507 Ginkgo has been found to increase coronary blood flow, as well as blood flow to the eyes, ears, skin, and brain in healthy people.506
In a randomized placebo-controlled trial in 40 patients with metabolic syndrome being treated with metformin, adding 120 mg ginkgo daily for 90 days decreased markers of insulin resistance, waist circumference and abdominal fat, and inflammatory marker levels, thereby lowering cardiovascular risk.508 A preliminary trial in 11 metabolic syndrome patients found ginkgo extract, taken for two months, decreased levels of hs-CRP, IL-6, and other markers of inflammation and oxidative stress, as well as early atherosclerotic plaque development.509 The same researchers found ginkgo extract, at 120 mg twice daily, reduced early atherosclerotic plaque formation in eight patients who had recently undergone coronary artery bypass surgery. Furthermore, ginkgo increased levels of endogenous antioxidant enzymes and reduced oxidized LDL levels.510 While older studies have also found ginkgo reduced claudication (pain while walking) in individuals with peripheral artery disease, more recent meta-analyses have not confirmed a benefit.511-513
Arjuna
The bark from the arjuna (Terminalia arjuna) tree has been used in traditional Ayurvedic medicine to treat angina, hypertension, heart failure, and dyslipidemia for centuries. Preclinical research indicates it reduces oxidative stress and inflammation and can improve lipid levels, inhibit platelet aggregation, increase coronary blood flow, and strengthen heart muscle contraction.514,515 Small uncontrolled clinical trials have reported that treatment with arjuna reduced angina frequency and improved cardiac function in patients with stable angina.514,516 In a randomized, placebo-controlled, crossover trial involving 58 men with stable angina, 500 mg arjuna bark extract three times daily for one week reduced angina episodes, reducing the need for acute isosorbide dinitrate therapy compared with placebo (18.22 mg of the drug used per week for the placebo group vs. 5.69 mg per week with arjuna extract), and improved treadmill performance. Furthermore, these effects were similar to those of daily isosorbide mononitrate therapy.517 A placebo-controlled trial in 116 subjects receiving standard care for stable coronary artery disease found 500 mg arjuna twice daily for six months reduced markers of inflammation.518 In a randomized placebo-controlled trial that included 100 heart failure patients, 750 mg arjuna twice daily for 12 weeks did not improve cardiac function, but did improve functional capacity, antioxidant status, and symptom-related quality of life in some participants.519 In other clinical studies, arjuna improved cardiac function in heart failure patients,520 lipid profiles in coronary artery disease patients,521 and endothelial function in smokers, and inhibited platelet aggregation in subjects with type 2 diabetes.514
Chili Pepper and Capsaicin
Chili peppers (Capsicum species) are a common culinary spice, estimated to be enjoyed daily by 25% of people around the world. Large observational studies and multiple meta-analyses have indicated regular chili-eaters live longer and healthier lives than non–chili-eaters.522-525 According to one meta-analysis of four studies that included nearly 565,000 people, those who consumed chili pepper ever or more than once a week had a 13% lower chance of dying from any cause and an 11% lower risk of dying from cardiovascular disease than those who rarely or never used chili pepper.526
Clinical trials further support the cardio-protective effects of chili peppers and their main active ingredients, capsaicin and related capsaicinoids. For example, multiple controlled trials have shown capsaicinoid supplements can reduce total and LDL-cholesterol levels, an effect that may contribute to cardiovascular protection.527 In a placebo-controlled crossover trial in 12 men with coronary artery disease, applying a capsaicin patch prior to an exercise stress test enhanced blood NO levels, increased coronary blood flow during exercise, and increased exercise tolerance as assessed by ECG.528 Clinical research has also shown capsaicin can support weight loss by increasing heat production and energy expenditure, and preclinical research suggests capsaicin reduces atherosclerotic plaque formation and oxidative stress, while increasing NO production and promoting vasodilation and blood flow.522
Gotu Kola
Gotu kola (Centella asiatica) is a plant used in traditional Ayurvedic and Chinese medicine for thousands of years. The most important bioactive components in gotu kola are triterpenes, which may have plaque-stabilizing abilities.529 Gotu kola extracts have demonstrated anti-inflammatory, oxidative stress-reducing, anti-hypertensive, and lipid-lowering actions, and improved endothelial function in preclinical studies.529,530
A single team of researchers has conducted multiple clinical studies using gotu kola extract, alone or combined with pycnogenol, in subjects with known coronary artery disease. In two placebo-controlled trials in atherosclerosis patients, 60 mg gotu kola extract three times daily for 12 months stabilized atherosclerotic plaques in femoral and carotid arteries.531,532 In an observational study in 391 people with asymptomatic atherosclerosis receiving standard management (education, exercise, diet, and lifestyle interventions), supplementing with gotu kola extract plus pycnogenol, at 100 mg per day each, was associated with greater reductions in plaque progression and oxidative stress after four years than pycnogenol alone or no supplements.533
An observational study monitored outcomes in 184 subjects with asymptomatic atherosclerosis receiving standard management, standard management plus 100 mg aspirin/day, or standard management, aspirin, and 450 mg gotu kola extract/day plus 150 mg pycnogenol/day. After three years, a substantially reduced rate of cardiovascular events was seen in participants who received gotu kola plus pycnogenol; the rate of cardiovascular events requiring hospitalization was <4% in the supplement group and >12% in those who received standard care, with or without aspirin.534 A similar study that involved 90 men with coronary artery calcifications found the same pycnogenol plus gotu kola combination improved patterns of calcification after one year.535 Other studies by the same research team consistently found that combinations of pycnogenol plus gotu kola extract increased plaque stability and/or reduced the number and size of plaques, as assessed by ultrasound, in atherosclerosis patients.536-540
Phytonutrients & Polyphenols
Curcumin
Curcumin is found in turmeric and has been shown to strongly inhibit inflammatory signaling.541 Clinical trials have shown curcumin can promote metabolic health, support weight loss, improve lipid levels, and lower high blood pressure, oxidative stress, and inflammation.14 According to a meta-analysis of findings from 11 randomized controlled trials, curcumin use for 12 weeks or longer may lower high systolic blood pressure.542 Other meta-analyses have indicated curcumin can improve lipid profiles,543 decrease hs-CRP levels,544 and improve flow-mediated dilation, a measure of endothelial function.545,546 Curcumin has also been found to reduce cardiovascular risk markers in patients with chronic kidney disease,547 non-alcoholic fatty liver disease,548,549 and type 2 diabetes,95,550 particularly when used long-term at doses of up to 1,500 mg per day.
In a randomized controlled trial in 64 subjects with type 2 diabetes and mild-to-moderate coronary artery disease, taking 80 mg nano-curcumin (a highly bioavailable form of curcumin) daily for three months reduced hs-CRP and lipoprotein (a) levels.551 In another trial, 90 patients undergoing coronary angioplasty (ie, percutaneous coronary intervention [PCI]) were assigned to receive 500 mg standard curcumin, 80 mg nano-curcumin, or placebo daily for eight weeks. Both forms of curcumin improved levels of lipids (total cholesterol, LDL-cholesterol, and triglycerides), markers of oxidative stress (total antioxidant capacity, superoxide dismutase, glutathione, and malondialdehyde), inflammatory markers (hs-CRP, interleukin-1-beta [IL-1β], and tumor necrosis factor-alpha [TNF-α]) relative to placebo, but nano-curcumin was more effective than standard curcumin at changing some of these parameters (total cholesterol, triglycerides, superoxide dismutase, malondialdehyde, and TNF-α).552 Taking 2,000 mg curcumin daily for 12 weeks was found to increase NO availability and reduce arterial stiffness in a placebo-controlled trial in 39 healthy middle-aged and older adults; in addition, flow-mediated dilation increased by 36%, indicating enhanced endothelial function, in those receiving curcumin.553
Tea Polyphenols
Tea is rich in flavanols called catechins. Green tea in particular contains the well-studied catechin epigallocatechin gallate (EGCG), which has been shown to prevent lipid peroxidation and lower oxidative stress, reduce inflammation, and improve glucose metabolism.554,555 In large observational studies, regular habitual tea-drinking has been associated with lower risks of coronary artery disease, peripheral artery disease, and all-cause mortality.556-559
Clinical evidence shows green tea polyphenols improve endothelial function and become incorporated into LDL particles where they protect against oxidation.560,561 In a randomized placebo-controlled trial in 20 subjects with type 2 diabetes, 400 mg green tea extract daily for 12 weeks reduced arterial stiffness.562 Another randomized controlled trial in 30 male smokers found 580 mg per day of green tea catechins for two weeks led to increased NO synthesis, improved forearm blood flow, and decreased levels of markers of inflammation and oxidative stress.563 Eight weeks of treatment with 250 mg green tea extract daily was found to improve lipid profiles compared with placebo in 33 patients with dyslipidemia participating in a randomized crossover trial.564 Other small placebo-controlled trials have shown green tea lowers platelet activation, CRP levels, and oxidized LDL levels in healthy adults.565,566
Resveratrol
Resveratrol is a polyphenol found in red grapes and red wine, berries, and peanuts. Preclinical studies have suggested resveratrol can slow aging and may have a positive effect on atherosclerosis by improving glucose and lipid metabolism and supporting healthy endothelial cell and vascular smooth muscle cell function.567-569
A randomized placebo-controlled trial in 50 patients with type 2 diabetes found 100 mg resveratrol daily for 12 weeks not only improved blood pressure and body weight, but also improved measures of arterial stiffness and oxidative stress.570 In another randomized controlled trial with 75 participants, 350 mg of resveratrol-enriched grape extract daily for six months lowered LDL-cholesterol, oxidized LDL, and ApoB levels.571 A randomized controlled trial in people with type 2 diabetes found resveratrol improved glucose control and insulin sensitivity, and combatted oxidative stress and inflammation—benefits that could also aid in prevention of atherosclerosis.572
Meta-analyses of findings from randomized controlled trials have shown resveratrol can improve flow-mediated dilation (a test of vascular function) and other markers of endothelial function.573,574 For example, in a randomized, placebo-controlled, crossover trial, 28 patients with obesity received 75 mg of trans-resveratrol per day for six weeks and placebo for six weeks, in random order; flow-mediated dilation improved by 23% after resveratrol compared with placebo.575 In another crossover trial, 24 participants with hypertension and endothelial dysfunction received a single dose of 300 mg trans-resveratrol and placebo on separate occasions in random order; flow-mediated dilation was found to improve in women after resveratrol treatment, especially in those with high LDL-cholesterol levels.576
In a trial in 85 patients with coronary artery disease, those who received 100 mg resveratrol daily in addition to standard therapies for two months had better cardiac function compared with those who received standard therapies alone.577 Another placebo-controlled trial in 40 subjects who had experienced a heart attack found 10 mg resveratrol daily for three months improved endothelial function, lowered LDL-cholesterol levels, and improved markers of blood clot susceptibility.578
Cocoa Flavanols
Flavanols are a type of polyphenol found in cocoa, as well as fruits, vegetables, and tea. Cocoa flavanols may support cardiovascular health by reducing lipid oxidation, inhibiting platelet aggregation, modulating inflammation, reducing blood pressure, and improving insulin sensitivity.19,579 Furthermore, a number of clinical trials in people with varying health statuses have indicated cocoa flavanols can enhance endothelial function.580 In a small trial involving patients with heart failure, eating flavanol-rich chocolate led to an almost immediate improvement in vascular endothelial function, when compared with plain chocolate, and sustained improvement after four weeks of daily consumption.581 In a placebo-controlled crossover trial in 11 type 2 diabetics and 11 healthy individuals, a single dose of 1,350 mg cocoa flavanols led to improvement on tests of vascular function in all participants.582 Two weeks of supplementing with 450 mg cocoa flavanols twice daily improved markers of endothelial function in 39 healthy young and elderly adults in another trial.583 In a controlled crossover trial in 30 middle-aged overweight men and women, 814 mg cocoa flavanols per day (in the form of dark chocolate and a sugar-free cocoa beverage) for four weeks improved vascular endothelial function. The women in the study also had improvement in arterial stiffness while receiving cocoa flavanols.584
In a large, randomized, controlled trial, 21,442 U.S. adults received either 500 mg cocoa flavanols (including 80 mg epicatechin) daily or placebo, along with a multivitamin supplement or another placebo. During a median follow-up of 3.6 years, cocoa flavanols were found to have reduced cardiovascular deaths by 27% but did not significantly affect other adverse cardiovascular outcomes or all-cause mortality.585
Green Coffee Bean Extract
Green coffee beans are higher in polyphenols called chlorogenic acids compared with roasted coffee beans, which are typically used to make coffee beverages. A meta-analysis of data from 14 randomized controlled trials with a combined total of 821 participants found green coffee bean extract, at doses of 180–376 mg daily, improved cardiometabolic health by reducing triglyceride levels, lowering systolic and diastolic blood pressures, and increasing HDL-cholesterol levels.586 Another meta-analysis that included 15 randomized controlled trials with a total of 637 participants found green coffee extract also lowered total cholesterol and fasting blood glucose levels.587 Other meta-analyses have also reported green coffee extract’s positive effects on lipid levels, glucose metabolism, and blood pressure.588-590 A placebo-controlled crossover trial in 21 healthy middle-aged volunteers found a single dose of 302 mg decaffeinated green coffee extract (but not higher doses) improved blood vessel function up to 24 hours post-dose.259 Another placebo-controlled trial in 16 healthy men found blood vessel function and a measure of arterial stiffness improved after two weeks of supplementing with a green coffee bean beverage.591
Oligomeric Proanthocyanidins
Oligomeric proanthocyanidins (OPCs) are polyphenols found in the flowers, pulp, seeds, and bark of many fruits, grains, and vegetables. Good sources of OPCs include grape seeds and skins, blueberries, and coffee.592 Preclinical research indicates OPCs may lower LDL-cholesterol and triglyceride levels, oxidative stress, and levels of markers of inflammatory immune activity, while increasing NO production and improving metabolic and cardiovascular risk factors.593 The health benefits of OPCs are related to their powerful free radical-scavenging properties.592,594
In a controlled trial involving 287 patients with known carotid artery thickening and/or plaque, diet and lifestyle counseling plus 100 mg grape seed OPCs twice daily for two years decreased carotid artery thickness by 5.8% and reduced plaque scores by 33.1%; these measures were stable or increased with diet and lifestyle counseling alone. Furthermore, those who received grape seed extract had lower rates of TIAs, coronary artery surgeries, and hospitalizations for unstable angina.595 In a randomized controlled trial that included 30 middle-aged adults with pre-hypertension, those who received 400 mg grape seed proanthocyanidins daily (but not 200 mg daily) for 12 weeks had greater reductions in systolic blood pressure than those receiving placebo; in addition, among non-smoking participants, 400 mg grape seed extract improved measures of vascular function.596
Aronia Melanocarpa
Aronia melanocarpa, also called black chokeberry, is a member of the rose family. It produces small berries rich in a wide variety of polyphenols.597 Aronia extract has been found to reduce lipid oxidation, improve blood lipid levels, and increase total blood antioxidant capacity in studies in humans.598 In an open-label trial in 143 adults with metabolic syndrome, treatment with a standardized aronia extract for 28 days led to improvements in body weight, cholesterol levels, blood pressure, and blood glucose levels.599 In a randomized placebo-controlled trial in 102 middle-aged participants with pre-hypertension, taking 106 mg of aronia polyphenols daily for 12 weeks resulted in improved endothelial function, as well as changes to the gut microbiome with a shift toward increased presence of beneficial bacteria.600 Similarly, in a controlled clinical trial in which 66 healthy male adults received an aronia extract containing 116 mg of polyphenols, a whole fruit powder, or a placebo daily for 12 weeks, those receiving aronia had improvement in a measure of vascular function and potentially beneficial modifications of the gut microbiota.601
A meta-analysis of findings from controlled trials indicated daily aronia use for six to eight weeks can reduce systolic blood pressure and total cholesterol levels, with stronger effects in individuals over 50 years of age.602 However, not all findings agree. In a randomized controlled trial that included 109 healthy men with mildly elevated cholesterol levels, 150 mg of aronia polyphenols daily for 90 days reduced total and LDL-cholesterol levels in men under 40 years of age, but not those 40 years and older, and had no effects on measures of glucose metabolism, blood pressure, oxidative stress, or inflammation.603 Another randomized controlled trial with 84 participants found neither a high-polyphenol nor low-polyphenol aronia juice, taken daily for four weeks, affected cholesterol levels or blood pressure compared with placebo.604
Hydroxytyrosol
Olive polyphenols, including hydroxytyrosol, have demonstrated anti-inflammatory, antioxidant, anti-hypertensive, anti-diabetic, anti-thrombotic, HDL-cholesterol-raising, and anti-atherosclerotic effects in preclinical research.605,606 Studies further indicate hydroxytyrosol, which is highly concentrated in extra virgin olive oil, may counteract endothelial dysfunction not only by reducing oxidative stress and inflammation, but also vascular aging and stiffness.607
Several clinical trials have found hydroxytyrosol can reduce levels of inflammatory markers, improve lipid profiles, enhance endothelial function, lower blood pressure, and reduce oxidative stress.608 For example, in a crossover trial, 30 patients with coronary artery disease took olive oil capsules providing 10 mg of hydroxytyrosol daily for one month and placebo for one month, in random order. Measures of coronary artery and cardiac function, as well as markers of oxidative stress and inflammation, were improved during treatment with hydroxytyrosol-rich olive oil.609 A randomized controlled crossover trial in 60 men with stage 1 hypertension found 6 mg hydroxytyrosol plus 136 mg oleuropein (another olive polyphenol) daily for six weeks significantly reduced blood pressure and total cholesterol, LDL-cholesterol, and triglyceride levels compared with a polyphenol-free supplement.610
Hesperidin
Hesperidin is a polyphenol that occurs in citrus fruits, especially their peels. Digestion of hesperidin by intestinal bacteria produces a compound called hesperetin, along with other metabolites. Hesperidin and hesperetin are powerful free radical scavengers and have demonstrated anti-inflammatory, insulin-sensitizing, and lipid-lowering activity in animal studies.611,612
Several controlled clinical trials have examined the effects of hesperidin on markers of cardiovascular risk. In one randomized controlled trial, 159 participants with pre- or stage 1 hypertension received either 500 mL of hesperidin-enriched orange juice (providing 600 mg hesperidin), standard orange juice (providing 345 mg hesperidin), or a control drink (providing no hesperidin) daily for 12 weeks. Hesperidin-enriched orange juice was found to have greater immediate and sustained blood pressure-lowering effects than the other drinks.613 A randomized controlled trial that had 68 participants with overweight or obesity found 450 mg hesperidin daily for six weeks did not improve vascular function overall; however, in a subgroup of 48 participants with baseline flow-mediated dilation of 3% or greater (healthy flow-mediated dilation is around 7%),614 hesperidin mitigated the negative effect of a high-fat meal on endothelial function.615 Another trial in 24 overweight men found 292 mg of hesperidin, taken daily in an orange juice or control drink, improved after-meal endothelial function as well as diastolic blood pressure after four weeks.616 In a crossover trial, 24 adults with metabolic syndrome were treated with 500 mg hesperidin per day or placebo for three weeks. Hesperidin improved endothelial function, reduced CRP levels by 32%, and decreased levels of total cholesterol, ApoB, and markers of vascular inflammation, relative to placebo.617
Pine Bark Extract (Pycnogenol)
Pycnogenol is obtained from the French maritime pine tree (Pinus pinaster) and contains several types of polyphenols, including catechins and epicatechins, proanthocyanidins, and cinnamic acids.618 Pycnogenol may benefit cardiovascular health through a variety of mechanisms, such as inhibiting inflammation and clotting, reducing oxidative stress, and improving endothelial function.619
In a randomized, placebo-controlled, crossover trial, 23 individuals with coronary artery disease received 200 mg pycnogenol daily for eight weeks followed by placebo for eight weeks, or vice versa. Treatment with pycnogenol resulted in a significant reduction in oxidative stress and a 32% increase in a measure of endothelial function.620 A small trial in 16 healthy young men found 180 mg pycnogenol daily for two weeks increased NO synthesis, vasodilation, and forearm blood flow, indicating improved endothelial function.621
Quercetin
Quercetin is a polyphenol found in virtually all plants and plant foods. Good sources of quercetin include apples, onions, cherries, berries, red grapes, red wine, and tea. Quercetin has well-established anti-inflammatory and antioxidant properties, and has been found to have beneficial effects on diabetes, obesity, dyslipidemia, high blood pressure, and atherosclerosis.622 Although quercetin is poorly absorbed in the intestines, it is digested by gut microbes, producing other compounds that may contribute to its cardio-protective effects; in addition, quercetin appears to support cardiovascular health by modulating gut microbiome composition.623
Although there have been mixed findings from clinical trials investigating the use of quercetin for cardiovascular prevention and treatment, some studies have found benefits.624 In a randomized, placebo-controlled, crossover trial that included 37 participants with pre-hypertension, 160 mg quercetin daily for four weeks led to reduced inflammatory marker levels and improved a marker of endothelial function.560 In another randomized, controlled, crossover trial that included 70 subjects with pre-hypertension and obesity or overweight, 162 mg quercetin daily for six weeks was found to reduce ambulatory blood pressure (an average calculated from 24 hours of monitoring), but did not affect markers of oxidation, inflammation, lipid or glucose metabolism, or endothelial function.625 Taking 150 mg quercetin daily for six weeks was also found to lower high blood pressure and levels of oxidized LDL in another crossover trial in 93 overweight or obese subjects with other components of metabolic syndrome.626
Vitamins & Minerals
B vitamins
The family of B vitamins includes eight compounds: thiamine (B1), riboflavin (B2), niacin (B3), pantothenic acid (B5), pyridoxine (B6), biotin (B7), folate (B9), and cobalamin (B12). B vitamins are found in many foods including fish, leafy greens, whole grains, legumes, meat, eggs, dairy products, and nutritional yeast. As coenzymes for enzymatic reactions that support every aspect of cellular function, B vitamins are essential for cardiovascular health.627 In a large observational study with 115,664 participants who were followed for 8‒12 years, higher folate intake in particular was associated with lower risks of stroke, heart attack, and cardiovascular mortality.628 Another study examined data from 55,569 participants in the ongoing National Health and Nutrition Examination Survey and found higher intakes of folate and B6 were associated with lower rates of all-cause, cardiovascular, and cancer deaths in men and all-cause and cardiovascular deaths in women.629
A randomized controlled trial in 60 individuals with pre-hypertension found supplementing with B vitamins (providing 10 mg B1, 10 mg B2, 100 mg B3, 50 mg B5, 3 mg B6, 1.5 mg B9, and 15 mcg B12) plus vitamin C (150 mg) daily for four months lowered levels of homocysteine and hs-CRP and reduced insulin resistance compared with placebo.630 In another trial involving 81 people with mild-to-moderate high blood pressure who were not on blood pressure medication, a supplement providing 2.4 grams L-arginine, 3 mg B6, 0.4 mg folic acid, and 2 mcg B12 per day for six months mitigated the negative effects of a high-fat meal on vascular function and lowered blood pressure and homocysteine levels compared with placebo.631
Vitamin E
Vitamin E is a family of fat-soluble antioxidant compounds comprising four tocopherols and four tocotrienols (each type has alpha, beta, gamma, and delta forms), with the highest concentrations found in oils, nuts, whole grains, and green leafy vegetables.632
Although findings from clinical trials have been mixed, multiple meta-analyses have found that supplementing with vitamin E, alone or in combination with other nutrients, reduced adverse cardiovascular outcomes including heart attacks and cardiovascular mortality.632-634 For example, a meta-analysis that included 10 randomized controlled trials found vitamin E supplementation reduced cardiovascular mortality by 12%.634 Another meta-analysis that included 27 trials found supplementing with 300–1,800 IU (201–1,206 mg d-alpha tocopherol equivalent) vitamin E per day improved endothelial function in those with low baseline serum vitamin E.635
Human bodies primarily use the alpha-tocopherol form of vitamin E, and alpha-tocopherol is necessary to treat vitamin E deficiency. However, preclinical evidence suggests gamma-tocopherol has stronger antioxidant, anti-inflammatory, and cardioprotective effects than alpha-tocopherol. Multiple clinical trials have found benefits of gamma-tocopherol supplementation for lowering oxidative stress, inhibiting platelet aggregation, and improving endothelial function.636 Further clinical research into the various forms of vitamin E is necessarily to more fully establish their effects on atherosclerosis and cardiovascular outcomes.
Vitamin K
Vitamin K is a fat-soluble vitamin with two naturally occurring forms: phylloquinone (K1) from plant sources and menaquinones (K2) from animal and bacterial sources. Much of the vitamin K1 in the diet is converted to K2 in the body. K1 is recognized for its involvement in blood clotting, while K2 plays an important role in healthy calcium metabolism in tissues throughout the body.637 Anticoagulant drugs that work by blocking the activity of vitamin K, including warfarin (Coumadin), are associated with increased vascular calcification.638-641 Also, kidney transplant recipients, in whom vitamin K deficiency is common, tend to experience increased vascular calcification and stiffness.642
An open-label randomized trial compared the effect of supplementing with 2 mg per day vitamin K1 with placebo in 72 patients with mild or moderate aortic valve calcification and thickening. After 12 months, the aortic valve calcification volume score increased by 22% in the placebo group compared with just 10% in the vitamin K group.643 In an earlier clinical trial, 388 healthy older men and women (aged 60–80 years) received either a daily multivitamin with 500 mcg vitamin K1 or a multivitamin alone for three years. In an analysis limited to participants who were at least 85% adherent to the supplement regimen, those receiving vitamin K had significantly less progression of coronary artery disease. In those with pre-existing CAC, there was 6% less progression in the vitamin K group.644
A meta-analysis of observational data from 21 studies with 222,592 participants found increased dietary intake of K1 and K2 were each associated with decreased risk of coronary artery disease, and vitamin K deficiency was linked to increased mortality from any cause.645 Similarly, another meta-analysis of data from three large U.S. cohorts, comprising a total of 3,891 participants, found the risk of all-cause mortality was 19% higher in those with the lowest circulating K1 levels compared to those with the highest levels. However, this study found no association between cardiovascular disease risk and K1 levels.646 An observational study in over 55,500 adults aged 50–64 years, followed for 21.5 years, found those in the highest 20% of dietary vitamin K1 intake—based on an initial diet survey—had a 23% lower risk of aortic stenosis and a 27% lower risk of aortic stenosis with complications.647
Analyses of controlled clinical trials have revealed some beneficial effects of vitamin K supplementation on vascular calcification. One systematic review and meta-analysis that included data from 13 controlled trials (2,162 subjects) and 14 longitudinal observational studies (10,726 subjects) concluded that vitamin K supplementation was associated with significant reductions in vascular calcification, undercarboxylated matrix Gla protein, and undercarboxylated osteocalcin.648 Another analysis concluded that although the benefit was not consistent across all studies, those with greater calcification at study entry had the greatest likelihood of benefit.648 A randomized controlled trial in 365 men with aortic valve calcification administered 720 mcg MK-7 (a form of vitamin K2) plus 1,000 IU (25 mcg) vitamin D daily for two years; this treatment had no significant effect on aortic or CAC scores or rates of heart valve surgeries, cardiovascular events, or all-cause deaths.649 A subsequent analysis of 304 subjects from this same trial found treatment slowed the progression of CAC in those with greater baseline CAC scores, but not in the overall set of participants, after two years. Additionally, a prespecified safety endpoint of the combined number of patients with heart attack, coronary revascularization, and all-cause mortality was reached in 10 placebo recipients but only three patients receiving the combined vitamin treatment.650
Vitamin C
Vitamin C (ascorbic acid) is a water-soluble vitamin with important free radical-scavenging effects. It is an essential cofactor in numerous enzymatic processes, including those related to wound healing, collagen synthesis, and control of gene expression. Observational studies have found that better vitamin C status is linked to protection against coronary artery disease, stroke, and high blood pressure. Controlled trials of vitamin C supplementation have demonstrated improvements in endothelial function, including in individuals with elevated cardiovascular risk.635,651,652 Nevertheless, randomized controlled trials using 120–1,000 mg vitamin C daily for eight weeks to nine years have so far not shown a benefit in terms of cardiovascular events or mortality.13,653
Vitamin D
Vitamin D is needed for immune regulation and cardiovascular health, and deficiency has been linked with atherosclerosis and cardiovascular disease.161,162 In a randomized controlled trial published in 2023, a total of 21,302 people aged 60–84 years received either 60,000 IU (15,000 mcg) vitamin D3 or placebo monthly for up to five years; those receiving vitamin D had a 19% lower risk of heart attack compared with placebo. Those in the vitamin D group also had a lower rate of major cardiovascular events in general, but this difference was not statistically significant.162
It is important to note that several randomized controlled trials have failed to show vitamin D supplementation improved cardiovascular outcomes.654-657 However, many of these trials used relatively low doses (eg, the equivalent of about 2,000 IU [50 mcg] per day or less) and/or high intermittent doses rather than regular daily doses. Furthermore, because these trials often have not assessed vitamin D status, it is still unclear whether supplementation would have demonstrated cardiovascular benefits in individuals with vitamin D deficiency. More trials that assess participants’ baseline vitamin D status and target optimal blood levels of 25-hydroxyvitamin D with appropriate daily doses are needed to establish vitamin D’s potential role in improving cardiovascular outcomes.
Magnesium
Magnesium deficiency, which is more common with aging, can contribute to atherosclerosis by promoting high blood pressure, lipid accumulation, disturbed calcium metabolism, heart rhythm abnormalities, impaired insulin sensitivity, and vascular stiffness.658,659 An observational study that monitored 14,446 participants for 27 years found lower blood magnesium levels were associated with a higher risk of developing coronary artery disease.660 In 13,826 individuals who were followed for about 24 years, low magnesium status was correlated with an increased risk of peripheral artery disease.661 Multiple studies have linked low magnesium intake and blood levels with higher risk of cardiovascular disease and events.662 Other observational evidence suggests healthy magnesium levels may help mitigate the effect of dyslipidemia on atherosclerosis risk.663
In a randomized placebo-controlled trial in 64 coronary artery disease patients, 300 mg magnesium sulfate (providing 30 mg of elemental magnesium) daily for six months improved markers of atherosclerotic risk, including levels of oxidized LDL, inflammatory markers, homocysteine, electrolytes, and thyroid hormone, as well as HbA1c.664,665 In another randomized placebo-controlled trial in 60 people with coronary artery disease, 300 mg magnesium sulfate for three months lowered gene expression and blood levels of two out of five inflammatory cytokines measured.666 A meta-analysis that included seven randomized controlled trials with a total of 306 participants found magnesium supplementation improved endothelial function but did not change carotid artery wall thickness.667 In addition, a randomized placebo-controlled trial in 124 subjects with overweight or mild obesity found 150 mg elemental magnesium three times daily for 24 weeks had no effect on arterial stiffness or blood pressure, regardless of whether it was in the form of magnesium citrate, oxide, or sulfate.668
2025
- Mar: Comprehensive update & review
2022
- Oct: Added section on coffee consumption and cardiovascular disease to Atherosclerosis Prevention
- Oct: Added section on green coffee bean extract to Nutrients
- Mar: Updated section on adopting a healthy diet in Atherosclerosis Prevention
- Mar: Updated section on potassium in Nutrients
2021
- Nov: Added section on TMAO and cardiovascular disease to Atherosclerosis Risk Factors
- May: Added section on carotenoids to Nutrients
- May: Updated section on physical activity in Atherosclerosis Prevention
2019
- Dec: Comprehensive update & review
Disclaimer and Safety Information
This information (and any accompanying material) is not intended to replace the attention or advice of a physician or other qualified health care professional. Anyone who wishes to embark on any dietary, drug, exercise, or other lifestyle change intended to prevent or treat a specific disease or condition should first consult with and seek clearance from a physician or other qualified health care professional. Pregnant women in particular should seek the advice of a physician before using any protocol listed on this website. The protocols described on this website are for adults only, unless otherwise specified. Product labels may contain important safety information and the most recent product information provided by the product manufacturers should be carefully reviewed prior to use to verify the dose, administration, and contraindications. National, state, and local laws may vary regarding the use and application of many of the therapies discussed. The reader assumes the risk of any injuries. The authors and publishers, their affiliates and assigns are not liable for any injury and/or damage to persons arising from this protocol and expressly disclaim responsibility for any adverse effects resulting from the use of the information contained herein.
The protocols raise many issues that are subject to change as new data emerge. None of our suggested protocol regimens can guarantee health benefits. Life Extension has not performed independent verification of the data contained in the referenced materials, and expressly disclaims responsibility for any error in the literature.
- Khandkar C, Madhavan MV, Weaver JC, Celermajer DS, Karimi Galougahi K. Atherothrombosis in Acute Coronary Syndromes-From Mechanistic Insights to Targeted Therapies. Cells. Apr 10 2021;10(4)doi:10.3390/cells10040865 https://www.ncbi.nlm.nih.gov/pubmed/33920201
- Jebari-Benslaiman S, Galicia-Garcia U, Larrea-Sebal A, et al. Pathophysiology of Atherosclerosis. Int J Mol Sci. Mar 20 2022;23(6)doi:10.3390/ijms23063346 https://www.ncbi.nlm.nih.gov/pubmed/35328769
- Higashi Y. Roles of Oxidative Stress and Inflammation in Vascular Endothelial Dysfunction-Related Disease. Antioxidants (Basel). Sep 30 2022;11(10)doi:10.3390/antiox11101958 https://www.ncbi.nlm.nih.gov/pubmed/36290681
- Baaten C, Nagy M, Bergmeier W, Spronk HMH, van der Meijden PEJ. Platelet biology and function: plaque erosion vs. rupture. Eur Heart J. Jan 1 2024;45(1):18-31. doi:10.1093/eurheartj/ehad720 https://www.ncbi.nlm.nih.gov/pubmed/37940193
- Henning RJ. Obesity and obesity-induced inflammatory disease contribute to atherosclerosis: a review of the pathophysiology and treatment of obesity. American journal of cardiovascular disease. 2021;11(4):504-529. https://www.ncbi.nlm.nih.gov/pubmed/34548951
- Yuan S, Mason AM, Burgess S, Larsson SC. Differentiating Associations of Glycemic Traits With Atherosclerotic and Thrombotic Outcomes: Mendelian Randomization Investigation. Diabetes. Oct 1 2022;71(10):2222-2232. doi:10.2337/db21-0905 https://www.ncbi.nlm.nih.gov/pubmed/35499407
- Kolte D, Yonetsu T, Ye JC, Libby P, Fuster V, Jang IK. Optical Coherence Tomography of Plaque Erosion: JACC Focus Seminar Part 2/3. J Am Coll Cardiol. Sep 21 2021;78(12):1266-1274. doi:10.1016/j.jacc.2021.07.030 https://www.ncbi.nlm.nih.gov/pubmed/34531028
- Libby P, Pasterkamp G, Crea F, Jang IK. Reassessing the Mechanisms of Acute Coronary Syndromes. Circ Res. Jan 4 2019;124(1):150-160. doi:10.1161/CIRCRESAHA.118.311098 https://www.ncbi.nlm.nih.gov/pubmed/30605419
- Bae JH, Lim H, Lim S. The Potential Cardiometabolic Effects of Long-Chain omega-3 Polyunsaturated Fatty Acids: Recent Updates and Controversies. Adv Nutr. Jul 2023;14(4):612-628. doi:10.1016/j.advnut.2023.03.014 https://www.ncbi.nlm.nih.gov/pubmed/37031750
- Jorat MV, Tabrizi R, Mirhosseini N, et al. The effects of coenzyme Q10 supplementation on lipid profiles among patients with coronary artery disease: a systematic review and meta-analysis of randomized controlled trials. Lipids in health and disease. Oct 9 2018;17(1):230. doi:10.1186/s12944-018-0876-4 https://www.ncbi.nlm.nih.gov/pubmed/30296936
- Sabbatinelli J, Orlando P, Galeazzi R, et al. Ubiquinol Ameliorates Endothelial Dysfunction in Subjects with Mild-to-Moderate Dyslipidemia: A Randomized Clinical Trial. Nutrients. Apr 15 2020;12(4)doi:10.3390/nu12041098 https://www.ncbi.nlm.nih.gov/pubmed/32326664
- Alehagen U, Johansson P, Bjornstedt M, Rosen A, Dahlstrom U. Cardiovascular mortality and N-terminal-proBNP reduced after combined selenium and coenzyme Q10 supplementation: a 5-year prospective randomized double-blind placebo-controlled trial among elderly Swedish citizens. Int J Cardiol. Sep 1 2013;167(5):1860-6. doi:10.1016/j.ijcard.2012.04.156 https://www.ncbi.nlm.nih.gov/pubmed/22626835
- Jenkins DJA, Spence JD, Giovannucci EL, et al. Supplemental Vitamins and Minerals for Cardiovascular Disease Prevention and Treatment: JACC Focus Seminar. J Am Coll Cardiol. Feb 2 2021;77(4):423-436. doi:10.1016/j.jacc.2020.09.619 https://www.ncbi.nlm.nih.gov/pubmed/33509399
- Surma S, Sahebkar A, Urbanski J, Penson PE, Banach M. Curcumin - The Nutraceutical With Pleiotropic Effects? Which Cardiometabolic Subjects Might Benefit the Most? Front Nutr. 2022;9:865497. doi:10.3389/fnut.2022.865497 https://www.ncbi.nlm.nih.gov/pubmed/35662932
- Hajizadeh-Sharafabad F, Sharifi Zahabi E. Role of alpha-lipoic acid in vascular function: A systematic review of human intervention studies. Crit Rev Food Sci Nutr. 2022;62(11):2928-2941. doi:10.1080/10408398.2020.1861425 https://www.ncbi.nlm.nih.gov/pubmed/33327738
- Przybylska S, Tokarczyk G. Lycopene in the Prevention of Cardiovascular Diseases. Int J Mol Sci. Feb 10 2022;23(4)doi:10.3390/ijms23041957 https://www.ncbi.nlm.nih.gov/pubmed/35216071
- Gadidala SK, Johny E, Thomas C, Nadella M, Undela K, Adela R. Effect of garlic extract on markers of lipid metabolism and inflammation in coronary artery disease (CAD) patients: A systematic review and meta-analysis. Phytother Res. Jun 2023;37(6):2242-2254. doi:10.1002/ptr.7729 https://www.ncbi.nlm.nih.gov/pubmed/36640154
- Mozos I, Flangea C, Vlad DC, et al. Effects of Anthocyanins on Vascular Health. Biomolecules. May 30 2021;11(6)doi:10.3390/biom11060811 https://www.ncbi.nlm.nih.gov/pubmed/34070757
- Behl T, Bungau S, Kumar K, et al. Pleotropic Effects of Polyphenols in Cardiovascular System. Biomed Pharmacother. Oct 2020;130:110714. doi:10.1016/j.biopha.2020.110714 https://www.ncbi.nlm.nih.gov/pubmed/34321158
- Libby P. The changing landscape of atherosclerosis. Nature. Apr 2021;592(7855):524-533. doi:10.1038/s41586-021-03392-8 https://www.ncbi.nlm.nih.gov/pubmed/33883728
- Bjorkegren JLM, Lusis AJ. Atherosclerosis: Recent developments. Cell. May 12 2022;185(10):1630-1645. doi:10.1016/j.cell.2022.04.004 https://www.ncbi.nlm.nih.gov/pubmed/35504280
- NHLBI. National Heart, Lung, and Blood Institute: Atherosclerosis. Updated 03/24/22. Accessed 08/02/23, https://www.nhlbi.nih.gov/health/atherosclerosis
- Kaski JC. Pathogenesis of atherosclerosis. UpToDate. Updated 4/15/2022. Accessed 9/6/2023, https://www.uptodate.com/contents/pathogenesis-of-atherosclerosis
- American Association of Neurological Surgeons. Cerebrovascular disease. Accessed March 5, 2021. https://www.aans.org/en/Patients/Neurosurgical-Conditions-and-Treatments/Cerebrovascular-Disease
- Duggan JP, Peters AS, Trachiotis GD, Antevil JL. Epidemiology of Coronary Artery Disease. The Surgical clinics of North America. Jun 2022;102(3):499-516. doi:10.1016/j.suc.2022.01.007 https://www.ncbi.nlm.nih.gov/pubmed/35671770
- Virani SS, Alonso A, Aparicio HJ, et al. Heart Disease and Stroke Statistics-2021 Update: A Report From the American Heart Association. Circulation. Feb 23 2021;143(8):e254-e743. doi:10.1161/CIR.0000000000000950 https://www.ncbi.nlm.nih.gov/pubmed/33501848
- Genovesi S, Parati G. Cardiovascular Risk in Children: Focus on Pathophysiological Aspects. Int J Mol Sci. Sep 10 2020;21(18)doi:10.3390/ijms21186612 https://www.ncbi.nlm.nih.gov/pubmed/32927656
- Gospodarczyk A, Marczewski K, Gospodarczyk N, Widuch M, Tkocz M, Zalejska-Fiolka J. Homocysteine and Cardiovascular Disease - a Current Review. Wiadomosci lekarskie (Warsaw, Poland : 1960). 2022;75(11 pt 2):2862-2866. doi:10.36740/WLek202211224 https://www.ncbi.nlm.nih.gov/pubmed/36591781
- Romero-Cabrera JL, Ankeny J, Fernandez-Montero A, Kales SN, Smith DL. A Systematic Review and Meta-Analysis of Advanced Biomarkers for Predicting Incident Cardiovascular Disease among Asymptomatic Middle-Aged Adults. Int J Mol Sci. Nov 4 2022;23(21)doi:10.3390/ijms232113540 https://www.ncbi.nlm.nih.gov/pubmed/36362325
- Stanhewicz AE, Kenney WL. Role of folic acid in nitric oxide bioavailability and vascular endothelial function. Nutr Rev. Jan 2017;75(1):61-70. doi:10.1093/nutrit/nuw053 https://www.ncbi.nlm.nih.gov/pubmed/27974600
- Rocha BS. The Nitrate-Nitrite-Nitric Oxide Pathway on Healthy Ageing: A Review of Pre-clinical and Clinical Data on the Impact of Dietary Nitrate in the Elderly. Mini Review. Front Aging. 2021-November-17 2021;2:778467. doi:10.3389/fragi.2021.778467 https://www.ncbi.nlm.nih.gov/pubmed/35821990
- Siragusa M, Fleming I. The eNOS signalosome and its link to endothelial dysfunction. Pflugers Arch. Jul 2016;468(7):1125-1137. doi:10.1007/s00424-016-1839-0 https://www.ncbi.nlm.nih.gov/pubmed/27184745
- Barrett TJ. Macrophages in Atherosclerosis Regression. Arterioscler Thromb Vasc Biol. Jan 2020;40(1):20-33. doi:10.1161/ATVBAHA.119.312802 https://www.ncbi.nlm.nih.gov/pubmed/31722535
- Fernandez DM, Rahman AH, Fernandez NF, et al. Single-cell immune landscape of human atherosclerotic plaques. Nat Med. Oct 2019;25(10):1576-1588. doi:10.1038/s41591-019-0590-4 https://www.ncbi.nlm.nih.gov/pubmed/31591603
- Sage AP, Tsiantoulas D, Binder CJ, Mallat Z. The role of B cells in atherosclerosis. Nature reviews Cardiology. Mar 2019;16(3):180-196. doi:10.1038/s41569-018-0106-9 https://www.ncbi.nlm.nih.gov/pubmed/30410107
- Libby P, Buring JE, Badimon L, et al. Atherosclerosis. Nat Rev Dis Primers. Aug 16 2019;5(1):56. doi:10.1038/s41572-019-0106-z https://www.ncbi.nlm.nih.gov/pubmed/31420554
- Pan W, Jie W, Huang H. Vascular calcification: Molecular mechanisms and therapeutic interventions. MedComm (2020). Feb 2023;4(1):e200. doi:10.1002/mco2.200 https://www.ncbi.nlm.nih.gov/pubmed/36620697
- Dzaye O, Razavi AC, Blaha MJ, Mortensen MB. Evaluation of coronary stenosis versus plaque burden for atherosclerotic cardiovascular disease risk assessment and management. Current opinion in cardiology. Nov 1 2021;36(6):769-775. doi:10.1097/HCO.0000000000000911 https://www.ncbi.nlm.nih.gov/pubmed/34620792
- Crea F, Kolodgie F, Finn A, Virmani R. Mechanisms of acute coronary syndromes related to atherosclerosis. UpToDate. Updated 9/19/2022. Accessed 9/6/2023, https://www.uptodate.com/contents/mechanisms-of-acute-coronary-syndromes-related-to-atherosclerosis
- Kolte D, Libby P, Jang IK. New Insights Into Plaque Erosion as a Mechanism of Acute Coronary Syndromes. JAMA. Mar 16 2021;325(11):1043-1044. doi:10.1001/jama.2021.0069 https://www.ncbi.nlm.nih.gov/pubmed/33616637
- Jia H, Kubo T, Akasaka T, Yu B. Optical Coherence Tomography Guidance in Management of Acute Coronary Syndrome Caused by Plaque Erosion. Circ J. Jan 25 2018;82(2):302-308. doi:10.1253/circj.CJ-17-1373 https://www.ncbi.nlm.nih.gov/pubmed/29332908
- Vergallo R, Jang IK, Crea F. New prediction tools and treatment for ACS patients with plaque erosion. Atherosclerosis. Feb 2021;318:45-51. doi:10.1016/j.atherosclerosis.2020.10.016 https://www.ncbi.nlm.nih.gov/pubmed/33127074
- Onea HL, Spinu M, Homorodean C, et al. Superficial Calcified Plates Associated to Plaque Erosions in Acute Coronary Syndromes. Life (Basel). Aug 11 2023;13(8)doi:10.3390/life13081732 https://www.ncbi.nlm.nih.gov/pubmed/37629589
- Mahler SA. UpToDate. Approach to the patient with suspected angina pectoris. UpToDate. Accessed 12/05/2023, https://www.uptodate.com/contents/approach-to-the-patient-with-suspected-angina-pectoris
- NICE. National Institute for Health and Care Excellence: Guidelines. Stable angina: management. National Institute for Health and Care Excellence (NICE)
- Sweis RN, Jivan A. Merck Manual. Professional Version. Overview of Acute Coronary Syndromes (ACS). Updated 9/2022. Accessed 9/7/2023, https://www.merckmanuals.com/professional/cardiovascular-disorders/coronary-artery-disease/overview-of-acute-coronary-syndromes-acs
- Jurgens CY, Lee CS, Aycock DM, et al. State of the Science: The Relevance of Symptoms in Cardiovascular Disease and Research: A Scientific Statement From the American Heart Association. Circulation. Sep 20 2022;146(12):e173-e184. doi:10.1161/CIR.0000000000001089 https://www.ncbi.nlm.nih.gov/pubmed/35979825
- Lineback CM, Stamm B, Sorond F, Caprio FZ. Carotid disease, cognition, and aging: time to redefine asymptomatic disease? Geroscience. Apr 2023;45(2):719-725. doi:10.1007/s11357-022-00688-z https://www.ncbi.nlm.nih.gov/pubmed/36376618
- Textor S. Chronic kidney disease resulting from atherosclerotic renal artery stenosis. UpToDate. Updated 1/18/2022. Accessed 9/7/2023, https://www.uptodate.com/contents/chronic-kidney-disease-resulting-from-atherosclerotic-renal-artery-stenosis
- Bokhari MR, Bokhari SRA. Renal Artery Stenosis. StatPearls. StatPearls Publishing. Copyright © 2023, StatPearls Publishing LLC.; 2025. https://www.ncbi.nlm.nih.gov/pubmed/28613469
- Allison MA, Armstrong DG, Goodney PP, et al. Health Disparities in Peripheral Artery Disease: A Scientific Statement From the American Heart Association. Circulation. Jul 18 2023;148(3):286-296. doi:10.1161/CIR.0000000000001153 https://www.ncbi.nlm.nih.gov/pubmed/37317860
- ACC. American College of Cardiology. Peripheral Matters. Peripheral and Coronary Artery Disease: Two Sides of the Same Coin. Updated 9/20/2019. Accessed 9/7/2023, https://www.acc.org/Latest-in-Cardiology/Articles/2019/09/14/24/42/Peripheral-and-Coronary-Artery-Disease-Two-Sides-of-the-Same-Coin
- Kotlyarov S. Immune and metabolic cross-links in the pathogenesis of comorbid non-alcoholic fatty liver disease. World J Gastroenterol. Jan 28 2023;29(4):597-615. doi:10.3748/wjg.v29.i4.597 https://www.ncbi.nlm.nih.gov/pubmed/36742172
- Stopic B, Medic-Brkic B, Savic-Vujovic K, Davidovic Z, Todorovic J, Dimkovic N. Biomarkers and Predictors of Adverse Cardiovascular Events in Different Stages of Chronic Kidney Disease. Dose-response : a publication of International Hormesis Society. Jul-Sep 2022;20(3):15593258221127568. doi:10.1177/15593258221127568 https://www.ncbi.nlm.nih.gov/pubmed/36118679
- Kushner P, Khunti K, Cebrian A, Deed G. Early Identification and Management of Chronic Kidney Disease: A Narrative Review of the Crucial Role of Primary Care Practitioners. Adv Ther. Oct 2024;41(10):3757-3770. doi:10.1007/s12325-024-02957-z https://www.ncbi.nlm.nih.gov/pubmed/39162984
- Scierka LE, Mena-Hurtado C, Ahmed ZV, et al. The association of depression with mortality and major adverse limb event outcomes in patients with peripheral artery disease: A systematic review and meta-analysis. J Affect Disord. Jan 1 2023;320:169-177. doi:10.1016/j.jad.2022.09.098 https://www.ncbi.nlm.nih.gov/pubmed/36179780
- Pivato CA, Chandiramani R, Petrovic M, et al. Depression and ischemic heart disease. Int J Cardiol. Oct 1 2022;364:9-15. doi:10.1016/j.ijcard.2022.05.056 https://www.ncbi.nlm.nih.gov/pubmed/35643217
- Tonhajzerova I, Sekaninova N, Bona Olexova L, Visnovcova Z. Novel Insight into Neuroimmune Regulatory Mechanisms and Biomarkers Linking Major Depression and Vascular Diseases: The Dilemma Continues. Int J Mol Sci. Mar 27 2020;21(7)doi:10.3390/ijms21072317 https://www.ncbi.nlm.nih.gov/pubmed/32230840
- Dhar AK, Barton DA. Depression and the Link with Cardiovascular Disease. Front Psychiatry. 2016;7:33. doi:10.3389/fpsyt.2016.00033 https://www.ncbi.nlm.nih.gov/pubmed/27047396
- Li Q, Ouyang X, Lin J. The impact of periodontitis on vascular endothelial dysfunction. Front Cell Infect Microbiol. 2022;12:998313. doi:10.3389/fcimb.2022.998313 https://www.ncbi.nlm.nih.gov/pubmed/36118034
- Ruan Q, Guan P, Qi W, et al. Porphyromonas gingivalis regulates atherosclerosis through an immune pathway. Front Immunol. 2023;14:1103592. doi:10.3389/fimmu.2023.1103592 https://www.ncbi.nlm.nih.gov/pubmed/36999040
- Kim S, Lee KY, Kim NH, et al. Relationship of obstructive sleep apnoea severity and subclinical systemic atherosclerosis. Eur Respir J. Feb 2020;55(2)doi:10.1183/13993003.00959-2019 https://www.ncbi.nlm.nih.gov/pubmed/31672758
- Lu M, Wang Z, Zhan X, Wei Y. Obstructive sleep apnea increases the risk of cardiovascular damage: a systematic review and meta-analysis of imaging studies. Syst Rev. Jul 30 2021;10(1):212. doi:10.1186/s13643-021-01759-6 https://www.ncbi.nlm.nih.gov/pubmed/34330323
- De Leonardis F, Colalillo G, Finazzi Agro E, Miano R, Fuschi A, Asimakopoulos AD. Endothelial Dysfunction, Erectile Deficit and Cardiovascular Disease: An Overview of the Pathogenetic Links. Biomedicines. Aug 1 2022;10(8)doi:10.3390/biomedicines10081848 https://www.ncbi.nlm.nih.gov/pubmed/36009395
- Parel PM, Berg AR, Hong CG, et al. Updates in the Impact of Chronic Systemic Inflammation on Vascular Inflammation by Positron Emission Tomography (PET). Current cardiology reports. Apr 2022;24(4):317-326. doi:10.1007/s11886-022-01651-2 https://www.ncbi.nlm.nih.gov/pubmed/35171444
- Choroszy M, Sobieszczanska B, Litwinowicz K, et al. Co-toxicity of Endotoxin and Indoxyl Sulfate, Gut-Derived Bacterial Metabolites, to Vascular Endothelial Cells in Coronary Arterial Disease Accompanied by Gut Dysbiosis. Nutrients. Jan 18 2022;14(3)doi:10.3390/nu14030424 https://www.ncbi.nlm.nih.gov/pubmed/35276782
- Manolis AA, Manolis TA, Melita H, Manolis AS. Gut Microbiota and Cardiovascular Disease: Symbiosis Versus Dysbiosis. Curr Med Chem. 2022;29(23):4050-4077. doi:10.2174/0929867328666211213112949 https://www.ncbi.nlm.nih.gov/pubmed/34961453
- MC. Mayo Clinic. Cholesterol ratio or non-HDL cholesterol: Which is most important? Available at https://www.mayoclinic.org/diseases-conditions/high-blood-cholesterol/expert-answers/cholesterol-ratio/faq-20058006 Last updated 01/12/2024. Accessed 03/04/2024. 2024;
- Greenland P, Blaha MJ, Budoff MJ, Erbel R, Watson KE. Coronary Calcium Score and Cardiovascular Risk. J Am Coll Cardiol. Jul 24 2018;72(4):434-447. doi:10.1016/j.jacc.2018.05.027 https://www.ncbi.nlm.nih.gov/pubmed/30025580
- Nasir K, Cainzos-Achirica M. Role of coronary artery calcium score in the primary prevention of cardiovascular disease. BMJ. May 4 2021;373:n776. doi:10.1136/bmj.n776 https://www.ncbi.nlm.nih.gov/pubmed/33947652
- Golub IS, Termeie OG, Kristo S, et al. Major Global Coronary Artery Calcium Guidelines. JACC Cardiovascular imaging. Jan 2023;16(1):98-117. doi:10.1016/j.jcmg.2022.06.018 https://www.ncbi.nlm.nih.gov/pubmed/36599573
- Khan SS, Matsushita K, Sang Y, et al. Development and Validation of the American Heart Association's PREVENT Equations. Circulation. Feb 6 2024;149(6):430-449. doi:10.1161/CIRCULATIONAHA.123.067626 https://www.ncbi.nlm.nih.gov/pubmed/37947085
- Badawy M, Naing L, Johar S, et al. Evaluation of cardiovascular diseases risk calculators for CVDs prevention and management: scoping review. BMC Public Health. Sep 14 2022;22(1):1742. doi:10.1186/s12889-022-13944-w https://www.ncbi.nlm.nih.gov/pubmed/36104666
- Grant JK, Ndumele CE, Martin SS. The Evolving Landscape of Cardiovascular Risk Assessment. JAMA. Sep 24 2024;332(12):967-969. doi:10.1001/jama.2024.13247 https://www.ncbi.nlm.nih.gov/pubmed/39073798
- Tesauro M, Mauriello A, Rovella V, et al. Arterial ageing: from endothelial dysfunction to vascular calcification. J Intern Med. May 2017;281(5):471-482. doi:10.1111/joim.12605 https://www.ncbi.nlm.nih.gov/pubmed/28345303
- Liberale L, Montecucco F, Tardif JC, Libby P, Camici GG. Inflamm-ageing: the role of inflammation in age-dependent cardiovascular disease. Eur Heart J. Aug 14 2020;41(31):2974-2982. doi:10.1093/eurheartj/ehz961 https://www.ncbi.nlm.nih.gov/pubmed/32006431
- Fuster JJ. Clonal Hematopoiesis and Coronary Artery Disease-A Deep Connection. JAMA Cardiol. Mar 1 2024;9(3):242-244. doi:10.1001/jamacardio.2023.5106 https://www.ncbi.nlm.nih.gov/pubmed/38198161
- Evans MA, Walsh K. Clonal hematopoiesis, somatic mosaicism, and age-associated disease. Physiol Rev. Jan 1 2023;103(1):649-716. doi:10.1152/physrev.00004.2022 https://www.ncbi.nlm.nih.gov/pubmed/36049115
- Memorial Sloan Kettering Cancer Center. Clonal Hematopoiesis (CH). Accessed 5/3/24, https://www.mskcc.org/cancer-care/types/leukemias/risk-factors/clonal-hematopoiesis-ch
- Hoermann G, Greiner G, Griesmacher A, Valent P. Clonal Hematopoiesis of Indeterminate Potential: A Multidisciplinary Challenge in Personalized Hematology. J Pers Med. Aug 20 2020;10(3)doi:10.3390/jpm10030094 https://www.ncbi.nlm.nih.gov/pubmed/32825226
- Liu R, Shao J. Research progress on risk factors related to intracranial artery, carotid artery, and coronary artery stenosis. Front Cardiovasc Med. 2022;9:970476. doi:10.3389/fcvm.2022.970476 https://www.ncbi.nlm.nih.gov/pubmed/36386370
- Miao G, Zhuo D, Han X, et al. From degenerative disease to malignant tumors: Insight to the function of ApoE. Biomed Pharmacother. Feb 2023;158:114127. doi:10.1016/j.biopha.2022.114127 https://www.ncbi.nlm.nih.gov/pubmed/36516696
- Alagarsamy J, Jaeschke A, Hui DY. Apolipoprotein E in Cardiometabolic and Neurological Health and Diseases. Int J Mol Sci. Aug 31 2022;23(17)doi:10.3390/ijms23179892 https://www.ncbi.nlm.nih.gov/pubmed/36077289
- Li Z, Shue F, Zhao N, Shinohara M, Bu G. APOE2: protective mechanism and therapeutic implications for Alzheimer's disease. Molecular neurodegeneration. Nov 4 2020;15(1):63. doi:10.1186/s13024-020-00413-4 https://www.ncbi.nlm.nih.gov/pubmed/33148290
- Vakhtangadze T, Singh Tak R, Singh U, Baig MS, Bezsonov E. Gender Differences in Atherosclerotic Vascular Disease: From Lipids to Clinical Outcomes. Front Cardiovasc Med. 2021;8:707889. doi:10.3389/fcvm.2021.707889 https://www.ncbi.nlm.nih.gov/pubmed/34262956
- Mital R, Bayne J, Rodriguez F, Ovbiagele B, Bhatt DL, Albert MA. Race and Ethnicity Considerations in Patients With Coronary Artery Disease and Stroke: JACC Focus Seminar 3/9. J Am Coll Cardiol. Dec 14 2021;78(24):2483-2492. doi:10.1016/j.jacc.2021.05.051 https://www.ncbi.nlm.nih.gov/pubmed/34886970
- Morton K, Heindl B, Clarkson S, Bittner V. Primordial Prevention of Atherosclerotic Cardiovascular Disease: A REVIEW OF THE LITERATURE. J Cardiopulm Rehabil Prev. Nov 1 2022;42(6):389-396. doi:10.1097/HCR.0000000000000748 https://www.ncbi.nlm.nih.gov/pubmed/36342681
- Wilson P. Overview of established risk factors for cardiovascular disease. UpToDate. Updated 7/22/2024. Accessed 9/24/2024, https://www.uptodate.com/contents/overview-of-established-risk-factors-for-cardiovascular-disease?source=history_widget
- Salehin S, Rasmussen P, Mai S, et al. Plant Based Diet and Its Effect on Cardiovascular Disease. Int J Environ Res Public Health. Feb 14 2023;20(4)doi:10.3390/ijerph20043337 https://www.ncbi.nlm.nih.gov/pubmed/36834032
- Anto L, Blesso CN. Interplay between diet, the gut microbiome, and atherosclerosis: Role of dysbiosis and microbial metabolites on inflammation and disordered lipid metabolism. J Nutr Biochem. Jul 2022;105:108991. doi:10.1016/j.jnutbio.2022.108991 https://www.ncbi.nlm.nih.gov/pubmed/35331903
- Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. Sep 10 2019;140(11):e563-e595. doi:10.1161/CIR.0000000000000677 https://www.ncbi.nlm.nih.gov/pubmed/30879339
- Meyer-Lindemann U, Moggio A, Dutsch A, Kessler T, Sager HB. The Impact of Exercise on Immunity, Metabolism, and Atherosclerosis. Int J Mol Sci. Feb 8 2023;24(4)doi:10.3390/ijms24043394 https://www.ncbi.nlm.nih.gov/pubmed/36834808
- Del Pozo-Cruz J, Garcia-Hermoso A, Alfonso-Rosa RM, et al. Replacing Sedentary Time: Meta-analysis of Objective-Assessment Studies. Am J Prev Med. Sep 2018;55(3):395-402. doi:10.1016/j.amepre.2018.04.042 https://www.ncbi.nlm.nih.gov/pubmed/30122216
- Makarem N, Castro-Diehl C, St-Onge MP, et al. Redefining Cardiovascular Health to Include Sleep: Prospective Associations With Cardiovascular Disease in the MESA Sleep Study. J Am Heart Assoc. Nov 2022;11(21):e025252. doi:10.1161/JAHA.122.025252 https://www.ncbi.nlm.nih.gov/pubmed/36259552
- Huang J, Qin S, Huang L, Tang Y, Ren H, Hu H. Efficacy and safety of Rhizoma curcumea longae with respect to improving the glucose metabolism of patients at risk for cardiovascular disease: a meta-analysis of randomised controlled trials. J Hum Nutr Diet. Oct 2019;32(5):591-606. doi:10.1111/jhn.12648 https://www.ncbi.nlm.nih.gov/pubmed/30983042
- Blinnikova K, Cohen CW, McKeag ID. Lifestyle Intervention for the Prevention of Cardiovascular Disease. Prim Care. Mar 2024;51(1):13-26. doi:10.1016/j.pop.2023.07.001 https://www.ncbi.nlm.nih.gov/pubmed/38278567
- DiGiacomo SI, Jazayeri MA, Barua RS, Ambrose JA. Environmental Tobacco Smoke and Cardiovascular Disease. Int J Environ Res Public Health. Dec 31 2018;16(1)doi:10.3390/ijerph16010096 https://www.ncbi.nlm.nih.gov/pubmed/30602668
- Munzel T, Hahad O, Kuntic M, Keaney JF, Deanfield JE, Daiber A. Effects of tobacco cigarettes, e-cigarettes, and waterpipe smoking on endothelial function and clinical outcomes. Eur Heart J. Nov 1 2020;41(41):4057-4070. doi:10.1093/eurheartj/ehaa460 https://www.ncbi.nlm.nih.gov/pubmed/32585699
- Zhou Z, Ong KL, Whelton SP, et al. Impact of Blood Lipids on 10-Year Cardiovascular Risk in Individuals Without Dyslipidemia and With Low Risk Factor Burden. Mayo Clin Proc. Oct 2022;97(10):1883-1893. doi:10.1016/j.mayocp.2022.03.025 https://www.ncbi.nlm.nih.gov/pubmed/35760597
- Alexander RW. Theodore Cooper Memorial Lecture. Hypertension and the pathogenesis of atherosclerosis. Oxidative stress and the mediation of arterial inflammatory response: a new perspective. Hypertension. Feb 1995;25(2):155-61. doi:10.1161/01.hyp.25.2.155 https://www.ncbi.nlm.nih.gov/pubmed/7843763
- Al-Mashhadi RH, Al-Mashhadi AL, Nasr ZP, et al. Local Pressure Drives Low-Density Lipoprotein Accumulation and Coronary Atherosclerosis in Hypertensive Minipigs. J Am Coll Cardiol. Feb 9 2021;77(5):575-589. doi:10.1016/j.jacc.2020.11.059 https://www.ncbi.nlm.nih.gov/pubmed/33538256
- Luo D, Cheng Y, Zhang H, et al. Association between high blood pressure and long term cardiovascular events in young adults: systematic review and meta-analysis. BMJ. Sep 9 2020;370:m3222. doi:10.1136/bmj.m3222 https://www.ncbi.nlm.nih.gov/pubmed/32907799
- Beverly JK, Budoff MJ. Atherosclerosis: Pathophysiology of insulin resistance, hyperglycemia, hyperlipidemia, and inflammation. Journal of diabetes. Feb 2020;12(2):102-104. doi:10.1111/1753-0407.12970 https://www.ncbi.nlm.nih.gov/pubmed/31411812
- Kosmas CE, Bousvarou MD, Kostara CE, Papakonstantinou EJ, Salamou E, Guzman E. Insulin resistance and cardiovascular disease. J Int Med Res. Mar 2023;51(3):3000605231164548. doi:10.1177/03000605231164548 https://www.ncbi.nlm.nih.gov/pubmed/36994866
- Lovren F, Teoh H, Verma S. Obesity and atherosclerosis: mechanistic insights. The Canadian journal of cardiology. Feb 2015;31(2):177-83. doi:10.1016/j.cjca.2014.11.031 https://www.ncbi.nlm.nih.gov/pubmed/25661552
- Katsiki N, Mikhailidis DP, Banach M. Leptin, cardiovascular diseases and type 2 diabetes mellitus. Acta Pharmacol Sin. Jul 2018;39(7):1176-1188. doi:10.1038/aps.2018.40 https://www.ncbi.nlm.nih.gov/pubmed/29877321
- Zhou Y, Li H, Xia N. The Interplay Between Adipose Tissue and Vasculature: Role of Oxidative Stress in Obesity. Front Cardiovasc Med. 2021;8:650214. doi:10.3389/fcvm.2021.650214 https://www.ncbi.nlm.nih.gov/pubmed/33748199
- Aboonabi A, Meyer RR, Singh I. The association between metabolic syndrome components and the development of atherosclerosis. Journal of human hypertension. Dec 2019;33(12):844-855. doi:10.1038/s41371-019-0273-0 https://www.ncbi.nlm.nih.gov/pubmed/31636352
- Ju SY, Lee JY, Kim DH. Association of metabolic syndrome and its components with all-cause and cardiovascular mortality in the elderly: A meta-analysis of prospective cohort studies. Medicine (Baltimore). Nov 2017;96(45):e8491. doi:10.1097/MD.0000000000008491 https://www.ncbi.nlm.nih.gov/pubmed/29137039
- Montone RA, Camilli M, Calvieri C, et al. Exposome in ischaemic heart disease: beyond traditional risk factors. Eur Heart J. Feb 7 2024;45(6):419-438. doi:10.1093/eurheartj/ehae001 https://www.ncbi.nlm.nih.gov/pubmed/38238478
- Avis SR, Vernon ST, Hagstrom E, Figtree GA. Coronary artery disease in the absence of traditional risk factors: a call for action. Eur Heart J. Oct 1 2021;42(37):3822-3824. doi:10.1093/eurheartj/ehab474 https://www.ncbi.nlm.nih.gov/pubmed/34293105
- Walldius G, de Faire U, Alfredsson L, et al. Long-term risk of a major cardiovascular event by apoB, apoA-1, and the apoB/apoA-1 ratio-Experience from the Swedish AMORIS cohort: A cohort study. PLoS Med. Dec 2021;18(12):e1003853. doi:10.1371/journal.pmed.1003853 https://www.ncbi.nlm.nih.gov/pubmed/34851955
- Lampsas S, Xenou M, Oikonomou E, et al. Lipoprotein(a) in Atherosclerotic Diseases: From Pathophysiology to Diagnosis and Treatment. Molecules. Jan 18 2023;28(3)doi:10.3390/molecules28030969 https://www.ncbi.nlm.nih.gov/pubmed/36770634
- Hong CG, Florida E, Li H, Parel PM, Mehta NN, Sorokin AV. Oxidized low-density lipoprotein associates with cardiovascular disease by a vicious cycle of atherosclerosis and inflammation: A systematic review and meta-analysis. Front Cardiovasc Med. 2022;9:1023651. doi:10.3389/fcvm.2022.1023651 https://www.ncbi.nlm.nih.gov/pubmed/36727024
- Hilvo M, Vasile VC, Donato LJ, Hurme R, Laaksonen R. Ceramides and Ceramide Scores: Clinical Applications for Cardiometabolic Risk Stratification. Front Endocrinol (Lausanne). 2020;11:570628. doi:10.3389/fendo.2020.570628 https://www.ncbi.nlm.nih.gov/pubmed/33133018
- National Lipid Association. Coronary Artery Calcium Testing. Accessed 1/3/2024, https://www.lipid.org/sites/default/files/coronary_artery_calcium_testing.pdf
- Kramer CM, Villines TC. Coronary artery calcium scoring (CAC): Overview and clinical utilization. UpToDate. Updated 10/10/24. Accessed 1/3/2024, https://www.uptodate.com/contents/coronary-artery-calcium-scoring-cac-overview-and-clinical-utilization
- Messenger B, Li D, Nasir K, Carr JJ, Blankstein R, Budoff MJ. Coronary calcium scans and radiation exposure in the multi-ethnic study of atherosclerosis. Int J Cardiovasc Imaging. Mar 2016;32(3):525-9. doi:10.1007/s10554-015-0799-3 https://www.ncbi.nlm.nih.gov/pubmed/26515964
- Sillesen H, Sartori S, Sandholt B, Baber U, Mehran R, Fuster V. Carotid plaque thickness and carotid plaque burden predict future cardiovascular events in asymptomatic adult Americans. Eur Heart J Cardiovasc Imaging. Sep 1 2018;19(9):1042-1050. doi:10.1093/ehjci/jex239 https://www.ncbi.nlm.nih.gov/pubmed/29059296
- Fuster V, Garcia-Alvarez A, Devesa A, et al. Influence of Subclinical Atherosclerosis Burden and Progression on Mortality. J Am Coll Cardiol. Oct 8 2024;84(15):1391-1403. doi:10.1016/j.jacc.2024.06.045 https://www.ncbi.nlm.nih.gov/pubmed/39357937
- Ihle-Hansen H, Vigen T, Berge T, et al. Carotid Plaque Score for Stroke and Cardiovascular Risk Prediction in a Middle-Aged Cohort From the General Population. J Am Heart Assoc. Sep 5 2023;12(17):e030739. doi:10.1161/JAHA.123.030739 https://www.ncbi.nlm.nih.gov/pubmed/37609981
- Carr SS, Hooper AJ, Sullivan DR, Burnett JR. Non-HDL-cholesterol and apolipoprotein B compared with LDL-cholesterol in atherosclerotic cardiovascular disease risk assessment. Pathology. Feb 2019;51(2):148-154. doi:10.1016/j.pathol.2018.11.006 https://www.ncbi.nlm.nih.gov/pubmed/30595507
- Bhale AS, Venkataraman K. Leveraging knowledge of HDLs major protein ApoA1: Structure, function, mutations, and potential therapeutics. Biomed Pharmacother. Oct 2022;154:113634. doi:10.1016/j.biopha.2022.113634 https://www.ncbi.nlm.nih.gov/pubmed/36063649
- Sniderman AD, Thanassoulis G, Glavinovic T, et al. Apolipoprotein B Particles and Cardiovascular Disease: A Narrative Review. JAMA Cardiol. Dec 1 2019;4(12):1287-1295. doi:10.1001/jamacardio.2019.3780 https://www.ncbi.nlm.nih.gov/pubmed/31642874
- Glavinovic T, Thanassoulis G, de Graaf J, Couture P, Hegele RA, Sniderman AD. Physiological Bases for the Superiority of Apolipoprotein B Over Low-Density Lipoprotein Cholesterol and Non-High-Density Lipoprotein Cholesterol as a Marker of Cardiovascular Risk. J Am Heart Assoc. Oct 18 2022;11(20):e025858. doi:10.1161/JAHA.122.025858 https://www.ncbi.nlm.nih.gov/pubmed/36216435
- Mach F, Baigent C, Catapano AL, et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. Jan 1 2020;41(1):111-188. doi:10.1093/eurheartj/ehz455 https://www.ncbi.nlm.nih.gov/pubmed/31504418
- Forte F, Calcaterra I, Lupoli R, et al. Association of apolipoprotein levels with peripheral arterial disease: a meta-analysis of literature studies. European journal of preventive cardiology. Feb 9 2022;28(18):1980-1990. doi:10.1093/eurjpc/zwaa029 https://www.ncbi.nlm.nih.gov/pubmed/33624016
- Thompson A, Danesh J. Associations between apolipoprotein B, apolipoprotein AI, the apolipoprotein B/AI ratio and coronary heart disease: a literature-based meta-analysis of prospective studies. J Intern Med. May 2006;259(5):481-92. doi:10.1111/j.1365-2796.2006.01644.x https://www.ncbi.nlm.nih.gov/pubmed/16629854
- Walldius G, Jungner I. The apoB/apoA-I ratio: a strong, new risk factor for cardiovascular disease and a target for lipid-lowering therapy--a review of the evidence. J Intern Med. May 2006;259(5):493-519. doi:10.1111/j.1365-2796.2006.01643.x https://www.ncbi.nlm.nih.gov/pubmed/16629855
- Walldius G, Jungner I, Holme I, Aastveit AH, Kolar W, Steiner E. High apolipoprotein B, low apolipoprotein A-I, and improvement in the prediction of fatal myocardial infarction (AMORIS study): a prospective study. Lancet. Dec 15 2001;358(9298):2026-33. doi:10.1016/S0140-6736(01)07098-2 https://www.ncbi.nlm.nih.gov/pubmed/11755609
- Reyes-Soffer G, Ginsberg HN, Berglund L, et al. Lipoprotein(a): A Genetically Determined, Causal, and Prevalent Risk Factor for Atherosclerotic Cardiovascular Disease: A Scientific Statement From the American Heart Association. Arterioscler Thromb Vasc Biol. Jan 2022;42(1):e48-e60. doi:10.1161/ATV.0000000000000147 https://www.ncbi.nlm.nih.gov/pubmed/34647487
- Nurmohamed NS, Gaillard EL, Malkasian S, et al. Lipoprotein(a) and Long-Term Plaque Progression, Low-Density Plaque, and Pericoronary Inflammation. JAMA Cardiol. Sep 1 2024;9(9):826-834. doi:10.1001/jamacardio.2024.1874 https://www.ncbi.nlm.nih.gov/pubmed/39018040
- Koschinsky ML, Bajaj A, Boffa MB, et al. A focused update to the 2019 NLA scientific statement on use of lipoprotein(a) in clinical practice. J Clin Lipidol. May-Jun 2024;18(3):e308-e319. doi:10.1016/j.jacl.2024.03.001 https://www.ncbi.nlm.nih.gov/pubmed/38565461
- Hackler E, 3rd, Lew J, Gore MO, et al. Racial Differences in Cardiovascular Biomarkers in the General Population. J Am Heart Assoc. Sep 17 2019;8(18):e012729. doi:10.1161/JAHA.119.012729 https://www.ncbi.nlm.nih.gov/pubmed/31514563
- Wilson DP, Jacobson TA, Jones PH, et al. Use of Lipoprotein(a) in clinical practice: A biomarker whose time has come. A scientific statement from the National Lipid Association. J Clin Lipidol. Sep-Oct 2022;16(5):e77-e95. doi:10.1016/j.jacl.2022.08.007 https://www.ncbi.nlm.nih.gov/pubmed/36068139
- Kaur G, Abdelrahman K, Berman AN, et al. Lipoprotein(a): Emerging insights and therapeutics. Am J Prev Cardiol. Jun 2024;18:100641. doi:10.1016/j.ajpc.2024.100641 https://www.ncbi.nlm.nih.gov/pubmed/38646022
- Rosenson RS, Stein JH, Durrington P. Lipoprotein(a). UpToDate. Updated 10/9/2024. Accessed 12/17/2024, https://www.uptodate.com/contents/lipoprotein-a#H14
- Schulz R, Schluter KD, Laufs U. Molecular and cellular function of the proprotein convertase subtilisin/kexin type 9 (PCSK9). Basic Res Cardiol. Mar 2015;110(2):4. doi:10.1007/s00395-015-0463-z https://www.ncbi.nlm.nih.gov/pubmed/25600226
- Tsimikas S, Karwatowska-Prokopczuk E, Gouni-Berthold I, et al. Lipoprotein(a) Reduction in Persons with Cardiovascular Disease. N Engl J Med. Jan 16 2020;382(3):244-255. doi:10.1056/NEJMoa1905239 https://www.ncbi.nlm.nih.gov/pubmed/31893580
- Nissen SE, Linnebjerg H, Shen X, et al. Lepodisiran, an Extended-Duration Short Interfering RNA Targeting Lipoprotein(a): A Randomized Dose-Ascending Clinical Trial. JAMA. Dec 5 2023;330(21):2075-2083. doi:10.1001/jama.2023.21835 https://www.ncbi.nlm.nih.gov/pubmed/37952254
- Nicholls SJ, Ni W, Rhodes GM, et al. Oral Muvalaplin for Lowering of Lipoprotein(a): A Randomized Clinical Trial. JAMA. Jan 21 2025;333(3):222-231. doi:10.1001/jama.2024.24017 https://www.ncbi.nlm.nih.gov/pubmed/39556768
- Kattoor AJ, Kanuri SH, Mehta JL. Role of Ox-LDL and LOX-1 in Atherogenesis. Curr Med Chem. 2019;26(9):1693-1700. doi:10.2174/0929867325666180508100950 https://www.ncbi.nlm.nih.gov/pubmed/29737246
- Jiang H, Zhou Y, Nabavi SM, et al. Mechanisms of Oxidized LDL-Mediated Endothelial Dysfunction and Its Consequences for the Development of Atherosclerosis. Front Cardiovasc Med. 2022;9:925923. doi:10.3389/fcvm.2022.925923 https://www.ncbi.nlm.nih.gov/pubmed/35722128
- Trpkovic A, Resanovic I, Stanimirovic J, et al. Oxidized low-density lipoprotein as a biomarker of cardiovascular diseases. Critical reviews in clinical laboratory sciences. 2015;52(2):70-85. doi:10.3109/10408363.2014.992063 https://www.ncbi.nlm.nih.gov/pubmed/25537066
- Xu L, Yan X, Tang Z, Feng B. Association between circulating oxidized OxLDL/LDL-C ratio and the severity of coronary atherosclerosis, along with other emerging biomarkers of cardiovascular disease in patients with type 2 diabetes. Diabetes Res Clin Pract. Sep 2022;191:110040. doi:10.1016/j.diabres.2022.110040 https://www.ncbi.nlm.nih.gov/pubmed/35985428
- Spaggiari R, Angelini S, Di Vincenzo A, et al. Ceramides as Emerging Players in Cardiovascular Disease: Focus on Their Pathogenetic Effects and Regulation by Diet. Adv Nutr. Jul 2024;15(7):100252. doi:10.1016/j.advnut.2024.100252 https://www.ncbi.nlm.nih.gov/pubmed/38876397
- Zietzer A, Dusing P, Reese L, Nickenig G, Jansen F. Ceramide Metabolism in Cardiovascular Disease: A Network With High Therapeutic Potential. Arterioscler Thromb Vasc Biol. Oct 2022;42(10):1220-1228. doi:10.1161/ATVBAHA.122.318048 https://www.ncbi.nlm.nih.gov/pubmed/36004640
- Wang DD, Toledo E, Hruby A, et al. Plasma Ceramides, Mediterranean Diet, and Incident Cardiovascular Disease in the PREDIMED Trial (Prevencion con Dieta Mediterranea). Circulation. May 23 2017;135(21):2028-2040. doi:10.1161/CIRCULATIONAHA.116.024261 https://www.ncbi.nlm.nih.gov/pubmed/28280233
- Nicholson RJ, Norris MK, Poss AM, Holland WL, Summers SA. The Lard Works in Mysterious Ways: Ceramides in Nutrition-Linked Chronic Disease. Annu Rev Nutr. Aug 22 2022;42:115-144. doi:10.1146/annurev-nutr-062220-112920 https://www.ncbi.nlm.nih.gov/pubmed/35584813
- Yamashita S, Kinoshita M, Miyazawa T. Dietary Sphingolipids Contribute to Health via Intestinal Maintenance. Int J Mol Sci. Jun 30 2021;22(13)doi:10.3390/ijms22137052 https://www.ncbi.nlm.nih.gov/pubmed/34208952
- Guillou S, Ghabri S, Jannot C, Gaillard E, Lamour I, Boisnic S. The moisturizing effect of a wheat extract food supplement on women's skin: a randomized, double-blind placebo-controlled trial. Int J Cosmet Sci. Apr 2011;33(2):138-43. doi:10.1111/j.1468-2494.2010.00600.x https://www.ncbi.nlm.nih.gov/pubmed/20646083
- Ridker PM, Rane M. Interleukin-6 Signaling and Anti-Interleukin-6 Therapeutics in Cardiovascular Disease. Circ Res. May 28 2021;128(11):1728-1746. doi:10.1161/CIRCRESAHA.121.319077 https://www.ncbi.nlm.nih.gov/pubmed/33998272
- Denegri A, Boriani G. High Sensitivity C-reactive Protein (hsCRP) and its Implications in Cardiovascular Outcomes. Curr Pharm Des. 2021;27(2):263-275. doi:10.2174/1381612826666200717090334 https://www.ncbi.nlm.nih.gov/pubmed/32679014
- Hoogeveen RC, Ballantyne CM. Residual Cardiovascular Risk at Low LDL: Remnants, Lipoprotein(a), and Inflammation. Clinical chemistry. Jan 8 2021;67(1):143-153. doi:10.1093/clinchem/hvaa252 https://www.ncbi.nlm.nih.gov/pubmed/33257928
- Maiocchi SL, Ku J, Thai T, Chan E, Rees MD, Thomas SR. Myeloperoxidase: A versatile mediator of endothelial dysfunction and therapeutic target during cardiovascular disease. Pharmacol Ther. May 2021;221:107711. doi:10.1016/j.pharmthera.2020.107711 https://www.ncbi.nlm.nih.gov/pubmed/33137376
- Ndrepepa G. Myeloperoxidase - A bridge linking inflammation and oxidative stress with cardiovascular disease. Clin Chim Acta. Jun 2019;493:36-51. doi:10.1016/j.cca.2019.02.022 https://www.ncbi.nlm.nih.gov/pubmed/30797769
- Surma S, Banach M. Fibrinogen and Atherosclerotic Cardiovascular Diseases-Review of the Literature and Clinical Studies. Int J Mol Sci. Dec 24 2021;23(1)doi:10.3390/ijms23010193 https://www.ncbi.nlm.nih.gov/pubmed/35008616
- Wang H, Wu P, Jiang D, et al. Relationship between serum homocysteine, fibrinogen, lipoprotein-a level, and peripheral arterial disease: a dose-response meta-analysis. Eur J Med Res. Nov 21 2022;27(1):261. doi:10.1186/s40001-022-00870-1 https://www.ncbi.nlm.nih.gov/pubmed/36411481
- OmegaQuant. Notable Publications from OmegaQuant Researchers. Accessed 1/3/2024, https://omegaquant.com/publications/
- Harris WS, Del Gobbo L, Tintle NL. The Omega-3 Index and relative risk for coronary heart disease mortality: Estimation from 10 cohort studies. Atherosclerosis. Jul 2017;262:51-54. doi:10.1016/j.atherosclerosis.2017.05.007 https://www.ncbi.nlm.nih.gov/pubmed/28511049
- Izzo M, Carrizzo A, Izzo C, et al. Vitamin D: Not Just Bone Metabolism but a Key Player in Cardiovascular Diseases. Life (Basel). May 18 2021;11(5)doi:10.3390/life11050452 https://www.ncbi.nlm.nih.gov/pubmed/34070202
- Thompson B, Waterhouse M, English DR, et al. Vitamin D supplementation and major cardiovascular events: D-Health randomised controlled trial. BMJ. Jun 28 2023;381:e075230. doi:10.1136/bmj-2023-075230 https://www.ncbi.nlm.nih.gov/pubmed/37380191
- Parva NR, Tadepalli S, Singh P, et al. Prevalence of Vitamin D Deficiency and Associated Risk Factors in the US Population (2011-2012). Cureus. Jun 5 2018;10(6):e2741. doi:10.7759/cureus.2741 https://www.ncbi.nlm.nih.gov/pubmed/30087817
- Khanna T, Shraim R, Zarkovic M, van Weele M, van Geffen J, Zgaga L. Comprehensive Analysis of Seasonal and Geographical Variation in UVB Radiation Relevant for Vitamin D Production in Europe. Nutrients. Dec 6 2022;14(23)doi:10.3390/nu14235189 https://www.ncbi.nlm.nih.gov/pubmed/36501219
- Herrmann W, Herrmann M. The Controversial Role of HCY and Vitamin B Deficiency in Cardiovascular Diseases. Nutrients. Mar 28 2022;14(7)doi:10.3390/nu14071412 https://www.ncbi.nlm.nih.gov/pubmed/35406025
- Yuan D, Chu J, Lin H, et al. Mechanism of homocysteine-mediated endothelial injury and its consequences for atherosclerosis. Front Cardiovasc Med. 2022;9:1109445. doi:10.3389/fcvm.2022.1109445 https://www.ncbi.nlm.nih.gov/pubmed/36727029
- Badri S, Vahdat S, Seirafian S, Pourfarzam M, Gholipur-Shahraki T, Ataei S. Homocysteine-Lowering Interventions in Chronic Kidney Disease. Journal of research in pharmacy practice. Jul-Sep 2021;10(3):114-124. doi:10.4103/jrpp.jrpp_75_21 https://www.ncbi.nlm.nih.gov/pubmed/35198504
- Gueant JL, Gueant-Rodriguez RM, Oussalah A, Zuily S, Rosenberg I. Hyperhomocysteinemia in Cardiovascular Diseases: Revisiting Observational Studies and Clinical Trials. Thromb Haemost. Mar 2023;123(3):270-282. doi:10.1055/a-1952-1946 https://www.ncbi.nlm.nih.gov/pubmed/36170884
- Boogaard H, Patton AP, Atkinson RW, et al. Long-term exposure to traffic-related air pollution and selected health outcomes: A systematic review and meta-analysis. Environ Int. Jun 2022;164:107262. doi:10.1016/j.envint.2022.107262 https://www.ncbi.nlm.nih.gov/pubmed/35569389
- UCS. Union of Concerned Scientists. Cars, Trucks, Buses and Air Pollution. Available at https://www.ucsusa.org/resources/cars-trucks-buses-and-air-pollution Last updated 07/19/2018. Accessed 07/27/2023. 2018 ;
- Chen J, Hoek G. Long-term exposure to PM and all-cause and cause-specific mortality: A systematic review and meta-analysis. Environ Int. Oct 2020;143:105974. doi:10.1016/j.envint.2020.105974 https://www.ncbi.nlm.nih.gov/pubmed/32703584
- Mannucci PM, Harari S, Franchini M. Novel evidence for a greater burden of ambient air pollution on cardiovascular disease. Haematologica. Dec 2019;104(12):2349-2357. doi:10.3324/haematol.2019.225086 https://www.ncbi.nlm.nih.gov/pubmed/31672903
- Kaufman JD, Adar SD, Barr RG, et al. Association between air pollution and coronary artery calcification within six metropolitan areas in the USA (the Multi-Ethnic Study of Atherosclerosis and Air Pollution): a longitudinal cohort study. Lancet. Aug 13 2016;388(10045):696-704. doi:10.1016/S0140-6736(16)00378-0 https://www.ncbi.nlm.nih.gov/pubmed/27233746
- Badida P, Krishnamurthy A, Jayaprakash J. Meta analysis of health effects of ambient air pollution exposure in low- and middle-income countries. Environ Res. Jan 1 2023;216(Pt 4):114604. doi:10.1016/j.envres.2022.114604 https://www.ncbi.nlm.nih.gov/pubmed/36375501
- Chen H, Samet JM, Bromberg PA, Tong H. Cardiovascular health impacts of wildfire smoke exposure. Part Fibre Toxicol. Jan 7 2021;18(1):2. doi:10.1186/s12989-020-00394-8 https://www.ncbi.nlm.nih.gov/pubmed/33413506
- Gao Y, Huang W, Yu P, et al. Long-term impacts of non-occupational wildfire exposure on human health: A systematic review. Environ Pollut. Mar 1 2023;320:121041. doi:10.1016/j.envpol.2023.121041 https://www.ncbi.nlm.nih.gov/pubmed/36639044
- Ma Y, Zang E, Liu Y, et al. Long-term exposure to wildland fire smoke PM(2.5) and mortality in the contiguous United States. medRxiv. Jun 11 2024;doi:10.1101/2023.01.31.23285059 https://www.ncbi.nlm.nih.gov/pubmed/36778437
- Sorensen M, Pershagen G, Thacher JD, et al. Health position paper and redox perspectives - Disease burden by transportation noise. Redox Biol. Feb 2024;69:102995. doi:10.1016/j.redox.2023.102995 https://www.ncbi.nlm.nih.gov/pubmed/38142584
- Daiber A, Lelieveld J, Steven S, et al. The "exposome" concept - how environmental risk factors influence cardiovascular health. Acta biochimica Polonica. Sep 10 2019;66(3):269-283. doi:10.18388/abp.2019_2853 https://www.ncbi.nlm.nih.gov/pubmed/31509369
- Bao YB, Wang CC, Peng WG, Nong DQ, Xiang P. [Human Accumulation and Toxic Effects of Microplastics:A Critical Review]. Huan Jing Ke Xue. Feb 8 2024;45(2):1173-1184. doi:10.13227/j.hjkx.202303260 https://www.ncbi.nlm.nih.gov/pubmed/38471954
- Li C, Li X, Bank MS, et al. The "Microplastome" - A Holistic Perspective to Capture the Real-World Ecology of Microplastics. Environ Sci Technol. Mar 5 2024;58(9):4060-4069. doi:10.1021/acs.est.3c08849 https://www.ncbi.nlm.nih.gov/pubmed/38331396
- Luo D, Chu X, Wu Y, et al. Micro- and nano-plastics in the atmosphere: A review of occurrence, properties and human health risks. J Hazard Mater. Mar 5 2024;465:133412. doi:10.1016/j.jhazmat.2023.133412 https://www.ncbi.nlm.nih.gov/pubmed/38218034
- Marfella R, Prattichizzo F, Sardu C, et al. Microplastics and Nanoplastics in Atheromas and Cardiovascular Events. N Engl J Med. Mar 7 2024;390(10):900-910. doi:10.1056/NEJMoa2309822 https://www.ncbi.nlm.nih.gov/pubmed/38446676
- National Institute of Environmental Health Sciences. Perfluoroalkyl and Polyfluoroalkyl Substances (PFAS). Accessed 5/3/24, https://www.niehs.nih.gov/health/topics/agents/pfc
- Schlezinger JJ, Gokce N. Perfluoroalkyl/Polyfluoroalkyl Substances: Links to Cardiovascular Disease Risk. Circ Res. Apr 26 2024;134(9):1136-1159. doi:10.1161/CIRCRESAHA.124.323697 https://www.ncbi.nlm.nih.gov/pubmed/38662859
- Lamas GA, Bhatnagar A, Jones MR, et al. Contaminant Metals as Cardiovascular Risk Factors: A Scientific Statement From the American Heart Association. J Am Heart Assoc. Jul 4 2023;12(13):e029852. doi:10.1161/JAHA.123.029852 https://www.ncbi.nlm.nih.gov/pubmed/37306302
- McGraw KE, Schilling K, Glabonjat RA, et al. Urinary Metal Levels and Coronary Artery Calcification: Longitudinal Evidence in the Multi-Ethnic Study of Atherosclerosis. J Am Coll Cardiol. Oct 15 2024;84(16):1545-1557. doi:10.1016/j.jacc.2024.07.020 https://www.ncbi.nlm.nih.gov/pubmed/39297845
- Writing Committee M, Virani SS, Newby LK, et al. 2023 AHA/ACC/ACCP/ASPC/NLA/PCNA Guideline for the Management of Patients With Chronic Coronary Disease: A Report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. Aug 29 2023;82(9):833-955. doi:10.1016/j.jacc.2023.04.003 https://www.ncbi.nlm.nih.gov/pubmed/37480922
- Ravalli F, Vela Parada X, Ujueta F, et al. Chelation Therapy in Patients With Cardiovascular Disease: A Systematic Review. J Am Heart Assoc. Mar 15 2022;11(6):e024648. doi:10.1161/JAHA.121.024648 https://www.ncbi.nlm.nih.gov/pubmed/35229619
- Villarruz-Sulit MV, Forster R, Dans AL, Tan FN, Sulit DV. Chelation therapy for atherosclerotic cardiovascular disease. Cochrane Database Syst Rev. May 5 2020;5(5):CD002785. doi:10.1002/14651858.CD002785.pub2 https://www.ncbi.nlm.nih.gov/pubmed/32367513
- Lamas GA, Goertz C, Boineau R, et al. Effect of disodium EDTA chelation regimen on cardiovascular events in patients with previous myocardial infarction: the TACT randomized trial. JAMA. Mar 27 2013;309(12):1241-50. doi:10.1001/jama.2013.2107 https://www.ncbi.nlm.nih.gov/pubmed/23532240
- Lamas GA, Anstrom KJ, Navas-Acien A, et al. Edetate Disodium-Based Chelation for Patients With a Previous Myocardial Infarction and Diabetes: TACT2 Randomized Clinical Trial. JAMA. Sep 10 2024;332(10):794-803. doi:10.1001/jama.2024.11463 https://www.ncbi.nlm.nih.gov/pubmed/39141382
- Carlsten C, Salvi S, Wong GWK, Chung KF. Personal strategies to minimise effects of air pollution on respiratory health: advice for providers, patients and the public. Eur Respir J. Jun 2020;55(6):1902056. doi:10.1183/13993003.02056-2019 https://www.ncbi.nlm.nih.gov/pubmed/32241830
- Wu W, Du Z, Wang Y, et al. The complex role of air pollution on the association between greenness and respiratory mortality: Insight from a large cohort, 2009-2020. Sci Total Environ. Nov 15 2023;899:165588. doi:10.1016/j.scitotenv.2023.165588 https://www.ncbi.nlm.nih.gov/pubmed/37474059
- Bianconi A, Longo G, Coa AA, Fiore M, Gori D. Impacts of Urban Green on Cardiovascular and Cerebrovascular Diseases-A Systematic Review and Meta-Analysis. Int J Environ Res Public Health. May 26 2023;20(11)doi:10.3390/ijerph20115966 https://www.ncbi.nlm.nih.gov/pubmed/37297570
- Environmental Protection Agency. Reducing PFAS in Your Drinking Water with a Home Filter. Accessed 5/3/24, https://www.epa.gov/system/files/documents/2024-04/water-filter-fact-sheet.pdf
- Munzel T, Schmidt FP, Steven S, Herzog J, Daiber A, Sorensen M. Environmental Noise and the Cardiovascular System. J Am Coll Cardiol. Feb 13 2018;71(6):688-697. doi:10.1016/j.jacc.2017.12.015 https://www.ncbi.nlm.nih.gov/pubmed/29420965
- Khoddam H, Maddah SA, Rezvani Khorshidi S, Zaman Kamkar M, Modanloo M. The effects of earplugs and eye masks on sleep quality of patients admitted to coronary care units: A randomised clinical trial. J Sleep Res. Apr 2022;31(2):e13473. doi:10.1111/jsr.13473 https://www.ncbi.nlm.nih.gov/pubmed/34514653
- Fang CS, Wang HH, Wang RH, Chou FH, Chang SL, Fang CJ. Effect of earplugs and eye masks on the sleep quality of intensive care unit patients: A systematic review and meta-analysis. J Adv Nurs. Nov 2021;77(11):4321-4331. doi:10.1111/jan.14914 https://www.ncbi.nlm.nih.gov/pubmed/34096647
- Ghamar Talepoor A, Doroudchi M. Immunosenescence in atherosclerosis: A role for chronic viral infections. Front Immunol. 2022;13:945016. doi:10.3389/fimmu.2022.945016 https://www.ncbi.nlm.nih.gov/pubmed/36059478
- NLM. National Library of Medicine: MedlinePlus. Aging changes in hormone production. Available at https://medlineplus.gov/ency/article/004000.htm Last update 07/21/2022. Accessed 04/11/2023. 2022 ;
- Fallara G, Pozzi E, Belladelli F, et al. Cardiovascular Morbidity and Mortality in Men - Findings From a Meta-analysis on the Time-related Measure of Risk of Exogenous Testosterone. The journal of sexual medicine. Aug 2022;19(8):1243-1254. doi:10.1016/j.jsxm.2022.05.145 https://www.ncbi.nlm.nih.gov/pubmed/35753891
- Hudson J, Cruickshank M, Quinton R, et al. Adverse cardiovascular events and mortality in men during testosterone treatment: an individual patient and aggregate data meta-analysis. Lancet Healthy Longev. Jun 2022;3(6):e381-e393. doi:10.1016/S2666-7568(22)00096-4 https://www.ncbi.nlm.nih.gov/pubmed/35711614
- Sood A, Hosseinpour A, Sood A, et al. Cardiovascular Outcomes of Hypogonadal Men Receiving Testosterone Replacement Therapy: A Meta-analysis of Randomized Controlled Trials. Endocr Pract. Jan 2024;30(1):2-10. doi:10.1016/j.eprac.2023.09.012 https://www.ncbi.nlm.nih.gov/pubmed/37797887
- Morgentaler A, Dhindsa S, Dobs AS, et al. Androgen Society Position Paper on Cardiovascular Risk With Testosterone Therapy. Mayo Clin Proc. Nov 2024;99(11):1785-1801. doi:10.1016/j.mayocp.2024.08.008 https://www.ncbi.nlm.nih.gov/pubmed/39436329
- Lincoff AM, Bhasin S, Flevaris P, et al. Cardiovascular Safety of Testosterone-Replacement Therapy. N Engl J Med. Jul 13 2023;389(2):107-117. doi:10.1056/NEJMoa2215025 https://www.ncbi.nlm.nih.gov/pubmed/37326322
- Jaiswal V, Sawhney A, Nebuwa C, et al. Association between testosterone replacement therapy and cardiovascular outcomes: A meta-analysis of 30 randomized controlled trials. Prog Cardiovasc Dis. Jul-Aug 2024;85:45-53. doi:10.1016/j.pcad.2024.04.001 https://www.ncbi.nlm.nih.gov/pubmed/38589271
- Mishra SR, Chung HF, Waller M, Mishra GD. Duration of estrogen exposure during reproductive years, age at menarche and age at menopause, and risk of cardiovascular disease events, all-cause and cardiovascular mortality: a systematic review and meta-analysis. BJOG : an international journal of obstetrics and gynaecology. Apr 2021;128(5):809-821. doi:10.1111/1471-0528.16524 https://www.ncbi.nlm.nih.gov/pubmed/32965759
- Jin J. Menopausal Hormone Therapy for Prevention of Chronic Conditions. JAMA. Nov 1 2022;328(17):1780. doi:10.1001/jama.2022.19855 https://www.ncbi.nlm.nih.gov/pubmed/36318132
- Mehta J, Kling JM, Manson JE. Risks, Benefits, and Treatment Modalities of Menopausal Hormone Therapy: Current Concepts. Front Endocrinol (Lausanne). 2021;12:564781. doi:10.3389/fendo.2021.564781 https://www.ncbi.nlm.nih.gov/pubmed/33841322
- Anagnostis P, Lambrinoudaki I, Stevenson JC, Goulis DG. Menopause-associated risk of cardiovascular disease. Endocr Connect. Apr 22 2022;11(4)doi:10.1530/EC-21-0537 https://www.ncbi.nlm.nih.gov/pubmed/35258483
- Sahu P, Gidwani B, Dhongade HJ. Pharmacological activities of dehydroepiandrosterone: A review. Steroids. Jan 2020;153:108507. doi:10.1016/j.steroids.2019.108507 https://www.ncbi.nlm.nih.gov/pubmed/31586606
- Klinge CM, Clark BJ, Prough RA. Dehydroepiandrosterone Research: Past, Current, and Future. Vitam Horm. 2018;108:1-28. doi:10.1016/bs.vh.2018.02.002 https://www.ncbi.nlm.nih.gov/pubmed/30029723
- Zhang S, Zhou J, Li L, et al. Effect of dehydroepiandrosterone on atherosclerosis in postmenopausal women. Bioscience trends. Jan 23 2022;15(6):353-364. doi:10.5582/bst.2021.01320 https://www.ncbi.nlm.nih.gov/pubmed/34759119
- Wu TT, Chen Y, Zhou Y, et al. Prognostic Value of Dehydroepiandrosterone Sulfate for Patients With Cardiovascular Disease: A Systematic Review and Meta-Analysis. J Am Heart Assoc. May 5 2017;6(5)doi:10.1161/JAHA.116.004896 https://www.ncbi.nlm.nih.gov/pubmed/28476876
- Li R, E L, Zha N. Circulating dehydroepiandrosterone sulfate level and cardiovascular or all-cause mortality in the elderly population: a meta-analysis. Ann Palliat Med. Sep 2020;9(5):3537-3545. doi:10.21037/apm-20-441 https://www.ncbi.nlm.nih.gov/pubmed/32921089
- Wu TT, Gao Y, Zheng YY, Ma YT, Xie X. Association of endogenous DHEA/DHEAS with coronary heart disease: A systematic review and meta-analysis. Clin Exp Pharmacol Physiol. Nov 2019;46(11):984-994. doi:10.1111/1440-1681.13146 https://www.ncbi.nlm.nih.gov/pubmed/31347187
- Qin Y, H OS, Khani V, Tan SC, Zhi Y. Effects of dehydroepiandrosterone (DHEA) supplementation on the lipid profile: A systematic review and dose-response meta-analysis of randomized controlled trials. Nutr Metab Cardiovasc Dis. Aug 28 2020;30(9):1465-1475. doi:10.1016/j.numecd.2020.05.015 https://www.ncbi.nlm.nih.gov/pubmed/32675010
- Boxer RS, Kleppinger A, Brindisi J, Feinn R, Burleson JA, Kenny AM. Effects of dehydroepiandrosterone (DHEA) on cardiovascular risk factors in older women with frailty characteristics. Age Ageing. Jul 2010;39(4):451-8. doi:10.1093/ageing/afq043 https://www.ncbi.nlm.nih.gov/pubmed/20484057
- Srinivasan M, Irving BA, Dhatariya K, et al. Effect of dehydroepiandrosterone replacement on lipoprotein profile in hypoadrenal women. J Clin Endocrinol Metab. Mar 2009;94(3):761-4. doi:10.1210/jc.2008-1774 https://www.ncbi.nlm.nih.gov/pubmed/19066301
- Srinivasan M, Irving BA, Frye RL, et al. Effects on lipoprotein particles of long-term dehydroepiandrosterone in elderly men and women and testosterone in elderly men. J Clin Endocrinol Metab. Apr 2010;95(4):1617-25. doi:10.1210/jc.2009-2000 https://www.ncbi.nlm.nih.gov/pubmed/20139233
- Rabijewski M, Zgliczynski W. [Positive effects of DHEA therapy on insulin resistance and lipids in men with angiographically verified coronary heart disease--preliminary study]. Endokrynologia Polska. Nov-Dec 2005;56(6):904-10. Korzystny wplyw stosowania DHEA na insulinoopornosc i lipidy osocza mezczyzn z koronarograficznie potwierdzona choroba wiencowa--doniesienie wstepne. https://www.ncbi.nlm.nih.gov/pubmed/16821209
- Shahid MA, Ashraf MA, Sharma S. Physiology, Thyroid Hormone. StatPearls. StatPearls Publishing. Copyright © 2023, StatPearls Publishing LLC.; 2025. https://www.ncbi.nlm.nih.gov/pubmed/29763182
- Yamakawa H, Kato TS, Noh JY, et al. Thyroid Hormone Plays an Important Role in Cardiac Function: From Bench to Bedside. Review. Front Physiol. 2021-October-18 2021;12:606931. doi:10.3389/fphys.2021.606931 https://www.ncbi.nlm.nih.gov/pubmed/34733168
- Cappola AR, Desai AS, Medici M, et al. Thyroid and Cardiovascular Disease: Research Agenda for Enhancing Knowledge, Prevention, and Treatment. Circulation. Jun 18 2019;139(25):2892-2909. doi:10.1161/CIRCULATIONAHA.118.036859 https://www.ncbi.nlm.nih.gov/pubmed/31081673
- Gluvic ZM, Zafirovic SS, Obradovic MM, Sudar-Milovanovic EM, Rizzo M, Isenovic ER. Hypothyroidism and Risk of Cardiovascular Disease. Curr Pharm Des. 2022;28(25):2065-2072. doi:10.2174/1381612828666220620160516 https://www.ncbi.nlm.nih.gov/pubmed/35726428
- Inoue K, Ritz B, Brent GA, Ebrahimi R, Rhee CM, Leung AM. Association of Subclinical Hypothyroidism and Cardiovascular Disease With Mortality. JAMA Netw Open. Feb 5 2020;3(2):e1920745. doi:10.1001/jamanetworkopen.2019.20745 https://www.ncbi.nlm.nih.gov/pubmed/32031647
- Biondi B. Levothyroxine and the Heart. In: Kahaly GJ, ed. 70 Years of Levothyroxine. Springer International Publishing; 2021:85-96. https://www.ncbi.nlm.nih.gov/pubmed/36315709
- Karam G, Agarwal A, Sadeghirad B, et al. Comparison of seven popular structured dietary programmes and risk of mortality and major cardiovascular events in patients at increased cardiovascular risk: systematic review and network meta-analysis. BMJ. Mar 29 2023;380:e072003. doi:10.1136/bmj-2022-072003 https://www.ncbi.nlm.nih.gov/pubmed/36990505
- Richardson LA, Izuora K, Basu A. Mediterranean Diet and Its Association with Cardiovascular Disease Risk Factors: A Scoping Review. Int J Environ Res Public Health. Oct 6 2022;19(19)doi:10.3390/ijerph191912762 https://www.ncbi.nlm.nih.gov/pubmed/36232062
- University of California San Francisco. Eating Right for Your Heart. Accessed 1/5/2024, https://www.ucsfhealth.org/education/eating-right-for-your-heart
- National Heart L, and Blood Institiute. Choose Heart - Healthy Foods. Updated 3/24/2022. Accessed 1/5/2024, https://www.nhlbi.nih.gov/health/heart-healthy-living/healthy-foods
- AHA. American Heart Association. The American Heart Association Diet and Lifestyle Recommendations. Accessed 1/5/2024, https://www.heart.org/en/healthy-living/healthy-eating/eat-smart/nutrition-basics/aha-diet-and-lifestyle-recommendations
- DHHS. U.S. Department of Health and Human Services. Office of Disease Prevention and Health Promotion. Heart-Healthy Foods: Shopping List. Updated 9/19/2023. Accessed 1/5/2024, https://health.gov/myhealthfinder/health-conditions/heart-health/heart-healthy-foods-shopping-list
- Wuopio J, Ling YT, Orho-Melander M, Engstrom G, Arnlov J. The association between sodium intake and coronary and carotid atherosclerosis in the general Swedish population. Eur Heart J Open. Mar 2023;3(2):oead024. doi:10.1093/ehjopen/oead024 https://www.ncbi.nlm.nih.gov/pubmed/37006408
- Sahranavard T, Carbone F, Montecucco F, et al. The role of potassium in atherosclerosis. Eur J Clin Invest. Mar 2021;51(3):e13454. doi:10.1111/eci.13454 https://www.ncbi.nlm.nih.gov/pubmed/33216974
- Ma Y, He FJ, Sun Q, et al. 24-Hour Urinary Sodium and Potassium Excretion and Cardiovascular Risk. N Engl J Med. Jan 20 2022;386(3):252-263. doi:10.1056/NEJMoa2109794 https://www.ncbi.nlm.nih.gov/pubmed/34767706
- McRae MP. Dietary Fiber Is Beneficial for the Prevention of Cardiovascular Disease: An Umbrella Review of Meta-analyses. J Chiropr Med. Dec 2017;16(4):289-299. doi:10.1016/j.jcm.2017.05.005 https://www.ncbi.nlm.nih.gov/pubmed/29276461
- OSU. Oregon State University: Linus Pauling Institute. Fiber. Data on file. ;
- Sacks FM, Lichtenstein AH, Wu JHY, et al. Dietary Fats and Cardiovascular Disease: A Presidential Advisory From the American Heart Association. Circulation. Jul 18 2017;136(3):e1-e23. doi:10.1161/CIR.0000000000000510 https://www.ncbi.nlm.nih.gov/pubmed/28620111
- Astrup A, Magkos F, Bier DM, et al. Saturated Fats and Health: A Reassessment and Proposal for Food-Based Recommendations: JACC State-of-the-Art Review. J Am Coll Cardiol. Aug 18 2020;76(7):844-857. doi:10.1016/j.jacc.2020.05.077 https://www.ncbi.nlm.nih.gov/pubmed/32562735
- Maki KC, Dicklin MR, Kirkpatrick CF. Saturated fats and cardiovascular health: Current evidence and controversies. J Clin Lipidol. Nov-Dec 2021;15(6):765-772. doi:10.1016/j.jacl.2021.09.049 https://www.ncbi.nlm.nih.gov/pubmed/34649831
- Jiang L, Wang J, Xiong K, Xu L, Zhang B, Ma A. Intake of Fish and Marine n-3 Polyunsaturated Fatty Acids and Risk of Cardiovascular Disease Mortality: A Meta-Analysis of Prospective Cohort Studies. Nutrients. Jul 9 2021;13(7)doi:10.3390/nu13072342 https://www.ncbi.nlm.nih.gov/pubmed/34371852
- Gorzynik-Debicka M, Przychodzen P, Cappello F, et al. Potential Health Benefits of Olive Oil and Plant Polyphenols. Int J Mol Sci. Feb 28 2018;19(3)doi:10.3390/ijms19030686 https://www.ncbi.nlm.nih.gov/pubmed/29495598
- Martínez-González MA, Sayón-Orea C, Bullón-Vela V, et al. Effect of olive oil consumption on cardiovascular disease, cancer, type 2 diabetes, and all-cause mortality: A systematic review and meta-analysis. Clinical Nutrition. 2022;41(12):2659-2682. doi:10.1016/j.clnu.2022.10.001 https://doi.org/10.1016/j.clnu.2022.10.001
- Xia M, Zhong Y, Peng Y, Qian C. Olive oil consumption and risk of cardiovascular disease and all-cause mortality: A meta-analysis of prospective cohort studies. Front Nutr. 2022;9:1041203. doi:10.3389/fnut.2022.1041203 https://www.ncbi.nlm.nih.gov/pubmed/36330142
- UCSF Health. Eating Right for Your Heart. Accessed 1/8/2024, https://www.ucsfhealth.org/education/eating-right-for-your-heart
- Perry CA, Gadde KM. The Role of Calorie Restriction in the Prevention of Cardiovascular Disease. Curr Atheroscler Rep. Apr 2022;24(4):235-242. doi:10.1007/s11883-022-00999-8 https://www.ncbi.nlm.nih.gov/pubmed/35107761
- de Souza AMA, Ecelbarger CM, Sandberg K. Caloric Restriction and Cardiovascular Health: the Good, the Bad, and the Renin-Angiotensin System. Physiology (Bethesda). Jul 1 2021;36(4):220-234. doi:10.1152/physiol.00002.2021 https://www.ncbi.nlm.nih.gov/pubmed/34159807
- Kirkham AA, Beka V, Prado CM. The effect of caloric restriction on blood pressure and cardiovascular function: A systematic review and meta-analysis of randomized controlled trials. Clin Nutr. Mar 2021;40(3):728-739. doi:10.1016/j.clnu.2020.06.029 https://www.ncbi.nlm.nih.gov/pubmed/32675017
- Allaf M, Elghazaly H, Mohamed OG, et al. Intermittent fasting for the prevention of cardiovascular disease. Cochrane Database Syst Rev. Jan 29 2021;1(1):CD013496. doi:10.1002/14651858.CD013496.pub2 https://www.ncbi.nlm.nih.gov/pubmed/33512717
- Cioffi I, Evangelista A, Ponzo V, et al. Intermittent versus continuous energy restriction on weight loss and cardiometabolic outcomes: a systematic review and meta-analysis of randomized controlled trials. J Transl Med. Dec 24 2018;16(1):371. doi:10.1186/s12967-018-1748-4 https://www.ncbi.nlm.nih.gov/pubmed/30583725
- Park J, Seo YG, Paek YJ, Song HJ, Park KH, Noh HM. Effect of alternate-day fasting on obesity and cardiometabolic risk: A systematic review and meta-analysis. Metabolism. Oct 2020;111:154336. doi:10.1016/j.metabol.2020.154336 https://www.ncbi.nlm.nih.gov/pubmed/32777443
- Ishizuka R, Otaki N, Tai Y, et al. Breakfast Skipping and Declines in Cognitive Score Among Community-Dwelling Older Adults: A Longitudinal Study of the HEIJO-KYO Cohort. Journal of geriatric psychiatry and neurology. Jul 2023;36(4):316-322. doi:10.1177/08919887221135551 https://www.ncbi.nlm.nih.gov/pubmed/36265459
- Wang Y, Li F, Li X, et al. Breakfast skipping and risk of all-cause, cardiovascular and cancer mortality among adults: a systematic review and meta-analysis of prospective cohort studies. Food Funct. Jun 4 2024;15(11):5703-5713. doi:10.1039/d3fo05705d https://www.ncbi.nlm.nih.gov/pubmed/38738978
- Rong S, Snetselaar LG, Xu G, et al. Association of Skipping Breakfast With Cardiovascular and All-Cause Mortality. J Am Coll Cardiol. Apr 30 2019;73(16):2025-2032. doi:10.1016/j.jacc.2019.01.065 https://www.ncbi.nlm.nih.gov/pubmed/31023424
- Young IE, Poobalan A, Steinbeck K, O'Connor HT, Parker HM. Distribution of energy intake across the day and weight loss: A systematic review and meta-analysis. Obes Rev. Mar 2023;24(3):e13537. doi:10.1111/obr.13537 https://www.ncbi.nlm.nih.gov/pubmed/36530130
- Rojas-Gonzalez A, Figueroa-Hernandez CY, Gonzalez-Rios O, et al. Coffee Chlorogenic Acids Incorporation for Bioactivity Enhancement of Foods: A Review. Molecules. May 25 2022;27(11)doi:10.3390/molecules27113400 https://www.ncbi.nlm.nih.gov/pubmed/35684338
- Naylor LH, Zimmermann D, Guitard-Uldry M, et al. Acute dose-response effect of coffee-derived chlorogenic acids on the human vasculature in healthy volunteers: a randomized controlled trial. Am J Clin Nutr. Feb 2 2021;113(2):370-379. doi:10.1093/ajcn/nqaa312 https://www.ncbi.nlm.nih.gov/pubmed/33330899
- Yanagimoto A, Matsui Y, Yamaguchi T, Hibi M, Kobayashi S, Osaki N. Effects of Ingesting Both Catechins and Chlorogenic Acids on Glucose, Incretin, and Insulin Sensitivity in Healthy Men: A Randomized, Double-Blinded, Placebo-Controlled Crossover Trial. Nutrients. Nov 28 2022;14(23)doi:10.3390/nu14235063 https://www.ncbi.nlm.nih.gov/pubmed/36501092
- Di Maso M, Boffetta P, Negri E, La Vecchia C, Bravi F. Caffeinated Coffee Consumption and Health Outcomes in the US Population: A Dose-Response Meta-Analysis and Estimation of Disease Cases and Deaths Avoided. Adv Nutr. Jul 30 2021;12(4):1160-1176. doi:10.1093/advances/nmaa177 https://www.ncbi.nlm.nih.gov/pubmed/33570108
- Poole R, Kennedy OJ, Roderick P, Fallowfield JA, Hayes PC, Parkes J. Coffee consumption and health: umbrella review of meta-analyses of multiple health outcomes. BMJ. Nov 22 2017;359:j5024. doi:10.1136/bmj.j5024 https://www.ncbi.nlm.nih.gov/pubmed/29167102
- Ribeiro EM, Alves M, Costa J, Ferreira JJ, Pinto FJ, Caldeira D. Safety of coffee consumption after myocardial infarction: A systematic review and meta-analysis. Nutr Metab Cardiovasc Dis. Nov 27 2020;30(12):2146-2158. doi:10.1016/j.numecd.2020.07.016 https://www.ncbi.nlm.nih.gov/pubmed/33158718
- Du Y, Lv Y, Zha W, Hong X, Luo Q. Effect of coffee consumption on dyslipidemia: A meta-analysis of randomized controlled trials. Nutr Metab Cardiovasc Dis. Nov 27 2020;30(12):2159-2170. doi:10.1016/j.numecd.2020.08.017 https://www.ncbi.nlm.nih.gov/pubmed/33239163
- Tverdal A, Selmer R, Cohen JM, Thelle DS. Coffee consumption and mortality from cardiovascular diseases and total mortality: Does the brewing method matter? European journal of preventive cardiology. Dec 2020;27(18):1986-1993. doi:10.1177/2047487320914443 https://www.ncbi.nlm.nih.gov/pubmed/32320635
- Surma S, Romanczyk M, Filipiak KJ, Lip GYH. Coffee and cardiac arrhythmias: Up-date review of the literature and clinical studies. Cardiol J. Aug 1 2023;30(4):654-667. doi:10.5603/CJ.a2022.0068 https://www.ncbi.nlm.nih.gov/pubmed/35912715
- Surma S, Oparil S. Coffee and Arterial Hypertension. Curr Hypertens Rep. Aug 9 2021;23(7):38. doi:10.1007/s11906-021-01156-3 https://www.ncbi.nlm.nih.gov/pubmed/34370111
- CDC. Centers for Disease Control and Prevention. How Much Physical Activity Do Adults Need? Available at https://www.cdc.gov/physicalactivity/basics/adults/index.htm Last reviewed 06/02/2022. Accessed 05/01/2023. 2022;
- Sulague RM, Suan NNM, Mendoza MF, Lavie CJ. The associations between exercise and lipid biomarkers. Prog Cardiovasc Dis. Nov-Dec 2022;75:59-68. doi:10.1016/j.pcad.2022.11.004 https://www.ncbi.nlm.nih.gov/pubmed/36400234
- Mendoza MVF, Kachur SM, Lavie CJ. The Effects of Exercise on Lipid Biomarkers. Methods Mol Biol. 2022;2343:93-117. doi:10.1007/978-1-0716-1558-4_6 https://www.ncbi.nlm.nih.gov/pubmed/34473317
- Mendoza MF, Lavie CJ. Clinical associations between exercise and lipoproteins. Curr Opin Lipidol. Dec 1 2022;33(6):364-373. doi:10.1097/MOL.0000000000000848 https://www.ncbi.nlm.nih.gov/pubmed/36305382
- Tirandi A, Montecucco F, Liberale L. Physical activity to reduce PCSK9 levels. Front Cardiovasc Med. 2022;9:988698. doi:10.3389/fcvm.2022.988698 https://www.ncbi.nlm.nih.gov/pubmed/36093150
- Shailendra P, Baldock KL, Li LSK, et al. Weight training and risk of all-cause, cardiovascular disease and cancer mortality among older adults. Int J Epidemiol. Apr 11 2024;53(3)doi:10.1093/ije/dyae074 https://www.ncbi.nlm.nih.gov/pubmed/38831478
- Gomes-Neto M, Duraes AR, Conceicao LSR, et al. Some types of exercise interventions are more effective than others in people with coronary heart disease: systematic review and network meta-analysis. J Physiother. Apr 2024;70(2):106-114. doi:10.1016/j.jphys.2024.02.018 https://www.ncbi.nlm.nih.gov/pubmed/38503676
- Katzmarzyk PT, Ross R, Blair SN, Despres JP. Should we target increased physical activity or less sedentary behavior in the battle against cardiovascular disease risk development? Atherosclerosis. Oct 2020;311:107-115. doi:10.1016/j.atherosclerosis.2020.07.010 https://www.ncbi.nlm.nih.gov/pubmed/32773106
- Germano-Soares AH, Andrade-Lima A, Meneses AL, et al. Association of time spent in physical activities and sedentary behaviors with carotid-femoral pulse wave velocity: A systematic review and meta-analysis. Atherosclerosis. Feb 2018;269:211-218. doi:10.1016/j.atherosclerosis.2018.01.009 https://www.ncbi.nlm.nih.gov/pubmed/29407596
- Nguyen CH, Marzolini S, Oh P, Thomas SG. A Retrospective Comparison of Fitness and Exercise Progression in Patients With Coronary and Peripheral Artery Disease in Cardiac Rehabilitation. The Canadian journal of cardiology. Feb 2021;37(2):260-268. doi:10.1016/j.cjca.2020.04.013 https://www.ncbi.nlm.nih.gov/pubmed/32818559
- AHA. American Heart Association. Body Mass Index (BMI) In Adults. Updated 8/1/2014. Accessed 1/8/2024, https://www.heart.org/en/healthy-living/healthy-eating/losing-weight/bmi-in-adults
- Manoharan MP, Raja R, Jamil A, et al. Obesity and Coronary Artery Disease: An Updated Systematic Review 2022. Cureus. Sep 2022;14(9):e29480. doi:10.7759/cureus.29480 https://www.ncbi.nlm.nih.gov/pubmed/36299943
- Lin DS, Lo HY, Yu AL, Lee JK, Yang WS, Hwang JJ. A Dose Response Association Between Body Mass Index and Mortality in Patients with Peripheral Artery Disease: A Meta-analysis Including 5 729 272 Individuals. European journal of vascular and endovascular surgery : the official journal of the European Society for Vascular Surgery. Mar 2022;63(3):495-502. doi:10.1016/j.ejvs.2021.11.016 https://www.ncbi.nlm.nih.gov/pubmed/35027277
- Drame M, Godaert L. The Obesity Paradox and Mortality in Older Adults: A Systematic Review. Nutrients. Apr 6 2023;15(7)doi:10.3390/nu15071780 https://www.ncbi.nlm.nih.gov/pubmed/37049633
- Liu C, Wong PY, Chung YL, et al. Deciphering the "obesity paradox" in the elderly: A systematic review and meta-analysis of sarcopenic obesity. Obes Rev. Feb 2023;24(2):e13534. doi:10.1111/obr.13534 https://www.ncbi.nlm.nih.gov/pubmed/36443946
- Katta N, Loethen T, Lavie CJ, Alpert MA. Obesity and Coronary Heart Disease: Epidemiology, Pathology, and Coronary Artery Imaging. Curr Probl Cardiol. Mar 2021;46(3):100655. doi:10.1016/j.cpcardiol.2020.100655 https://www.ncbi.nlm.nih.gov/pubmed/32843206
- Csige I, Ujvarosy D, Szabo Z, et al. The Impact of Obesity on the Cardiovascular System. J Diabetes Res. 2018;2018:3407306. doi:10.1155/2018/3407306 https://www.ncbi.nlm.nih.gov/pubmed/30525052
- Valenzuela PL, Carrera-Bastos P, Castillo-Garcia A, Lieberman DE, Santos-Lozano A, Lucia A. Obesity and the risk of cardiometabolic diseases. Nature reviews Cardiology. Jul 2023;20(7):475-494. doi:10.1038/s41569-023-00847-5 https://www.ncbi.nlm.nih.gov/pubmed/36927772
- Mahajan R, Stokes M, Elliott A, et al. Complex interaction of obesity, intentional weight loss and heart failure: a systematic review and meta-analysis. Heart. Jan 2020;106(1):58-68. doi:10.1136/heartjnl-2019-314770 https://www.ncbi.nlm.nih.gov/pubmed/31530572
- Joris PJ, Zeegers MP, Mensink RP. Weight loss improves fasting flow-mediated vasodilation in adults: a meta-analysis of intervention studies. Atherosclerosis. Mar 2015;239(1):21-30. doi:10.1016/j.atherosclerosis.2014.12.056 https://www.ncbi.nlm.nih.gov/pubmed/25568949
- Pietrzykowska NB. Obesity Action Coalition. Benefits of 5-10 Percent Weight-loss. Updated 2013. Accessed 1/8/2024, https://www.obesityaction.org/resources/benefits-of-5-10-percent-weight-loss/
- Baranwal N, Yu PK, Siegel NS. Sleep physiology, pathophysiology, and sleep hygiene. Prog Cardiovasc Dis. Mar-Apr 2023;77:59-69. doi:10.1016/j.pcad.2023.02.005 https://www.ncbi.nlm.nih.gov/pubmed/36841492
- Lloyd-Jones DM, Allen NB, Anderson CAM, et al. Life's Essential 8: Updating and Enhancing the American Heart Association's Construct of Cardiovascular Health: A Presidential Advisory From the American Heart Association. Circulation. Aug 2 2022;146(5):e18-e43. doi:10.1161/CIR.0000000000001078 https://www.ncbi.nlm.nih.gov/pubmed/35766027
- Sun J, Li Y, Zhao M, et al. Association of the American Heart Association's new "Life's Essential 8" with all-cause and cardiovascular disease-specific mortality: prospective cohort study. BMC Med. Mar 29 2023;21(1):116. doi:10.1186/s12916-023-02824-8 https://www.ncbi.nlm.nih.gov/pubmed/36978123
- Huang T, Redline S. Cross-sectional and Prospective Associations of Actigraphy-Assessed Sleep Regularity With Metabolic Abnormalities: The Multi-Ethnic Study of Atherosclerosis. Diabetes Care. Aug 2019;42(8):1422-1429. doi:10.2337/dc19-0596 https://www.ncbi.nlm.nih.gov/pubmed/31167888
- Huang T, Mariani S, Redline S. Sleep Irregularity and Risk of Cardiovascular Events: The Multi-Ethnic Study of Atherosclerosis. J Am Coll Cardiol. Mar 10 2020;75(9):991-999. doi:10.1016/j.jacc.2019.12.054 https://www.ncbi.nlm.nih.gov/pubmed/32138974
- Chen LD, Lin L, Lin XJ, et al. Effect of continuous positive airway pressure on carotid intima-media thickness in patients with obstructive sleep apnea: A meta-analysis. PLoS One. 2017;12(9):e0184293. doi:10.1371/journal.pone.0184293 https://www.ncbi.nlm.nih.gov/pubmed/28863162
- Sanchez-de-la-Torre M, Gracia-Lavedan E, Benitez ID, et al. Adherence to CPAP Treatment and the Risk of Recurrent Cardiovascular Events: A Meta-Analysis. JAMA. Oct 3 2023;330(13):1255-1265. doi:10.1001/jama.2023.17465 https://www.ncbi.nlm.nih.gov/pubmed/37787793
- Rapelli G, Pietrabissa G, Manzoni GM, et al. Improving CPAP Adherence in Adults With Obstructive Sleep Apnea Syndrome: A Scoping Review of Motivational Interventions. Frontiers in psychology. 2021;12:705364. doi:10.3389/fpsyg.2021.705364 https://www.ncbi.nlm.nih.gov/pubmed/34475840
- Feng G, Zhuge P, Zhang Z, Ma J. The impact of continuous positive airway pressure therapy on cardiovascular events in patients with obstructive sleep apnoea: an updated systematic review and meta-analysis. Sleep Breath. Oct 2024;28(5):2095-2105. doi:10.1007/s11325-024-03107-z https://www.ncbi.nlm.nih.gov/pubmed/39083193
- Shea S, Lionis C, Kite C, et al. Non-Alcoholic Fatty Liver Disease (NAFLD) and Potential Links to Depression, Anxiety, and Chronic Stress. Biomedicines. Nov 16 2021;9(11)doi:10.3390/biomedicines9111697 https://www.ncbi.nlm.nih.gov/pubmed/34829926
- Silverman AL, Herzog AA, Silverman DI. Hearts and Minds: Stress, Anxiety, and Depression: Unsung Risk Factors for Cardiovascular Disease. Cardiol Rev. Jul/Aug 2019;27(4):202-207. doi:10.1097/CRD.0000000000000228 https://www.ncbi.nlm.nih.gov/pubmed/30130257
- Krittanawong C, Maitra NS, Khawaja M, et al. Association of pessimism with cardiovascular events and all-cause mortality. Prog Cardiovasc Dis. Jan-Feb 2023;76:91-98. doi:10.1016/j.pcad.2022.11.018 https://www.ncbi.nlm.nih.gov/pubmed/36462555
- Sharma T, Padala PR, Mehta JL. Loneliness and Social Isolation: Determinants of Cardiovascular Outcomes. Curr Cardiol Rev. 2021;17(6):e051121190873. doi:10.2174/1573403X17666210129101845 https://www.ncbi.nlm.nih.gov/pubmed/33511946
- Christiansen J, Qualter P, Friis K, et al. Associations of loneliness and social isolation with physical and mental health among adolescents and young adults. Perspect Public Health. Jul 2021;141(4):226-236. doi:10.1177/17579139211016077 https://www.ncbi.nlm.nih.gov/pubmed/34148462
- Pourriyahi H, Yazdanpanah N, Saghazadeh A, Rezaei N. Loneliness: An Immunometabolic Syndrome. Int J Environ Res Public Health. Nov 19 2021;18(22)doi:10.3390/ijerph182212162 https://www.ncbi.nlm.nih.gov/pubmed/34831917
- Li H, Xia N. The role of oxidative stress in cardiovascular disease caused by social isolation and loneliness. Redox Biol. Oct 2020;37:101585. doi:10.1016/j.redox.2020.101585 https://www.ncbi.nlm.nih.gov/pubmed/32709420
- Ahmed M, Cerda I, Maloof M. Breaking the vicious cycle: The interplay between loneliness, metabolic illness, and mental health. Front Psychiatry. 2023;14:1134865. doi:10.3389/fpsyt.2023.1134865 https://www.ncbi.nlm.nih.gov/pubmed/36970267
- Klein LW. Pathophysiologic Mechanisms of Tobacco Smoke Producing Atherosclerosis. Curr Cardiol Rev. 2022;18(6):e110422203389. doi:10.2174/1573403X18666220411113112 https://www.ncbi.nlm.nih.gov/pubmed/35410615
- NIH. Naional Institutes of Health: How Smoking Affects the Heart and Blood Vessels. Available at https://www.nhlbi.nih.gov/health/heart/smoking Last updated 03/24/2023. Accessed 05/05/2023. 2022;
- NIH. National Institutes of Health: Strategies to Quit Smoking. Available at https://www.nhlbi.nih.gov/health/heart/smoking/tips-to-quit Last updated 03/24/2022. Accessed 05/05/2023. 2022;
- Chudzinska M, Wolowiec L, Banach J, Rogowicz D, Grzesk G. Alcohol and Cardiovascular Diseases-Do the Consumption Pattern and Dose Make the Difference? J Cardiovasc Dev Dis. Sep 22 2022;9(10)doi:10.3390/jcdd9100317 https://www.ncbi.nlm.nih.gov/pubmed/36286269
- Vacca A, Bulfone L, Cicco S, et al. Alcohol Intake and Arterial Hypertension: Retelling of a Multifaceted Story. Nutrients. Feb 15 2023;15(4)doi:10.3390/nu15040958 https://www.ncbi.nlm.nih.gov/pubmed/36839317
- Hwang CL, Muchira J, Hibner BA, Phillips SA, Piano MR. Alcohol Consumption: A New Risk Factor for Arterial Stiffness? Cardiovascular toxicology. Mar 2022;22(3):236-245. doi:10.1007/s12012-022-09728-8 https://www.ncbi.nlm.nih.gov/pubmed/35195845
- Zhong L, Chen W, Wang T, et al. Alcohol and Health Outcomes: An Umbrella Review of Meta-Analyses Base on Prospective Cohort Studies. Front Public Health. 2022;10:859947. doi:10.3389/fpubh.2022.859947 https://www.ncbi.nlm.nih.gov/pubmed/35602135
- DC. Centers for Disease Control and Prevention: Dietary Guidelines for Alcohol. Available at https://www.cdc.gov/alcohol/fact-sheets/moderate-drinking.htm Last updated 04/19/2022. Accessed 05/05/2023. 2022;
- Jacobsen AP, Raber I, McCarthy CP, et al. Lifelong Aspirin for All in the Secondary Prevention of Chronic Coronary Syndrome: Still Sacrosanct or Is Reappraisal Warranted? Circulation. Oct 20 2020;142(16):1579-1590. doi:10.1161/CIRCULATIONAHA.120.045695 https://www.ncbi.nlm.nih.gov/pubmed/32886529
- CC. American College of Cardiology. New USPSTF Recommendation on Aspirin in CVD: No For Primary Prevention, Yes For Secondary Prevention. Updated 4/27/2022. Accessed 1/8/2024, https://www.acc.org/Latest-in-Cardiology/Articles/2022/04/27/20/41/New-USPSTF-Recommendation-on-Aspirin-in-CVD
- Spencer FA, Guyatt G, Tampi M, Golemiec B. Aspirin in the primary prevention of cardiovascular disease and cancer. UpToDate. Updated 9/13/2023. Accessed 1/8/2024, https://www.uptodate.com/contents/aspirin-in-the-primary-prevention-of-cardiovascular-disease-and-cancer
- Cofer LB, Barrett TJ, Berger JS. Aspirin for the Primary Prevention of Cardiovascular Disease: Time for a Platelet-Guided Approach. Arterioscler Thromb Vasc Biol. Oct 2022;42(10):1207-1216. doi:10.1161/ATVBAHA.122.318020 https://www.ncbi.nlm.nih.gov/pubmed/36047408
- Force USPST, Davidson KW, Barry MJ, et al. Aspirin Use to Prevent Cardiovascular Disease: US Preventive Services Task Force Recommendation Statement. JAMA. Apr 26 2022;327(16):1577-1584. doi:10.1001/jama.2022.4983 https://www.ncbi.nlm.nih.gov/pubmed/35471505
- Hira RS, Gosch KL, Kazi DS, et al. Potential Impact of the 2019 ACC/AHA Guidelines on the Primary Prevention of Cardiovascular Disease Recommendations on the Inappropriate Routine Use of Aspirin and Aspirin Use Without a Recommended Indication for Primary Prevention of Cardiovascular Disease in Cardiology Practices: Insights From the NCDR PINNACLE Registry. Circulation Cardiovascular quality and outcomes. Mar 2022;15(3):e007979. doi:10.1161/CIRCOUTCOMES.121.007979 https://www.ncbi.nlm.nih.gov/pubmed/35098732
- Gragnano F, Cao D, Pirondini L, et al. P2Y(12) Inhibitor or Aspirin Monotherapy for Secondary Prevention of Coronary Events. J Am Coll Cardiol. Jul 11 2023;82(2):89-105. doi:10.1016/j.jacc.2023.04.051 https://www.ncbi.nlm.nih.gov/pubmed/37407118
- Ray KK, Ference BA, Severin T, et al. World Heart Federation Cholesterol Roadmap 2022. Glob Heart. 2022;17(1):75. doi:10.5334/gh.1154 https://www.ncbi.nlm.nih.gov/pubmed/36382159
- AHA. American Heart Association: Cholesterol Medications. Available at https://www.heart.org/en/health-topics/cholesterol/prevention-and-treatment-of-high-cholesterol-hyperlipidemia/cholesterol-medications Last updated 11/11/2020. Accessed 05/06/2023. 2020;
- Davidson MH, Pradeep P. Dyslipidemia. Merck Manual. Profressional Version. Updated 5/2023. Accessed 1/8/2024, https://www.merckmanuals.com/professional/endocrine-and-metabolic-disorders/lipid-disorders/dyslipidemia
- Rosenson RS, Eckel RH. Hypertriglyceridemia in adults: Management. UpToDate. Updated 6/19/2024. Accessed 11/22/2024, https://www.uptodate.com/contents/hypertriglyceridemia-in-adults-management
- Laufs U, Parhofer KG, Ginsberg HN, Hegele RA. Clinical review on triglycerides. Eur Heart J. Jan 1 2020;41(1):99-109c. doi:10.1093/eurheartj/ehz785 https://www.ncbi.nlm.nih.gov/pubmed/31764986
- Huston J, Schaffner H, Cox A, et al. A Critical Review of Icosapent Ethyl in Cardiovascular Risk Reduction. Am J Cardiovasc Drugs. Jul 2023;23(4):393-406. doi:10.1007/s40256-023-00583-8 https://www.ncbi.nlm.nih.gov/pubmed/37188993
- Bittner V. Eicosapentanoic Acid Supplementation for Atherosclerotic Cardiovascular Disease Prevention. Circulation. Aug 6 2024;150(6):435-438. doi:10.1161/CIRCULATIONAHA.124.069881 https://www.ncbi.nlm.nih.gov/pubmed/39102483
- Sheppard JP, Lakshmanan S, Dahal S, et al. EPA Versus Mixed EPA/DHA Plus Statin for Coronary Atherosclerosis: Meta-Analysis of Prospective Imaging Trials. JACC Cardiovascular imaging. Oct 2022;15(10):1825-1828. doi:10.1016/j.jcmg.2022.04.014 https://www.ncbi.nlm.nih.gov/pubmed/36202462
- Bhatt DL, Steg PG, Miller M, et al. Cardiovascular Risk Reduction with Icosapent Ethyl for Hypertriglyceridemia. N Engl J Med. Jan 3 2019;380(1):11-22. doi:10.1056/NEJMoa1812792 https://www.ncbi.nlm.nih.gov/pubmed/30415628
- Yokoyama M, Origasa H, Matsuzaki M, et al. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open-label, blinded endpoint analysis. Lancet. Mar 31 2007;369(9567):1090-8. doi:10.1016/S0140-6736(07)60527-3 https://www.ncbi.nlm.nih.gov/pubmed/17398308
- Saito Y, Yokoyama M, Origasa H, et al. Effects of EPA on coronary artery disease in hypercholesterolemic patients with multiple risk factors: sub-analysis of primary prevention cases from the Japan EPA Lipid Intervention Study (JELIS). Atherosclerosis. Sep 2008;200(1):135-40. doi:10.1016/j.atherosclerosis.2008.06.003 https://www.ncbi.nlm.nih.gov/pubmed/18667204
- Miyauchi K, Iwata H, Nishizaki Y, et al. Randomized Trial for Evaluation in Secondary Prevention Efficacy of Combination Therapy-Statin and Eicosapentaenoic Acid (RESPECT-EPA). Circulation. Aug 6 2024;150(6):425-434. doi:10.1161/CIRCULATIONAHA.123.065520 https://www.ncbi.nlm.nih.gov/pubmed/38873793
- Budoff MJ, Bhatt DL, Kinninger A, et al. Effect of icosapent ethyl on progression of coronary atherosclerosis in patients with elevated triglycerides on statin therapy: final results of the EVAPORATE trial. Eur Heart J. Oct 21 2020;41(40):3925-3932. doi:10.1093/eurheartj/ehaa652 https://www.ncbi.nlm.nih.gov/pubmed/32860032
- Nicholls SJ, Lincoff AM, Garcia M, et al. Effect of High-Dose Omega-3 Fatty Acids vs Corn Oil on Major Adverse Cardiovascular Events in Patients at High Cardiovascular Risk: The STRENGTH Randomized Clinical Trial. JAMA. Dec 8 2020;324(22):2268-2280. doi:10.1001/jama.2020.22258 https://www.ncbi.nlm.nih.gov/pubmed/33190147
- Basile J, Bloch MJ. Overview of hypertension in adults. UpToDate. Updated 12/6/2023. Accessed 1/8/2024, https://www.uptodate.com/contents/overview-of-hypertension-in-adults#H27
- Whelton PK, Bundy JD, Carey RM. Intensive Blood Pressure Treatment Goals: Evidence for Cardiovascular Protection From Observational Studies and Clinical Trials. Am J Hypertens. Nov 2 2022;35(11):905-914. doi:10.1093/ajh/hpac045 https://www.ncbi.nlm.nih.gov/pubmed/35390116
- Liu Y, Cotillard A, Vatier C, et al. A Dietary Supplement Containing Cinnamon, Chromium and Carnosine Decreases Fasting Plasma Glucose and Increases Lean Mass in Overweight or Obese Pre-Diabetic Subjects: A Randomized, Placebo-Controlled Trial. PLoS One. 2015;10(9):e0138646. doi:10.1371/journal.pone.0138646 https://www.ncbi.nlm.nih.gov/pubmed/26406981
- Joseph JJ, Deedwania P, Acharya T, et al. Comprehensive Management of Cardiovascular Risk Factors for Adults With Type 2 Diabetes: A Scientific Statement From the American Heart Association. Circulation. Mar 2022;145(9):e722-e759. doi:10.1161/CIR.0000000000001040 https://www.ncbi.nlm.nih.gov/pubmed/35000404
- American Diabetes Association Professional Practice C. 6. Glycemic Targets: Standards of Medical Care in Diabetes-2022. Diabetes Care. Jan 1 2022;45(Suppl 1):S83-S96. doi:10.2337/dc22-S006 https://www.ncbi.nlm.nih.gov/pubmed/34964868
- Wexler DJ. Metformin in the treatment of adults with type 2 diabetes mellitus. UpToDate. Updated 5/4/2023. Accessed 1/8/2024, https://www.uptodate.com/contents/metformin-in-the-treatment-of-adults-with-type-2-diabetes-mellitus
- Han Y, Xie H, Liu Y, Gao P, Yang X, Shen Z. Effect of metformin on all-cause and cardiovascular mortality in patients with coronary artery diseases: a systematic review and an updated meta-analysis. Cardiovasc Diabetol. Jul 30 2019;18(1):96. doi:10.1186/s12933-019-0900-7 https://www.ncbi.nlm.nih.gov/pubmed/31362743
- Zhang K, Yang W, Dai H, Deng Z. Cardiovascular risk following metformin treatment in patients with type 2 diabetes mellitus: Results from meta-analysis. Diabetes Res Clin Pract. Feb 2020;160:108001. doi:10.1016/j.diabres.2020.108001 https://www.ncbi.nlm.nih.gov/pubmed/31904444
- Hu Y, Lei M, Ke G, et al. Metformin Use and Risk of All-Cause Mortality and Cardiovascular Events in Patients With Chronic Kidney Disease-A Systematic Review and Meta-Analysis. Front Endocrinol (Lausanne). 2020;11:559446. doi:10.3389/fendo.2020.559446 https://www.ncbi.nlm.nih.gov/pubmed/33117278
- UpToDate. Dapagliflozin: Drug Information. Accessed 1/8/2024, https://www.uptodate.com/contents/dapagliflozin-drug-information
- He G, Yang G, Huang X, Luo D, Tang C, Zhang Z. SGLT2 inhibitors for prevention of primary and secondary cardiovascular outcomes: A meta-analysis of randomized controlled trials. Heart & lung : the journal of critical care. May-Jun 2023;59:109-116. doi:10.1016/j.hrtlng.2023.02.009 https://www.ncbi.nlm.nih.gov/pubmed/36801545
- Wu JH, Foote C, Blomster J, et al. Effects of sodium-glucose cotransporter-2 inhibitors on cardiovascular events, death, and major safety outcomes in adults with type 2 diabetes: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. May 2016;4(5):411-9. doi:10.1016/S2213-8587(16)00052-8 https://www.ncbi.nlm.nih.gov/pubmed/27009625
- Mascolo A, Scavone C, Scisciola L, Chiodini P, Capuano A, Paolisso G. SGLT-2 inhibitors reduce the risk of cerebrovascular/cardiovascular outcomes and mortality: A systematic review and meta-analysis of retrospective cohort studies. Pharmacol Res. Oct 2021;172:105836. doi:10.1016/j.phrs.2021.105836 https://www.ncbi.nlm.nih.gov/pubmed/34418562
- Wang W, Chen LY, Walker RF, et al. SGLT2 Inhibitors Are Associated With Reduced Cardiovascular Disease in Patients With Type 2 Diabetes: An Analysis of Real-World Data. Mayo Clin Proc. Jul 2023;98(7):985-996. doi:10.1016/j.mayocp.2023.01.023 https://www.ncbi.nlm.nih.gov/pubmed/37419588
- FDA. U.S. Food & Drug Administration. FDA News Release: FDA Approves First Treatment to Reduce Risk of Serious Heart Problems Specifically in Adults with Obesity or Overweight. Updated 3/8/2024. Accessed 5/7/2024, https://www.fda.gov/news-events/press-announcements/fda-approves-first-treatment-reduce-risk-serious-heart-problems-specifically-adults-obesity-or
- Hinnen D, Kruger D, Magwire M. Type 2 diabetes and cardiovascular disease: risk reduction and early intervention. Postgrad Med. Jan 2023;135(1):2-12. doi:10.1080/00325481.2022.2126235 https://www.ncbi.nlm.nih.gov/pubmed/36154802
- Lopaschuk GD, Verma S. Mechanisms of Cardiovascular Benefits of Sodium Glucose Co-Transporter 2 (SGLT2) Inhibitors: A State-of-the-Art Review. JACC Basic Transl Sci. Jun 2020;5(6):632-644. doi:10.1016/j.jacbts.2020.02.004 https://www.ncbi.nlm.nih.gov/pubmed/32613148
- Simms-Williams N, Treves N, Yin H, et al. Effect of combination treatment with glucagon-like peptide-1 receptor agonists and sodium-glucose cotransporter-2 inhibitors on incidence of cardiovascular and serious renal events: population based cohort study. BMJ. Apr 25 2024;385:e078242. doi:10.1136/bmj-2023-078242 https://www.ncbi.nlm.nih.gov/pubmed/38663919
- DAIC. Diagnostic and Interventional Cardiology. U.S. FDA Approves First Anti-Inflammatory Drug for Cardiovascular Disease. Updated 6/20/2023. Accessed 1/8/2024, https://www.dicardiology.com/content/us-fda-approves-first-anti-inflammatory-drug-cardiovascular-disease
- Nidorf SM, Fiolet ATL, Mosterd A, et al. Colchicine in Patients with Chronic Coronary Disease. N Engl J Med. Nov 5 2020;383(19):1838-1847. doi:10.1056/NEJMoa2021372 https://www.nejm.org/doi/pdf/10.1056/NEJMoa2021372?articleTools=true
- Dasgeb B, Kornreich D, McGuinn K, Okon L, Brownell I, Sackett DL. Colchicine: an ancient drug with novel applications. Br J Dermatol. Feb 2018;178(2):350-356. doi:10.1111/bjd.15896 https://www.ncbi.nlm.nih.gov/pubmed/28832953
- Telmesani A, Moss E, Chetrit M. The Use of Colchicine in Periodontal Diseases. American College of Cardiology. Updated 12/5/2019. Accessed 1/8/2024, https://www.acc.org/Latest-in-Cardiology/Articles/2019/12/04/08/22/The-Use-of-Colchicine-in-Pericardial-Diseases
- Cimmino G, Loffredo FS, De Rosa G, Cirillo P. Colchicine in Athero-Thrombosis: Molecular Mechanisms and Clinical Evidence. Int J Mol Sci. Jan 27 2023;24(3)doi:10.3390/ijms24032483 https://www.ncbi.nlm.nih.gov/pubmed/36768804
- Gonzalez L, Bulnes JF, Orellana MP, Munoz Venturelli P, Martinez Rodriguez G. The Role of Colchicine in Atherosclerosis: From Bench to Bedside. Pharmaceutics. Jul 1 2022;14(7)doi:10.3390/pharmaceutics14071395 https://www.ncbi.nlm.nih.gov/pubmed/35890291
- Ma Z, Chen J, Jin K, Chen X. Colchicine and coronary heart disease risks: A meta-analysis of randomized controlled clinical trials. Front Cardiovasc Med. 2022;9:947959. doi:10.3389/fcvm.2022.947959 https://www.ncbi.nlm.nih.gov/pubmed/36176989
- Nogic J, Mehta O, Tong D, Brown AJ, Layland J. Colchicine in the Management of Acute Coronary Syndrome: A Meta-analysis. Cardiology and therapy. Mar 2023;12(1):171-181. doi:10.1007/s40119-022-00298-y https://www.ncbi.nlm.nih.gov/pubmed/36609745
- Aw KL, Koh A, Lee HL, Kudzinskas A, De Palma R. Colchicine for symptomatic coronary artery disease after percutaneous coronary intervention. Open Heart. Jan 2022;9(1)doi:10.1136/openhrt-2021-001887 https://www.ncbi.nlm.nih.gov/pubmed/34992158
- Adams JA, Uryash A, Lopez JR. Non-Invasive Pulsatile Shear Stress Modifies Endothelial Activation; A Narrative Review. Biomedicines. Nov 28 2022;10(12)doi:10.3390/biomedicines10123050 https://www.ncbi.nlm.nih.gov/pubmed/36551807
- Lin S, Xiao-Ming W, Gui-Fu W. Expert consensus on the clinical application of enhanced external counterpulsation in elderly people (2019). Aging Med (Milton). Mar 2020;3(1):16-24. doi:10.1002/agm2.12097 https://www.ncbi.nlm.nih.gov/pubmed/32232188
- Brix M, Buschmann EE, Zietzer A, et al. Long-term individual shear rate therapy counterpulsation enhances plasma nitrite release in patients with PAD. VASA Zeitschrift fur Gefasskrankheiten. Jan 2017;46(1):37-45. doi:10.1024/0301-1526/a000600 https://www.ncbi.nlm.nih.gov/pubmed/27960614
- Nyvad J, Lerman A, Lerman LO. With a Little Help From My Friends: the Role of the Renal Collateral Circulation in Atherosclerotic Renovascular Disease. Hypertension. Apr 2022;79(4):717-725. doi:10.1161/HYPERTENSIONAHA.121.17960 https://www.ncbi.nlm.nih.gov/pubmed/35135307
- Caceres J, Atal P, Arora R, Yee D. Enhanced external counterpulsation: A unique treatment for the "No-Option" refractory angina patient. J Clin Pharm Ther. Apr 2021;46(2):295-303. doi:10.1111/jcpt.13330 https://www.ncbi.nlm.nih.gov/pubmed/33410549
- Zhou ZF, Wang DJ, Li XM, Zhang CL, Wu CY. Effects of enhanced external counterpulsation on exercise capacity and quality of life in patients with chronic heart failure: A meta-analysis. Medicine (Baltimore). Jul 9 2021;100(27):e26536. doi:10.1097/MD.0000000000026536 https://www.ncbi.nlm.nih.gov/pubmed/34232191
- Soubh N, Hillmeister P, Buschmann E, Klaproth C, Buschmann I. Tolerability safety and effectiveness of enhanced external counterpulsation versus individual shear rate therapy in patients with lower extremity atherosclerotic disease: A prospective pilot clinical trial. Acta physiologica (Oxford, England). Mar 2023;237(3):e13913. doi:10.1111/apha.13913 https://www.ncbi.nlm.nih.gov/pubmed/36599365
- Lederman S, Ottery FD, Cano A, et al. Fezolinetant for treatment of moderate-to-severe vasomotor symptoms associated with menopause (SKYLIGHT 1): a phase 3 randomised controlled study. Lancet. Apr 1 2023;401(10382):1091-1102. doi:10.1016/S0140-6736(23)00085-5 https://www.ncbi.nlm.nih.gov/pubmed/36924778
- Liu JY, Xiong L, Stinear CM, Leung H, Leung TW, Wong KSL. External counterpulsation enhances neuroplasticity to promote stroke recovery. J Neurol Neurosurg Psychiatry. Mar 2019;90(3):361-363. doi:10.1136/jnnp-2018-318185 https://www.ncbi.nlm.nih.gov/pubmed/29844246
- NIH. What Is Coronary Artery Bypass Grafting? Accessed 12/05/2023, https://www.nhlbi.nih.gov/health/coronary-artery-bypass-grafting
- Aranki S, Suri RM. Early noncardiac complications of coronary artery bypass graft surgery. UpToDate. Updated 8/28/2023. Accessed 1/8/2024, https://www.uptodate.com/contents/early-noncardiac-complications-of-coronary-artery-bypass-graft-surgery
- Sweis RN, Jivan A. Overview of Coronary Artery Disease. Merck Manual. Profressional Version. Updated 9/2022. Accessed 1/8/2024, https://www.merckmanuals.com/professional/cardiovascular-disorders/coronary-artery-disease/overview-of-coronary-artery-disease#v934055
- Zeng Y, Xu J, Deng Y, Li X, Chen W, Tang Y. Drug-eluting stents for coronary artery disease in the perspective of bibliometric analysis. Front Cardiovasc Med. 2024;11:1288659. doi:10.3389/fcvm.2024.1288659 https://www.ncbi.nlm.nih.gov/pubmed/38440210
- Akkus NI, Abdulbaki A, Jimenez E, Tandon N. Atherectomy devices: technology update. Med Devices (Auckl). 2014/12/17 2015;8(null):1-10. doi:10.2147/MDER.S50594 https://www.ncbi.nlm.nih.gov/pubmed/25565904
- AHA. American Heart Association. Prevention and Treatment of PAD. Available at https://www.heart.org/en/health-topics/peripheral-artery-disease/prevention-and-treatment-of-pad Last reviewed 02/13/24. Accessed 03/07/24. 2024;
- Krishnan P, Tarricone A, Chen S, Sharma S. The role of directional atherectomy in critical-limb ischemia. Therapeutic advances in cardiovascular disease. Jan-Dec 2021;135:17539447211046953. doi:10.1177/17539447211046953 https://www.ncbi.nlm.nih.gov/pubmed/34796770
- Wardle BG, Ambler GK, Radwan RW, Hinchliffe RJ, Twine CP. Atherectomy for peripheral arterial disease. Cochrane Database Syst Rev. Sep 29 2020;9(9):CD006680. doi:10.1002/14651858.CD006680.pub3 https://www.ncbi.nlm.nih.gov/pubmed/32990327
- AHA. American Hear Association. Heart Procedures and Surgeries. https://www.heart.org/en/health-topics/heart-attack/treatment-of-a-heart-attack/cardiac-procedures-and-surgeries Last reviewed 10/06/2023. Accessed 03/07/2024. 2023;
- Mouawad NJ. Successful Use of Adjunctive Orbital Atherectomy for Extensively Calcified Carotid Artery Lesions Using Flow Reversal Neuroprotection Technique. Annals of vascular surgery. Nov 2020;69:449 e1-449 e6. doi:10.1016/j.avsg.2020.05.016 https://www.ncbi.nlm.nih.gov/pubmed/32473307
- Abdelaziz A, Elsayed H, Hamdaalah A, et al. Safety and feasibility of rotational atherectomy (RA) versus conventional stenting in patients with chronic total occlusion (CTO) lesions: a systematic review and meta-analysis. BMC cardiovascular disorders. Jan 2 2024;24(1):4. doi:10.1186/s12872-023-03673-2 https://www.ncbi.nlm.nih.gov/pubmed/38166554
- Allali A, Abdel-Wahab M, Elbasha K, et al. Rotational atherectomy of calcified coronary lesions: current practice and insights from two randomized trials. Clin Res Cardiol. Sep 2023;112(9):1143-1163. doi:10.1007/s00392-022-02013-2 https://www.ncbi.nlm.nih.gov/pubmed/35482101
- Maron DJ, Hochman JS, Reynolds HR, et al. Initial Invasive or Conservative Strategy for Stable Coronary Disease. N Engl J Med. Apr 9 2020;382(15):1395-1407. doi:10.1056/NEJMoa1915922 https://www.ncbi.nlm.nih.gov/pubmed/32227755
- Cutlip D, Levin T. Revascularization in patients with stable coronary artery disease: Coronary artery bypass graft surgery versus percutaneous coronary intervention. UpToDate. Updated 8/23/2023. Accessed 1/8/2024, https://www.uptodate.com/contents/revascularization-in-patients-with-stable-coronary-artery-disease-coronary-artery-bypass-graft-surgery-versus-percutaneous-coronary-intervention
- Hochman JS, Anthopolos R, Reynolds HR, et al. Survival After Invasive or Conservative Management of Stable Coronary Disease. Circulation. Jan 3 2023;147(1):8-19. doi:10.1161/CIRCULATIONAHA.122.062714 https://www.ncbi.nlm.nih.gov/pubmed/36335918
- Redfors B, Stone GW, Alexander JH, et al. Outcomes According to Coronary Revascularization Modality in the ISCHEMIA Trial. J Am Coll Cardiol. Feb 6 2024;83(5):549-558. doi:10.1016/j.jacc.2023.11.002 https://www.ncbi.nlm.nih.gov/pubmed/37956961
- Stone GW, Ali ZA, O'Brien SM, et al. Impact of Complete Revascularization in the ISCHEMIA Trial. J Am Coll Cardiol. Sep 19 2023;82(12):1175-1188. doi:10.1016/j.jacc.2023.06.015 https://www.ncbi.nlm.nih.gov/pubmed/37462593
- Bangalore S, Maron DJ, O'Brien SM, et al. Management of Coronary Disease in Patients with Advanced Kidney Disease. N Engl J Med. Apr 23 2020;382(17):1608-1618. doi:10.1056/NEJMoa1915925 https://www.ncbi.nlm.nih.gov/pubmed/32227756
- Rezkalla SH, Kloner RA. Invasive versus Conservative Management in Coronary Artery Disease. Clin Med Res. Jun 2023;21(2):95-104. doi:10.3121/cmr.2023.1806 https://www.ncbi.nlm.nih.gov/pubmed/37407216
- Sud M, Han L, Koh M, et al. Low-Density Lipoprotein Cholesterol and Adverse Cardiovascular Events After Percutaneous Coronary Intervention. J Am Coll Cardiol. Sep 22 2020;76(12):1440-1450. doi:10.1016/j.jacc.2020.07.033 https://www.ncbi.nlm.nih.gov/pubmed/32943162
- AHA. American Heart Association. Unstable Angina. Updated 12/5/2022. Accessed 1/8/2024, https://www.heart.org/en/health-topics/heart-attack/angina-chest-pain/unstable-angina
- NLM. National Library of Medicine. MedlinePlus. Angina. Updated 1/3/2017. Accessed 1/8/2024, https://medlineplus.gov/angina.html
- AHA. American Heart Association. Angina Pectoris (Stable Angina). Updated 12/5/2022. Accessed 1/8/2024, https://www.heart.org/en/health-topics/heart-attack/angina-chest-pain/angina-pectoris-stable-angina
- Kannam JP, Gersh BJ. Beta blockers in the management of chronic coronary syndrome. UpToDate. Updated 5/16/2023. Accessed 11/22/2024, https://www.uptodate.com/contents/beta-blockers-in-the-management-of-chronic-coronary-syndrome
- Kannam JP, Gersh BJ. Nitrates in the management of chronic coronary syndrome. UpToDate. Updated 10/17/2023. Accessed 11/22/2024, https://www.uptodate.com/contents/nitrates-in-the-management-of-chronic-coronary-syndrome
- Reed M, Kerndt CC, Gopal S, Pellegrini MV, Nicolas D. Ranolazine. StatPearls. StatPearls Publishing. Copyright © 2024, StatPearls Publishing LLC.; 2025. https://www.ncbi.nlm.nih.gov/pubmed/29939605
- Qu H, Meng YY, Chai H, et al. The effect of statin treatment on circulating coenzyme Q10 concentrations: an updated meta-analysis of randomized controlled trials. Eur J Med Res. Nov 10 2018;23(1):57. doi:10.1186/s40001-018-0353-6 https://www.ncbi.nlm.nih.gov/pubmed/30414615
- Suarez-Rivero JM, Pastor-Maldonado CJ, de la Mata M, et al. Atherosclerosis and Coenzyme Q(10). Int J Mol Sci. Oct 20 2019;20(20)doi:10.3390/ijms20205195 https://www.ncbi.nlm.nih.gov/pubmed/31635164
- Gutierrez-Mariscal FM, de la Cruz-Ares S, Torres-Pena JD, Alcala-Diaz JF, Yubero-Serrano EM, Lopez-Miranda J. Coenzyme Q(10) and Cardiovascular Diseases. Antioxidants (Basel). Jun 3 2021;10(6)doi:10.3390/antiox10060906 https://www.ncbi.nlm.nih.gov/pubmed/34205085
- Lee BJ, Tseng YF, Yen CH, Lin PT. Effects of coenzyme Q10 supplementation (300 mg/day) on antioxidation and anti-inflammation in coronary artery disease patients during statins therapy: a randomized, placebo-controlled trial. Nutr J. Nov 6 2013;12(1):142. doi:10.1186/1475-2891-12-142 https://www.ncbi.nlm.nih.gov/pubmed/24192015
- Park SY, Pekas EJ, Headid RJ, 3rd, et al. Acute mitochondrial antioxidant intake improves endothelial function, antioxidant enzyme activity, and exercise tolerance in patients with peripheral artery disease. American journal of physiology Heart and circulatory physiology. Aug 1 2020;319(2):H456-H467. doi:10.1152/ajpheart.00235.2020 https://www.ncbi.nlm.nih.gov/pubmed/32706261
- Lei L, Liu Y. Efficacy of coenzyme Q10 in patients with cardiac failure: a meta-analysis of clinical trials. BMC cardiovascular disorders. Jul 24 2017;17(1):196. doi:10.1186/s12872-017-0628-9 https://www.ncbi.nlm.nih.gov/pubmed/28738783
- Kawashima C, Matsuzawa Y, Konishi M, et al. Ubiquinol Improves Endothelial Function in Patients with Heart Failure with Reduced Ejection Fraction: A Single-Center, Randomized Double-Blind Placebo-Controlled Crossover Pilot Study. Am J Cardiovasc Drugs. Aug 2020;20(4):363-372. doi:10.1007/s40256-019-00384-y https://www.ncbi.nlm.nih.gov/pubmed/31713723
- Alehagen U, Aaseth J, Alexander J, Johansson P. Still reduced cardiovascular mortality 12 years after supplementation with selenium and coenzyme Q10 for four years: A validation of previous 10-year follow-up results of a prospective randomized double-blind placebo-controlled trial in elderly. PLoS One. 2018;13(4):e0193120. doi:10.1371/journal.pone.0193120 https://www.ncbi.nlm.nih.gov/pubmed/29641571
- Dunning BJ, Bourgonje AR, Bulthuis MLC, et al. Selenium and coenzyme Q(10) improve the systemic redox status while reducing cardiovascular mortality in elderly population-based individuals. Free Radic Biol Med. Aug 1 2023;204:207-214. doi:10.1016/j.freeradbiomed.2023.04.024 https://www.ncbi.nlm.nih.gov/pubmed/37179031
- Alehagen U, Aaseth J, Lindahl TL, Larsson A, Alexander J. Dietary Supplementation with Selenium and Coenzyme Q(10) Prevents Increase in Plasma D-Dimer While Lowering Cardiovascular Mortality in an Elderly Swedish Population. Nutrients. Apr 17 2021;13(4)doi:10.3390/nu13041344 https://www.ncbi.nlm.nih.gov/pubmed/33920725
- Alehagen U, Aaseth J, Alexander J, Johansson P, Larsson A. Supplemental selenium and coenzyme Q10 reduce glycation along with cardiovascular mortality in an elderly population with low selenium status - A four-year, prospective, randomised, double-blind placebo-controlled trial. Journal of trace elements in medicine and biology : organ of the Society for Minerals and Trace Elements (GMS). May 4 2020;61:126541. doi:10.1016/j.jtemb.2020.126541 https://www.ncbi.nlm.nih.gov/pubmed/32417634
- Opstad TB, Alexander J, Aaseth J, Larsson A, Seljeflot I, Alehagen U. Increased SIRT1 Concentration Following Four Years of Selenium and Q(10) Intervention Associated with Reduced Cardiovascular Mortality at 10-Year Follow-Up-Sub-Study of a Previous Prospective Double-Blind Placebo-Controlled Randomized Clinical Trial. Antioxidants (Basel). Mar 21 2023;12(3)doi:10.3390/antiox12030759 https://www.ncbi.nlm.nih.gov/pubmed/36979007
- Opstad TB, Alexander J, Aaseth JO, Larsson A, Seljeflot I, Alehagen U. Selenium and Coenzyme Q(10) Intervention Prevents Telomere Attrition, with Association to Reduced Cardiovascular Mortality-Sub-Study of a Randomized Clinical Trial. Nutrients. Aug 15 2022;14(16)doi:10.3390/nu14163346 https://www.ncbi.nlm.nih.gov/pubmed/36014852
- Park HY, Kim SW, Seo J, et al. Dietary Arginine and Citrulline Supplements for Cardiovascular Health and Athletic Performance: A Narrative Review. Nutrients. Mar 3 2023;15(5)doi:10.3390/nu15051268 https://www.ncbi.nlm.nih.gov/pubmed/36904267
- Gambardella J, Khondkar W, Morelli MB, Wang X, Santulli G, Trimarco V. Arginine and Endothelial Function. Biomedicines. Aug 6 2020;8(8)doi:10.3390/biomedicines8080277 https://www.ncbi.nlm.nih.gov/pubmed/32781796
- Rodrigues-Krause J, Krause M, Rocha I, Umpierre D, Fayh APT. Association of l-Arginine Supplementation with Markers of Endothelial Function in Patients with Cardiovascular or Metabolic Disorders: A Systematic Review and Meta-Analysis. Nutrients. Dec 20 2018;11(1)doi:10.3390/nu11010015 https://www.ncbi.nlm.nih.gov/pubmed/30577559
- McNeal CJ, Meininger CJ, Reddy D, Wilborn CD, Wu G. Safety and Effectiveness of Arginine in Adults. J Nutr. Dec 2016;146(12):2587S-2593S. doi:10.3945/jn.116.234740 https://www.ncbi.nlm.nih.gov/pubmed/27934649
- Shiraseb F, Asbaghi O, Bagheri R, Wong A, Figueroa A, Mirzaei K. Effect of l-Arginine Supplementation on Blood Pressure in Adults: A Systematic Review and Dose-Response Meta-analysis of Randomized Clinical Trials. Adv Nutr. Aug 1 2022;13(4):1226-1242. doi:10.1093/advances/nmab155 https://www.ncbi.nlm.nih.gov/pubmed/34967840
- Sun T, Zhou WB, Luo XP, Tang YL, Shi HM. Oral L-arginine supplementation in acute myocardial infarction therapy: a meta-analysis of randomized controlled trials. Clin Cardiol. Nov 2009;32(11):649-52. doi:10.1002/clc.20616 https://www.ncbi.nlm.nih.gov/pubmed/19938054
- Deveaux A, Fouillet H, Petzke KJ, et al. A Slow- Compared with a Fast-Release Form of Oral Arginine Increases Its Utilization for Nitric Oxide Synthesis in Overweight Adults with Cardiometabolic Risk Factors in a Randomized Controlled Study. J Nutr. Jul 2016;146(7):1322-9. doi:10.3945/jn.116.231910 https://www.ncbi.nlm.nih.gov/pubmed/27281799
- Tripathi AK, Ray AK, Mishra SK, Bishen SM, Mishra H, Khurana A. Molecular and Therapeutic Insights of Alpha-Lipoic Acid as a Potential Molecule for Disease Prevention. Rev Bras Farmacogn. 2023;33(2):272-287. doi:10.1007/s43450-023-00370-1 https://www.ncbi.nlm.nih.gov/pubmed/36778891
- Jalilpiran Y, Hajishafiee M, Khorshidi M, et al. The effect of Alpha-lipoic acid supplementation on endothelial function: A systematic review and meta-analysis. Phytother Res. May 2021;35(5):2386-2395. doi:10.1002/ptr.6959 https://www.ncbi.nlm.nih.gov/pubmed/33205568
- Rahimlou M, Asadi M, Banaei Jahromi N, Mansoori A. Alpha-lipoic acid (ALA) supplementation effect on glycemic and inflammatory biomarkers: A Systematic Review and meta- analysis. Clin Nutr ESPEN. Aug 2019;32:16-28. doi:10.1016/j.clnesp.2019.03.015 https://www.ncbi.nlm.nih.gov/pubmed/31221283
- Ahmadi M, Keshavarz SA, Abbasi B. Effects of alpha lipoic acid supplementation on serum lipid profile in patients with metabolic syndrome: A randomized, double-blind, placebo-controlled clinical trial. ARYA atherosclerosis. Jul 2022;18(4):1-8. doi:10.22122/arya.2022.26181 https://www.ncbi.nlm.nih.gov/pubmed/36817351
- Mohammadi V, Khorvash F, Feizi A, Askari G. Does Alpha-lipoic Acid Supplementation Modulate Cardiovascular Risk Factors in Patients with Stroke? A Randomized, Double-blind Clinical Trial. International journal of preventive medicine. 2018;9:34. doi:10.4103/ijpvm.IJPVM_32_17 https://www.ncbi.nlm.nih.gov/pubmed/29721235
- Modanloo M, Shokrzadeh M. Analyzing Mitochondrial Dysfunction, Oxidative Stress, and Apoptosis: Potential Role of L-carnitine. Iran J Kidney Dis. Mar 2019;13(2):74-86. https://www.ncbi.nlm.nih.gov/pubmed/30988244
- Mingorance C, Rodriguez-Rodriguez R, Justo ML, Herrera MD, de Sotomayor MA. Pharmacological effects and clinical applications of propionyl-L-carnitine. Nutr Rev. May 2011;69(5):279-90. doi:10.1111/j.1753-4887.2011.00387.x https://www.ncbi.nlm.nih.gov/pubmed/21521230
- Mingorance C, Duluc L, Chalopin M, et al. Propionyl-L-carnitine corrects metabolic and cardiovascular alterations in diet-induced obese mice and improves liver respiratory chain activity. PLoS One. 2012;7(3):e34268. doi:10.1371/journal.pone.0034268 https://www.ncbi.nlm.nih.gov/pubmed/22457831
- Kamoen V, Vander Stichele R, Campens L, De Bacquer D, Van Bortel L, de Backer TL. Propionyl-L-carnitine for intermittent claudication. Cochrane Database Syst Rev. Dec 26 2021;12(12):CD010117. doi:10.1002/14651858.CD010117.pub2 https://www.ncbi.nlm.nih.gov/pubmed/34954832
- Katoh A, Kai H, Harada H, Niiyama H, Ikeda H. Oral Administration of Glucosamine Improves Vascular Endothelial Function by Modulating Intracellular Redox State. Int Heart J. Dec 12 2017;58(6):926-932. doi:10.1536/ihj.16-534 https://www.ncbi.nlm.nih.gov/pubmed/29151484
- Li ZH, Gao X, Chung VC, et al. Associations of regular glucosamine use with all-cause and cause-specific mortality: a large prospective cohort study. Ann Rheum Dis. Jun 2020;79(6):829-836. doi:10.1136/annrheumdis-2020-217176 https://www.ncbi.nlm.nih.gov/pubmed/32253185
- Ma H, Li X, Sun D, et al. Association of habitual glucosamine use with risk of cardiovascular disease: prospective study in UK Biobank. BMJ. May 14 2019;365:l1628. doi:10.1136/bmj.l1628 https://www.ncbi.nlm.nih.gov/pubmed/31088786
- Mazzucchelli R, Rodriguez-Martin S, Garcia-Vadillo A, et al. Risk of acute myocardial infarction among new users of chondroitin sulfate: A nested case-control study. PLoS One. 2021;16(7):e0253932. doi:10.1371/journal.pone.0253932 https://www.ncbi.nlm.nih.gov/pubmed/34252115
- Bhimani J, O'Connell K, Kuk D, Du M, Navarro SL, Kantor ED. Glucosamine and Chondroitin Use and Mortality Among Adults in the United States from 1999 to 2014. J Integr Complement Med. Aug 2023;29(8):492-500. doi:10.1089/jicm.2022.0783 https://www.ncbi.nlm.nih.gov/pubmed/36971848
- Khan UM, Sevindik M, Zarrabi A, et al. Lycopene: Food Sources, Biological Activities, and Human Health Benefits. Oxid Med Cell Longev. 2021;2021:2713511. doi:10.1155/2021/2713511 https://www.ncbi.nlm.nih.gov/pubmed/34840666
- Chiva-Blanch G, Jimenez C, Pinyol M, et al. 5-cis-, Trans- and Total Lycopene Plasma Concentrations Inversely Relate to Atherosclerotic Plaque Burden in Newly Diagnosed Type 2 Diabetes Subjects. Nutrients. Jun 6 2020;12(6)doi:10.3390/nu12061696 https://www.ncbi.nlm.nih.gov/pubmed/32517202
- Rissanen TH, Voutilainen S, Nyyssonen K, Salonen R, Kaplan GA, Salonen JT. Serum lycopene concentrations and carotid atherosclerosis: the Kuopio Ischaemic Heart Disease Risk Factor Study. Am J Clin Nutr. Jan 2003;77(1):133-8. doi:10.1093/ajcn/77.1.133 https://www.ncbi.nlm.nih.gov/pubmed/12499332
- Gianetti J, Pedrinelli R, Petrucci R, et al. Inverse association between carotid intima-media thickness and the antioxidant lycopene in atherosclerosis. Am Heart J. Mar 2002;143(3):467-74. doi:10.1067/mhj.2002.120776 https://www.ncbi.nlm.nih.gov/pubmed/11868053
- Rissanen T, Voutilainen S, Nyyssonen K, Salonen R, Salonen JT. Low plasma lycopene concentration is associated with increased intima-media thickness of the carotid artery wall. Arterioscler Thromb Vasc Biol. Dec 2000;20(12):2677-81. doi:10.1161/01.atv.20.12.2677 https://www.ncbi.nlm.nih.gov/pubmed/11116071
- Cheng HM, Koutsidis G, Lodge JK, Ashor AW, Siervo M, Lara J. Lycopene and tomato and risk of cardiovascular diseases: A systematic review and meta-analysis of epidemiological evidence. Crit Rev Food Sci Nutr. 2019;59(1):141-158. doi:10.1080/10408398.2017.1362630 https://www.ncbi.nlm.nih.gov/pubmed/28799780
- Cheng HM, Koutsidis G, Lodge JK, Ashor A, Siervo M, Lara J. Tomato and lycopene supplementation and cardiovascular risk factors: A systematic review and meta-analysis. Atherosclerosis. Feb 2017;257:100-108. doi:10.1016/j.atherosclerosis.2017.01.009 https://www.ncbi.nlm.nih.gov/pubmed/28129549
- Gajendragadkar PR, Hubsch A, Maki-Petaja KM, Serg M, Wilkinson IB, Cheriyan J. Effects of oral lycopene supplementation on vascular function in patients with cardiovascular disease and healthy volunteers: a randomised controlled trial. PLoS One. 2014;9(6):e99070. doi:10.1371/journal.pone.0099070 https://www.ncbi.nlm.nih.gov/pubmed/24911964
- Zou ZY, Xu XR, Lin XM, et al. Effects of lutein and lycopene on carotid intima-media thickness in Chinese subjects with subclinical atherosclerosis: a randomised, double-blind, placebo-controlled trial. Br J Nutr. Feb 2014;111(3):474-80. doi:10.1017/S0007114513002730 https://www.ncbi.nlm.nih.gov/pubmed/24047757
- Colman-Martinez M, Martinez-Huelamo M, Valderas-Martinez P, et al. trans-Lycopene from tomato juice attenuates inflammatory biomarkers in human plasma samples: An intervention trial. Mol Nutr Food Res. Nov 2017;61(11)doi:10.1002/mnfr.201600993 https://www.ncbi.nlm.nih.gov/pubmed/28688174
- Ahn YJ, Kim H. Lutein as a Modulator of Oxidative Stress-Mediated Inflammatory Diseases. Antioxidants (Basel). Sep 13 2021;10(9)doi:10.3390/antiox10091448 https://www.ncbi.nlm.nih.gov/pubmed/34573081
- Higdon J. Carotenoids. Oregon State University. Linus Pauling Institute. Micronutrient Information Center. Updated 9/2023. Accessed 1/8/2024, Data on file.
- Hajizadeh-Sharafabad F, Ghoreishi Z, Maleki V, Tarighat-Esfanjani A. Mechanistic insights into the effect of lutein on atherosclerosis, vascular dysfunction, and related risk factors: A systematic review of in vivo, ex vivo and in vitro studies. Pharmacol Res. Nov 2019;149:104477. doi:10.1016/j.phrs.2019.104477 https://www.ncbi.nlm.nih.gov/pubmed/31605782
- Xu XR, Zou ZY, Xiao X, Huang YM, Wang X, Lin XM. Effects of lutein supplement on serum inflammatory cytokines, ApoE and lipid profiles in early atherosclerosis population. Journal of atherosclerosis and thrombosis. 2013;20(2):170-7. doi:10.5551/jat.14365 https://www.ncbi.nlm.nih.gov/pubmed/23154578
- Wang MX, Jiao JH, Li ZY, Liu RR, Shi Q, Ma L. Lutein supplementation reduces plasma lipid peroxidation and C-reactive protein in healthy nonsmokers. Atherosclerosis. Apr 2013;227(2):380-5. doi:10.1016/j.atherosclerosis.2013.01.021 https://www.ncbi.nlm.nih.gov/pubmed/23398944
- Chung RWS, Leanderson P, Lundberg AK, Jonasson L. Lutein exerts anti-inflammatory effects in patients with coronary artery disease. Atherosclerosis. Jul 2017;262:87-93. doi:10.1016/j.atherosclerosis.2017.05.008 https://www.ncbi.nlm.nih.gov/pubmed/28527371
- Leermakers ET, Darweesh SK, Baena CP, et al. The effects of lutein on cardiometabolic health across the life course: a systematic review and meta-analysis. Am J Clin Nutr. Feb 2016;103(2):481-94. doi:10.3945/ajcn.115.120931 https://www.ncbi.nlm.nih.gov/pubmed/26762372
- Li N, Wu X, Zhuang W, et al. Green leafy vegetable and lutein intake and multiple health outcomes. Food Chem. Oct 30 2021;360:130145. doi:10.1016/j.foodchem.2021.130145 https://www.ncbi.nlm.nih.gov/pubmed/34034049
- Zou Z, Xu X, Huang Y, et al. High serum level of lutein may be protective against early atherosclerosis: the Beijing atherosclerosis study. Atherosclerosis. Dec 2011;219(2):789-93. doi:10.1016/j.atherosclerosis.2011.08.006 https://www.ncbi.nlm.nih.gov/pubmed/21872250
- Fredman G, Khan S. Specialized pro-resolving mediators enhance the clearance of dead cells. Immunol Rev. Oct 2023;319(1):151-157. doi:10.1111/imr.13278 https://www.ncbi.nlm.nih.gov/pubmed/37787174
- Gonzalez AL, Dungan MM, Smart CD, Madhur MS, Doran AC. Inflammation Resolution in the Cardiovascular System: Arterial Hypertension, Atherosclerosis, and Ischemic Heart Disease. Antioxid Redox Signal. Feb 2024;40(4-6):292-316. doi:10.1089/ars.2023.0284 https://www.ncbi.nlm.nih.gov/pubmed/37125445
- Yang B, Tseng PT, Hu X, et al. Comparative efficacy of omega-3 polyunsaturated fatty acids on major cardiovascular events: A network meta-analysis of randomized controlled trials. Prog Lipid Res. Nov 2022;88:101196. doi:10.1016/j.plipres.2022.101196 https://www.ncbi.nlm.nih.gov/pubmed/36341839
- Rodriguez D, Lavie CJ, Elagizi A, Milani RV. Update on Omega-3 Polyunsaturated Fatty Acids on Cardiovascular Health. Nutrients. Dec 3 2022;14(23)doi:10.3390/nu14235146 https://www.ncbi.nlm.nih.gov/pubmed/36501174
- Markozannes G, Ntzani EE, Tsapas A, et al. Dose-related meta-analysis for Omega-3 fatty acids supplementation on major adverse cardiovascular events. Clin Nutr. Apr 2022;41(4):923-930. doi:10.1016/j.clnu.2022.02.022 https://www.ncbi.nlm.nih.gov/pubmed/35290840
- Yu F, Qi S, Ji Y, Wang X, Fang S, Cao R. Effects of omega-3 fatty acid on major cardiovascular outcomes: A systematic review and meta-analysis. Medicine (Baltimore). Jul 29 2022;101(30):e29556. doi:10.1097/MD.0000000000029556 https://www.ncbi.nlm.nih.gov/pubmed/35905212
- Yokoyama Y, Kuno T, Morita SX, et al. Eicosapentaenoic Acid for Cardiovascular Events Reduction- Systematic Review and Network Meta-Analysis of Randomized Controlled Trials. Journal of cardiology. Nov 2022;80(5):416-422. doi:10.1016/j.jjcc.2022.07.008 https://www.ncbi.nlm.nih.gov/pubmed/35914996
- Nayda NC, Thomas JM, Delaney CL, Miller MD. The effect of omega-3 polyunsaturated fatty acid intake on blood levels of omega-3s in people with chronic atherosclerotic disease: a systematic review. Nutr Rev. Oct 10 2023;81(11):1447-1461. doi:10.1093/nutrit/nuad020 https://www.ncbi.nlm.nih.gov/pubmed/36882117
- FDA. Food and Drug Administration. FDA Announces New Qualified Health Claims for EPA and DHA Omega-3 Consumption and the Risk of Hypertension and Coronary Heart Disease. Available at https://www.fda.gov/food/cfsan-constituent-updates/fda-announces-new-qualified-health-claims-epa-and-dha-omega-3-consumption-and-risk-hypertension-and. Published 06/19/2019. Accessed 04/18/23. 2019;
- Marcus MD, Link MS. Omega-3 Fatty Acids and Arrhythmias. Circulation. Aug 6 2024;150(6):488-503. doi:10.1161/CIRCULATIONAHA.123.065769 https://www.ncbi.nlm.nih.gov/pubmed/39102482
- Bork CS, Myhre PL, Schmidt EB. Do omega-3 fatty acids increase risk of atrial fibrillation? Curr Opin Clin Nutr Metab Care. Mar 1 2023;26(2):78-82. doi:10.1097/MCO.0000000000000907 https://www.ncbi.nlm.nih.gov/pubmed/36892957
- Chen G, Qian ZM, Zhang J, et al. Regular use of fish oil supplements and course of cardiovascular diseases: prospective cohort study. BMJ Med. 2024;3(1):e000451. doi:10.1136/bmjmed-2022-000451 https://www.ncbi.nlm.nih.gov/pubmed/38800667
- Yamaguchi A, Botta E, Holinstat M. Eicosanoids in inflammation in the blood and the vessel. Front Pharmacol. 2022;13:997403. doi:10.3389/fphar.2022.997403 https://www.ncbi.nlm.nih.gov/pubmed/36238558
- Fredman G, Serhan CN. Specialized pro-resolving mediators in vascular inflammation and atherosclerotic cardiovascular disease. Nature reviews Cardiology. Nov 2024;21(11):808-823. doi:10.1038/s41569-023-00984-x https://www.ncbi.nlm.nih.gov/pubmed/38216693
- Doran AC. Inflammation Resolution: Implications for Atherosclerosis. Circ Res. Jan 7 2022;130(1):130-148. doi:10.1161/CIRCRESAHA.121.319822 https://www.ncbi.nlm.nih.gov/pubmed/34995137
- Back M, Yurdagul A, Jr., Tabas I, Oorni K, Kovanen PT. Inflammation and its resolution in atherosclerosis: mediators and therapeutic opportunities. Nature reviews Cardiology. Jul 2019;16(7):389-406. doi:10.1038/s41569-019-0169-2 https://www.ncbi.nlm.nih.gov/pubmed/30846875
- Schaller MS, Chen M, Colas RA, et al. Treatment With a Marine Oil Supplement Alters Lipid Mediators and Leukocyte Phenotype in Healthy Patients and Those With Peripheral Artery Disease. J Am Heart Assoc. Aug 4 2020;9(15):e016113. doi:10.1161/JAHA.120.016113 https://www.ncbi.nlm.nih.gov/pubmed/32696697
- Kraft JD, Blomgran R, Bergstrom I, et al. Lipoxins modulate neutrophil oxidative burst, integrin expression and lymphatic transmigration differentially in human health and atherosclerosis. FASEB J. Mar 2022;36(3):e22173. doi:10.1096/fj.202101219RR https://www.ncbi.nlm.nih.gov/pubmed/35104001
- Rangarajan S, Orujyan D, Rangchaikul P, Radwan MM. Critical Role of Inflammation and Specialized Pro-Resolving Mediators in the Pathogenesis of Atherosclerosis. Biomedicines. Nov 6 2022;10(11)doi:10.3390/biomedicines10112829 https://www.ncbi.nlm.nih.gov/pubmed/36359349
- Reynolds AN, Akerman A, Kumar S, Diep Pham HT, Coffey S, Mann J. Dietary fibre in hypertension and cardiovascular disease management: systematic review and meta-analyses. BMC Med. Apr 22 2022;20(1):139. doi:10.1186/s12916-022-02328-x https://www.ncbi.nlm.nih.gov/pubmed/35449060
- Jovanovski E, Yashpal S, Komishon A, et al. Effect of psyllium (Plantago ovata) fiber on LDL cholesterol and alternative lipid targets, non-HDL cholesterol and apolipoprotein B: a systematic review and meta-analysis of randomized controlled trials. Am J Clin Nutr. Nov 1 2018;108(5):922-932. doi:10.1093/ajcn/nqy115 https://www.ncbi.nlm.nih.gov/pubmed/30239559
- Ho HVT, Jovanovski E, Zurbau A, et al. A systematic review and meta-analysis of randomized controlled trials of the effect of konjac glucomannan, a viscous soluble fiber, on LDL cholesterol and the new lipid targets non-HDL cholesterol and apolipoprotein B. Am J Clin Nutr. May 2017;105(5):1239-1247. doi:10.3945/ajcn.116.142158 https://www.ncbi.nlm.nih.gov/pubmed/28356275
- de Morais Junior AC, Schincaglia RM, Viana RB, et al. The separate effects of whole oats and isolated beta-glucan on lipid profile: A systematic review and meta-analysis of randomized controlled trials. Clin Nutr ESPEN. Feb 2023;53:224-237. doi:10.1016/j.clnesp.2022.12.019 https://www.ncbi.nlm.nih.gov/pubmed/36657917
- Llanaj E, Dejanovic GM, Valido E, et al. Effect of oat supplementation interventions on cardiovascular disease risk markers: a systematic review and meta-analysis of randomized controlled trials. Eur J Nutr. Jun 2022;61(4):1749-1778. doi:10.1007/s00394-021-02763-1 https://www.ncbi.nlm.nih.gov/pubmed/34977959
- Zurbau A, Noronha JC, Khan TA, Sievenpiper JL, Wolever TMS. The effect of oat beta-glucan on postprandial blood glucose and insulin responses: a systematic review and meta-analysis. Eur J Clin Nutr. Nov 2021;75(11):1540-1554. doi:10.1038/s41430-021-00875-9 https://www.ncbi.nlm.nih.gov/pubmed/33608654
- Mansuri NM, Mann NK, Rizwan S, et al. Role of Gut Microbiome in Cardiovascular Events: A Systematic Review. Cureus. Dec 2022;14(12):e32465. doi:10.7759/cureus.32465 https://www.ncbi.nlm.nih.gov/pubmed/36644080
- Khalili L, Centner AM, Salazar G. Effects of Berries, Phytochemicals, and Probiotics on Atherosclerosis through Gut Microbiota Modification: A Meta-Analysis of Animal Studies. Int J Mol Sci. Feb 4 2023;24(4)doi:10.3390/ijms24043084 https://www.ncbi.nlm.nih.gov/pubmed/36834497
- Naruszewicz M, Johansson ML, Zapolska-Downar D, Bukowska H. Effect of Lactobacillus plantarum 299v on cardiovascular disease risk factors in smokers. Am J Clin Nutr. Dec 2002;76(6):1249-55. doi:10.1093/ajcn/76.6.1249 https://www.ncbi.nlm.nih.gov/pubmed/12450890
- Jones ML, Martoni CJ, Prakash S. Cholesterol lowering and inhibition of sterol absorption by Lactobacillus reuteri NCIMB 30242: a randomized controlled trial. Eur J Clin Nutr. Nov 2012;66(11):1234-41. doi:10.1038/ejcn.2012.126 https://www.ncbi.nlm.nih.gov/pubmed/22990854
- Ahn HY, Kim M, Chae JS, et al. Supplementation with two probiotic strains, Lactobacillus curvatus HY7601 and Lactobacillus plantarum KY1032, reduces fasting triglycerides and enhances apolipoprotein A-V levels in non-diabetic subjects with hypertriglyceridemia. Atherosclerosis. Aug 2015;241(2):649-56. doi:10.1016/j.atherosclerosis.2015.06.030 https://www.ncbi.nlm.nih.gov/pubmed/26117402
- Boren J, Chapman MJ, Krauss RM, et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease: pathophysiological, genetic, and therapeutic insights: a consensus statement from the European Atherosclerosis Society Consensus Panel. Eur Heart J. Jun 21 2020;41(24):2313-2330. doi:10.1093/eurheartj/ehz962 https://www.ncbi.nlm.nih.gov/pubmed/32052833
- Li M, Yun W, Wang G, Li A, Gao J, He Q. Roles and mechanisms of garlic and its extracts on atherosclerosis: A review. Front Pharmacol. 2022;13:954938. doi:10.3389/fphar.2022.954938 https://www.ncbi.nlm.nih.gov/pubmed/36263122
- Sobenin IA, Myasoedova VA, Iltchuk MI, Zhang DW, Orekhov AN. Therapeutic effects of garlic in cardiovascular atherosclerotic disease. Chinese journal of natural medicines. Oct 2019;17(10):721-728. doi:10.1016/S1875-5364(19)30088-3 https://www.ncbi.nlm.nih.gov/pubmed/31703752
- Taghizadeh M, Hamedifard Z, Jafarnejad S. Effect of garlic supplementation on serum C-reactive protein level: A systematic review and meta-analysis of randomized controlled trials. Phytother Res. Feb 2019;33(2):243-252. doi:10.1002/ptr.6225 https://www.ncbi.nlm.nih.gov/pubmed/30370629
- Ried K. Garlic Lowers Blood Pressure in Hypertensive Individuals, Regulates Serum Cholesterol, and Stimulates Immunity: An Updated Meta-analysis and Review. J Nutr. Feb 2016;146(2):389S-396S. doi:10.3945/jn.114.202192 https://www.ncbi.nlm.nih.gov/pubmed/26764326
- Shabani E, Sayemiri K, Mohammadpour M. The effect of garlic on lipid profile and glucose parameters in diabetic patients: A systematic review and meta-analysis. Prim Care Diabetes. Feb 2019;13(1):28-42. doi:10.1016/j.pcd.2018.07.007 https://www.ncbi.nlm.nih.gov/pubmed/30049636
- Kodera Y, Kurita M, Nakamoto M, Matsutomo T. Chemistry of aged garlic: Diversity of constituents in aged garlic extract and their production mechanisms via the combination of chemical and enzymatic reactions. Exp Ther Med. Feb 2020;19(2):1574-1584. doi:10.3892/etm.2019.8393 https://www.ncbi.nlm.nih.gov/pubmed/32010342
- Capasso A. Antioxidant action and therapeutic efficacy of Allium sativum L. Molecules. Jan 4 2013;18(1):690-700. doi:10.3390/molecules18010690 https://www.ncbi.nlm.nih.gov/pubmed/23292331
- Emamat H, Tangestani H, Totmaj AS, Ghalandari H, Nasrollahzadeh J. The effect of garlic on vascular function: A systematic review of randomized clinical trials. Clin Nutr. Dec 2020;39(12):3563-3570. doi:10.1016/j.clnu.2020.02.016 https://www.ncbi.nlm.nih.gov/pubmed/32143889
- Lindstedt S, Wlosinska M, Nilsson AC, Hlebowicz J, Fakhro M, Sheikh R. Successful improved peripheral tissue perfusion was seen in patients with atherosclerosis after 12 months of treatment with aged garlic extract. Int Wound J. Oct 2021;18(5):681-691. doi:10.1111/iwj.13570 https://www.ncbi.nlm.nih.gov/pubmed/33590955
- Wlosinska M, Nilsson AC, Hlebowicz J, Malmsjo M, Fakhro M, Lindstedt S. Aged garlic extract preserves cutaneous microcirculation in patients with increased risk for cardiovascular diseases: A double-blinded placebo-controlled study. Int Wound J. Dec 2019;16(6):1487-1493. doi:10.1111/iwj.13220 https://www.ncbi.nlm.nih.gov/pubmed/31518044
- Wlosinska M, Nilsson AC, Hlebowicz J, et al. The effect of aged garlic extract on the atherosclerotic process - a randomized double-blind placebo-controlled trial. BMC Complement Med Ther. Apr 29 2020;20(1):132. doi:10.1186/s12906-020-02932-5 https://www.ncbi.nlm.nih.gov/pubmed/32349742
- Matsumoto S, Nakanishi R, Li D, et al. Aged Garlic Extract Reduces Low Attenuation Plaque in Coronary Arteries of Patients with Metabolic Syndrome in a Prospective Randomized Double-Blind Study. J Nutr. Feb 2016;146(2):427S-432S. doi:10.3945/jn.114.202424 https://www.ncbi.nlm.nih.gov/pubmed/26764322
- Shaikh K, Kinninger A, Cherukuri L, et al. Aged garlic extract reduces low attenuation plaque in coronary arteries of patients with diabetes: A randomized, double-blind, placebo-controlled study. Exp Ther Med. Feb 2020;19(2):1457-1461. doi:10.3892/etm.2019.8371 https://www.ncbi.nlm.nih.gov/pubmed/32010322
- Nirvanashetty S, Panda S. High Potency Aged Garlic Extract reduces Cardiovascular Disease Risk Factors in Healthy Participants: A Randomized, Double-Blind, Placebo Controlled Study. Open Acc J Comp & Alt Med. 10/20/2023 2023;5(1):585-593. doi:10.32474/OAJCAM.2023.05.000203 https://lupinepublishers.com/complementary-alternative-medicine-journal/fulltext/high-potency-ginger-extract-reduces-menstrual-discomfort-in-healthy.ID.000203.php
- Villano D, Marhuenda J, Arcusa R, et al. Effect of Black Garlic Consumption on Endothelial Function and Lipid Profile: A Before-and-After Study in Hypercholesterolemic and Non-Hypercholesterolemic Subjects. Nutrients. Jul 14 2023;15(14)doi:10.3390/nu15143138 https://www.ncbi.nlm.nih.gov/pubmed/37513556
- Jung ES, Park SH, Choi EK, et al. Reduction of blood lipid parameters by a 12-wk supplementation of aged black garlic: a randomized controlled trial. Nutrition. Sep 2014;30(9):1034-9. doi:10.1016/j.nut.2014.02.014 https://www.ncbi.nlm.nih.gov/pubmed/24976429
- Zhang J, Chai X, Zhao F, Hou G, Meng Q. Food Applications and Potential Health Benefits of Hawthorn. Foods. Sep 15 2022;11(18)doi:10.3390/foods11182861 https://www.ncbi.nlm.nih.gov/pubmed/36140986
- Wu M, Liu L, Xing Y, Yang S, Li H, Cao Y. Roles and Mechanisms of Hawthorn and Its Extracts on Atherosclerosis: A Review. Front Pharmacol. 2020;11:118. doi:10.3389/fphar.2020.00118 https://www.ncbi.nlm.nih.gov/pubmed/32153414
- Dehghani S, Mehri S, Hosseinzadeh H. The effects of Crataegus pinnatifida (Chinese hawthorn) on metabolic syndrome: A review. Iranian journal of basic medical sciences. May 2019;22(5):460-468. doi:10.22038/IJBMS.2019.31964.7678 https://www.ncbi.nlm.nih.gov/pubmed/31217924
- Kadas ZE, G. A.; Heper, G. The Metabolic Effects of Hawthorn Vinegar in Patients with High Cardiovascular Risk Group. J Food Nutr Res. 2014;2(9):539–545. https://pubs.sciepub.com/jfnr/2/9/2/index.html
- Walker AF, Marakis G, Simpson E, et al. Hypotensive effects of hawthorn for patients with diabetes taking prescription drugs: a randomised controlled trial. The British journal of general practice : the journal of the Royal College of General Practitioners. Jun 2006;56(527):437-43. https://www.ncbi.nlm.nih.gov/pubmed/16762125
- Asgary S, Naderi GH, Sadeghi M, Kelishadi R, Amiri M. Antihypertensive effect of Iranian Crataegus curvisepala Lind.: a randomized, double-blind study. Drugs under experimental and clinical research. 2004;30(5-6):221-5. https://www.ncbi.nlm.nih.gov/pubmed/15700749
- Walker AF, Marakis G, Morris AP, Robinson PA. Promising hypotensive effect of hawthorn extract: a randomized double-blind pilot study of mild, essential hypertension. Phytother Res. Feb 2002;16(1):48-54. doi:10.1002/ptr.947 https://www.ncbi.nlm.nih.gov/pubmed/11807965
- Noor ET, Das R, Lami MS, et al. Ginkgo biloba: A Treasure of Functional Phytochemicals with Multimedicinal Applications. Evid Based Complement Alternat Med. 2022;2022:8288818. doi:10.1155/2022/8288818 https://www.ncbi.nlm.nih.gov/pubmed/35265150
- Tao Y, Zhu F, Pan M, Liu Q, Wang P. Pharmacokinetic, Metabolism, and Metabolomic Strategies Provide Deep Insight Into the Underlying Mechanism of Ginkgo biloba Flavonoids in the Treatment of Cardiovascular Disease. Front Nutr. 2022;9:857370. doi:10.3389/fnut.2022.857370 https://www.ncbi.nlm.nih.gov/pubmed/35399672
- Silva H, Martins FG. Cardiovascular Activity of Ginkgo biloba-An Insight from Healthy Subjects. Biology. Dec 21 2022;12(1)doi:10.3390/biology12010015 https://www.ncbi.nlm.nih.gov/pubmed/36671707
- Li X, Lu L, Chen J, Zhang C, Chen H, Huang H. New Insight into the Mechanisms of Ginkgo Biloba Extract in Vascular Aging Prevention. Curr Vasc Pharmacol. 2020;18(4):334-345. doi:10.2174/1570161117666190621150725 https://www.ncbi.nlm.nih.gov/pubmed/31223090
- Aziz TA, Hussain SA, Mahwi TO, Ahmed ZA. Efficacy and safety of Ginkgo biloba extract as an "add-on" treatment to metformin for patients with metabolic syndrome: a pilot clinical study. Ther Clin Risk Manag. 2018;14:1219-1226. doi:10.2147/TCRM.S169503 https://www.ncbi.nlm.nih.gov/pubmed/30034238
- Siegel G, Ermilov E, Knes O, Rodriguez M. Combined lowering of low grade systemic inflammation and insulin resistance in metabolic syndrome patients treated with Ginkgo biloba. Atherosclerosis. Dec 2014;237(2):584-8. doi:10.1016/j.atherosclerosis.2014.10.023 https://www.ncbi.nlm.nih.gov/pubmed/25463092
- Rodriguez M, Ringstad L, Schafer P, et al. Reduction of atherosclerotic nanoplaque formation and size by Ginkgo biloba (EGb 761) in cardiovascular high-risk patients. Atherosclerosis. Jun 2007;192(2):438-44. doi:10.1016/j.atherosclerosis.2007.02.021 https://www.ncbi.nlm.nih.gov/pubmed/17397850
- Pittler MH, Ernst E. Ginkgo biloba extract for the treatment of intermittent claudication: a meta-analysis of randomized trials. Am J Med. Mar 2000;108(4):276-81. doi:10.1016/s0002-9343(99)00454-4 https://www.ncbi.nlm.nih.gov/pubmed/11014719
- Nicolai SP, Kruidenier LM, Bendermacher BL, et al. Ginkgo biloba for intermittent claudication. Cochrane Database Syst Rev. Jun 6 2013;2013(6):CD006888. doi:10.1002/14651858.CD006888.pub3 https://www.ncbi.nlm.nih.gov/pubmed/23744597
- Nicolai SP, Gerardu VC, Kruidenier LM, Prins MH, Teijink JA. From the Cochrane library: Ginkgo biloba for intermittent claudication. VASA Zeitschrift fur Gefasskrankheiten. May 2010;39(2):153-8. doi:10.1024/0301-1526/a000021 https://www.ncbi.nlm.nih.gov/pubmed/20464671
- Dwivedi S, Chopra D. Revisiting Terminalia arjuna - An Ancient Cardiovascular Drug. Journal of traditional and complementary medicine. Oct 2014;4(4):224-31. doi:10.4103/2225-4110.139103 https://www.ncbi.nlm.nih.gov/pubmed/25379463
- Kapoor D, Vijayvergiya R, Dhawan V. Terminalia arjuna in coronary artery disease: ethnopharmacology, pre-clinical, clinical & safety evaluation. J Ethnopharmacol. Sep 11 2014;155(2):1029-45. doi:10.1016/j.jep.2014.06.056 https://www.ncbi.nlm.nih.gov/pubmed/25014508
- Dwivedi S, Agarwal MP. Antianginal and cardioprotective effects of Terminalia arjuna, an indigenous drug, in coronary artery disease. J Assoc Physicians India. Apr 1994;42(4):287-9. https://www.ncbi.nlm.nih.gov/pubmed/7741874
- Bharani A, Ganguli A, Mathur LK, Jamra Y, Raman PG. Efficacy of Terminalia arjuna in chronic stable angina: a double-blind, placebo-controlled, crossover study comparing Terminalia arjuna with isosorbide mononitrate. Indian heart journal. Mar-Apr 2002;54(2):170-5. https://www.ncbi.nlm.nih.gov/pubmed/12086380
- Kapoor D, Trikha D, Vijayvergiya R, Parashar KK, Kaul D, Dhawan V. Short-Term Adjuvant Therapy with Terminalia arjuna Attenuates Ongoing Inflammation and Immune Imbalance in Patients with Stable Coronary Artery Disease: In Vitro and In Vivo Evidence. Journal of cardiovascular translational research. Apr 2015;8(3):173-86. doi:10.1007/s12265-015-9620-x https://www.ncbi.nlm.nih.gov/pubmed/25827448
- Maulik SK, Wilson V, Seth S, et al. Clinical efficacy of water extract of stem bark of Terminalia arjuna (Roxb. ex DC.) Wight & Arn. in patients of chronic heart failure: a double-blind, randomized controlled trial. Phytomedicine. Oct 15 2016;23(11):1211-9. doi:10.1016/j.phymed.2016.02.007 https://www.ncbi.nlm.nih.gov/pubmed/26988798
- Bharani A, Ganguly A, Bhargava KD. Salutary effect of Terminalia Arjuna in patients with severe refractory heart failure. Int J Cardiol. May 1995;49(3):191-9. doi:10.1016/0167-5273(95)02320-v https://www.ncbi.nlm.nih.gov/pubmed/7649665
- Gupta R, Singhal S, Goyle A, Sharma VN. Antioxidant and hypocholesterolaemic effects of Terminalia arjuna tree-bark powder: a randomised placebo-controlled trial. J Assoc Physicians India. Feb 2001;49:231-5. https://www.ncbi.nlm.nih.gov/pubmed/11225136
- Szallasi A. Dietary Capsaicin: A Spicy Way to Improve Cardio-Metabolic Health? Biomolecules. Nov 29 2022;12(12)doi:10.3390/biom12121783 https://www.ncbi.nlm.nih.gov/pubmed/36551210
- Kaur M, Verma BR, Zhou L, et al. Association of pepper intake with all-cause and specific cause mortality - A systematic review and meta-analysis. Am J Prev Cardiol. Mar 2022;9:100301. doi:10.1016/j.ajpc.2021.100301 https://www.ncbi.nlm.nih.gov/pubmed/34977833
- Yamani N, Musheer A, Gosain P, et al. Meta-analysis evaluating the impact of chili-pepper intake on all-cause and cardiovascular mortality: A systematic review. Ann Med Surg (Lond). Oct 2021;70:102774. doi:10.1016/j.amsu.2021.102774 https://www.ncbi.nlm.nih.gov/pubmed/34603712
- Ofori-Asenso R, Mohsenpour MA, Nouri M, Faghih S, Liew D, Mazidi M. Association of Spicy Chilli Food Consumption With Cardiovascular and All-Cause Mortality: A Meta-Analysis of Prospective Cohort Studies. Angiology. Aug 2021;72(7):625-632. doi:10.1177/0003319721995666 https://www.ncbi.nlm.nih.gov/pubmed/33657876
- Yang L, Sun J, Zhao M, Xi B. Chili pepper intake and all-cause and disease-specific mortality. Int J Vitam Nutr Res. Aug 2023;93(4):378-384. doi:10.1024/0300-9831/a000746 https://www.ncbi.nlm.nih.gov/pubmed/35038885
- Kelava L, Nemeth D, Hegyi P, et al. Dietary supplementation of transient receptor potential vanilloid-1 channel agonists reduces serum total cholesterol level: a meta-analysis of controlled human trials. Crit Rev Food Sci Nutr. 2022;62(25):7025-7035. doi:10.1080/10408398.2021.1910138 https://www.ncbi.nlm.nih.gov/pubmed/33840333
- Fragasso G, Palloshi A, Piatti PM, et al. Nitric-oxide mediated effects of transdermal capsaicin patches on the ischemic threshold in patients with stable coronary disease. Journal of cardiovascular pharmacology. Sep 2004;44(3):340-7. doi:10.1097/01.fjc.0000137161.76616.85 https://www.ncbi.nlm.nih.gov/pubmed/15475832
- Sun B, Wu L, Wu Y, et al. Therapeutic Potential of Centella asiatica and Its Triterpenes: A Review. Front Pharmacol. 2020;11:568032. doi:10.3389/fphar.2020.568032 https://www.ncbi.nlm.nih.gov/pubmed/33013406
- Razali NNM, Ng CT, Fong LY. Cardiovascular Protective Effects of Centella asiatica and Its Triterpenes: A Review. Planta Med. Nov 2019;85(16):1203-1215. doi:10.1055/a-1008-6138 https://www.ncbi.nlm.nih.gov/pubmed/31539918
- Cesarone MR, Belcaro G, Nicolaides AN, et al. Increase in echogenicity of echolucent carotid plaques after treatment with total triterpenic fraction of Centella asiatica: a prospective, placebo-controlled, randomized trial. Angiology. Oct 2001;52 Suppl 2:S19-25. https://www.ncbi.nlm.nih.gov/pubmed/11666118
- Incandela L, Belcaro G, Nicolaides AN, et al. Modification of the echogenicity of femoral plaques after treatment with total triterpenic fraction of Centella asiatica: a prospective, randomized, placebo-controlled trial. Angiology. Oct 2001;52 Suppl 2:S69-73. https://www.ncbi.nlm.nih.gov/pubmed/11666127
- Belcaro G, Dugall M, Ippolito E, et al. Pycnogenol(R) and Centella asiatica to prevent asymptomatic atherosclerosis progression in clinical events. Minerva cardioangiologica. Feb 2017;65(1):24-31. doi:10.23736/S0026-4725.16.04008-1 https://www.ncbi.nlm.nih.gov/pubmed/26505327
- Belcaro G, Cesarone MR, Scipione C, et al. Delayed progression of atherosclerosis and cardiovascular events in asymptomatic patients with atherosclerotic plaques: 3-year prevention with the supplementation with Pycnogenol(R)+Centellicum(R). Minerva cardioangiologica. Feb 2020;68(1):15-21. doi:10.23736/S0026-4725.19.05051-5 https://www.ncbi.nlm.nih.gov/pubmed/31625707
- Hu S, Belcaro G, Cesarone MR, et al. Central cardiovascular calcifications: supplementation with Pycnogenol(R) and Centellicum(R): variations over 12 months. Minerva cardioangiologica. Feb 2020;68(1):22-26. doi:10.23736/S0026-4725.19.05052-7 https://www.ncbi.nlm.nih.gov/pubmed/31633315
- Belcaro G, Dugall M, Hosoi M, et al. Pycnogenol(R) and Centella Asiatica for asymptomatic atherosclerosis progression. Int Angiol. Feb 2014;33(1):20-6. https://www.ncbi.nlm.nih.gov/pubmed/24452082
- Belcaro G, Ippolito E, Dugall M, et al. Pycnogenol(R) and Centella asiatica in the management of asymptomatic atherosclerosis progression. Int Angiol. Apr 2015;34(2):150-7. https://www.ncbi.nlm.nih.gov/pubmed/25519846
- Luzzi R, Belcaro G, Ippolito E. Carotid plaque stabilization induced by the supplement association Pycnogenol(R) and centella asiatica (Centellicum(R)). Minerva cardioangiologica. Dec 2016;64(6):603-9. https://www.ncbi.nlm.nih.gov/pubmed/26496510
- Belcaro G, Cornelli U. Variations in Echogenicity in Carotid and Femoral Atherosclerotic Plaques with Pycnogenol + Centella Asiatica Supplementation. The International journal of angiology : official publication of the International College of Angiology, Inc. Jun 2017;26(2):95-101. doi:10.1055/s-0036-1594292 https://www.ncbi.nlm.nih.gov/pubmed/28566935
- Belcaro G, Cesarone MR, Scipione C, et al. Pycnogenol(R)+Centellicum(R), post-stent evaluation: prevention of neointima and plaque re-growth. Minerva cardioangiologica. Dec 2019;67(6):450-455. doi:10.23736/S0026-4725.19.05048-5 https://www.ncbi.nlm.nih.gov/pubmed/31850725
- Benameur T, Frota Gaban SV, Giacomucci G, et al. The Effects of Curcumin on Inflammasome: Latest Update. Molecules. Jan 11 2023;28(2)doi:10.3390/molecules28020742 https://www.ncbi.nlm.nih.gov/pubmed/36677800
- Hadi A, Pourmasoumi M, Ghaedi E, Sahebkar A. The effect of Curcumin/Turmeric on blood pressure modulation: A systematic review and meta-analysis. Pharmacol Res. Dec 2019;150:104505. doi:10.1016/j.phrs.2019.104505 https://www.ncbi.nlm.nih.gov/pubmed/31647981
- Musazadeh V, Roshanravan N, Mohammadizadeh M, Kavyani Z, Dehghan P, Mosharkesh E. Curcumin as a novel approach in improving lipid profile: An umbrella meta-analysis. Nutr Metab Cardiovasc Dis. Nov 2022;32(11):2493-2504. doi:10.1016/j.numecd.2022.07.021 https://www.ncbi.nlm.nih.gov/pubmed/36058763
- Gorabi AM, Abbasifard M, Imani D, et al. Effect of curcumin on C-reactive protein as a biomarker of systemic inflammation: An updated meta-analysis of randomized controlled trials. Phytother Res. Jan 2022;36(1):85-97. doi:10.1002/ptr.7284 https://www.ncbi.nlm.nih.gov/pubmed/34586711
- Changal KH, Khan MS, Bashir R, Sheikh MA. Curcumin Preparations Can Improve Flow-Mediated Dilation and Endothelial Function: A Meta-Analysis. Complement Med Res. 2020;27(4):272-281. Curcuminhaltige Praparate konnen die flussvermittelte Vasodilatation und die Endothelfunktion verbessern: Eine Metaanalyse. doi:10.1159/000506180 https://www.ncbi.nlm.nih.gov/pubmed/32101871
- Hallajzadeh J, Milajerdi A, Kolahdooz F, Amirani E, Mirzaei H, Asemi Z. The effects of curcumin supplementation on endothelial function: A systematic review and meta-analysis of randomized controlled trials. Phytother Res. Nov 2019;33(11):2989-2995. doi:10.1002/ptr.6477 https://www.ncbi.nlm.nih.gov/pubmed/31423626
- Futuhi F, Naghibzadeh Tahami A, Azmandian J, Saber A. The effects of curcumin-containing supplementations on inflammatory markers and lipid profiles in patients with chronic kidney diseases: a systematic review and meta-analysis of randomized controlled trials. Journal of complementary & integrative medicine. Sep 1 2022;19(3):531-541. doi:10.1515/jcim-2022-0082 https://www.ncbi.nlm.nih.gov/pubmed/35649583
- Yang K, Chen J, Zhang T, et al. Efficacy and safety of dietary polyphenol supplementation in the treatment of non-alcoholic fatty liver disease: A systematic review and meta-analysis. Front Immunol. 2022;13:949746. doi:10.3389/fimmu.2022.949746 https://www.ncbi.nlm.nih.gov/pubmed/36159792
- Jalali M, Mahmoodi M, Mosallanezhad Z, Jalali R, Imanieh MH, Moosavian SP. The effects of curcumin supplementation on liver function, metabolic profile and body composition in patients with non-alcoholic fatty liver disease: A systematic review and meta-analysis of randomized controlled trials. Complement Ther Med. Jan 2020;48:102283. doi:10.1016/j.ctim.2019.102283 https://www.ncbi.nlm.nih.gov/pubmed/31987259
- Altobelli E, Angeletti PM, Marziliano C, Mastrodomenico M, Giuliani AR, Petrocelli R. Potential Therapeutic Effects of Curcumin on Glycemic and Lipid Profile in Uncomplicated Type 2 Diabetes-A Meta-Analysis of Randomized Controlled Trial. Nutrients. Jan 27 2021;13(2)doi:10.3390/nu13020404 https://www.ncbi.nlm.nih.gov/pubmed/33514002
- Dastani M, Rahimi HR, Askari VR, et al. Three months of combination therapy with nano-curcumin reduces the inflammation and lipoprotein (a) in type 2 diabetic patients with mild to moderate coronary artery disease: Evidence of a randomized, double-blinded, placebo-controlled clinical trial. Biofactors. Jan 2023;49(1):108-118. doi:10.1002/biof.1874 https://www.ncbi.nlm.nih.gov/pubmed/35674733
- Helli B, Gerami H, Kavianpour M, Heybar H, Hosseini SK, Haghighian HK. Curcumin Nanomicelle Improves Lipid Profile, Stress Oxidative Factors and Inflammatory Markers in Patients Undergoing Coronary Elective Angioplasty; A Randomized Clinical Trial. Endocr Metab Immune Disord Drug Targets. 2021;21(11):2090-2098. doi:10.2174/1871530321666210104145231 https://www.ncbi.nlm.nih.gov/pubmed/33397249
- Santos-Parker JR, Strahler TR, Bassett CJ, Bispham NZ, Chonchol MB, Seals DR. Curcumin supplementation improves vascular endothelial function in healthy middle-aged and older adults by increasing nitric oxide bioavailability and reducing oxidative stress. Aging (Albany NY). Jan 3 2017;9(1):187-208. doi:10.18632/aging.101149 https://www.ncbi.nlm.nih.gov/pubmed/28070018
- Sheng Y, Sun Y, Tang Y, et al. Catechins: Protective mechanism of antioxidant stress in atherosclerosis. Front Pharmacol. 2023;14:1144878. doi:10.3389/fphar.2023.1144878 https://www.ncbi.nlm.nih.gov/pubmed/37033663
- Eng QY, Thanikachalam PV, Ramamurthy S. Molecular understanding of Epigallocatechin gallate (EGCG) in cardiovascular and metabolic diseases. J Ethnopharmacol. Jan 10 2018;210:296-310. doi:10.1016/j.jep.2017.08.035 https://www.ncbi.nlm.nih.gov/pubmed/28864169
- Gu L, Liu X, Wu S, Chu K, Bao JJ. A cross-sectional study on the tea consumption effects of ankle-brachial index. Vascular. Apr 2023;31(2):341-349. doi:10.1177/17085381211064745 https://www.ncbi.nlm.nih.gov/pubmed/34957865
- Wang X, Liu F, Li J, et al. Tea consumption and the risk of atherosclerotic cardiovascular disease and all-cause mortality: The China-PAR project. European journal of preventive cardiology. Dec 2020;27(18):1956-1963. doi:10.1177/2047487319894685 https://www.ncbi.nlm.nih.gov/pubmed/31914807
- Kishimoto Y, Saita E, Taguchi C, et al. Associations between Green Tea Consumption and Coffee Consumption and the Prevalence of Coronary Artery Disease. J Nutr Sci Vitaminol (Tokyo). 2020;66(3):237-245. doi:10.3177/jnsv.66.237 https://www.ncbi.nlm.nih.gov/pubmed/32612086
- Sajjadieh Khajouui AR, Najafian J, Talebzadeh R, Nejati M, Behjati M. The relationship between regular tea drinking and calcification of the coronary arteries. J Cardiovasc Thorac Res. 2022;14(2):95-100. doi:10.34172/jcvtr.2022.12 https://www.ncbi.nlm.nih.gov/pubmed/35935391
- Dower JI, Geleijnse JM, Gijsbers L, Schalkwijk C, Kromhout D, Hollman PC. Supplementation of the Pure Flavonoids Epicatechin and Quercetin Affects Some Biomarkers of Endothelial Dysfunction and Inflammation in (Pre)Hypertensive Adults: A Randomized Double-Blind, Placebo-Controlled, Crossover Trial. J Nutr. Jul 2015;145(7):1459-63. doi:10.3945/jn.115.211888 https://www.ncbi.nlm.nih.gov/pubmed/25972527
- Suzuki-Sugihara N, Kishimoto Y, Saita E, et al. Green tea catechins prevent low-density lipoprotein oxidation via their accumulation in low-density lipoprotein particles in humans. Nutr Res. Jan 2016;36(1):16-23. doi:10.1016/j.nutres.2015.10.012 https://www.ncbi.nlm.nih.gov/pubmed/26773777
- Quezada-Fernandez P, Trujillo-Quiros J, Pascoe-Gonzalez S, et al. Effect of green tea extract on arterial stiffness, lipid profile and sRAGE in patients with type 2 diabetes mellitus: a randomised, double-blind, placebo-controlled trial. Int J Food Sci Nutr. Dec 2019;70(8):977-985. doi:10.1080/09637486.2019.1589430 https://www.ncbi.nlm.nih.gov/pubmed/31084381
- Oyama J, Maeda T, Kouzuma K, et al. Green tea catechins improve human forearm endothelial dysfunction and have antiatherosclerotic effects in smokers. Circ J. Mar 2010;74(3):578-88. doi:10.1253/circj.cj-09-0692 https://www.ncbi.nlm.nih.gov/pubmed/20134098
- Batista Gde A, Cunha CL, Scartezini M, von der Heyde R, Bitencourt MG, Melo SF. Prospective double-blind crossover study of Camellia sinensis (green tea) in dyslipidemias. Arq Bras Cardiol. Aug 2009;93(2):128-34. doi:10.1590/s0066-782x2009000800010 https://www.ncbi.nlm.nih.gov/pubmed/19838489
- Inami S, Takano M, Yamamoto M, et al. Tea catechin consumption reduces circulating oxidized low-density lipoprotein. Int Heart J. Nov 2007;48(6):725-32. doi:10.1536/ihj.48.725 https://www.ncbi.nlm.nih.gov/pubmed/18160764
- Steptoe A, Gibson EL, Vuononvirta R, et al. The effects of chronic tea intake on platelet activation and inflammation: a double-blind placebo controlled trial. Atherosclerosis. Aug 2007;193(2):277-82. doi:10.1016/j.atherosclerosis.2006.08.054 https://www.ncbi.nlm.nih.gov/pubmed/17010979
- Fan D, Liu C, Zhang Z, et al. Progress in the Preclinical and Clinical Study of Resveratrol for Vascular Metabolic Disease. Molecules. Nov 3 2022;27(21)doi:10.3390/molecules27217524 https://www.ncbi.nlm.nih.gov/pubmed/36364370
- Guo S, Zhou Y, Xie X. Resveratrol inhibiting TGF/ERK signaling pathway can improve atherosclerosis: backgrounds, mechanisms and effects. Biomed Pharmacother. Nov 2022;155:113775. doi:10.1016/j.biopha.2022.113775 https://www.ncbi.nlm.nih.gov/pubmed/36271557
- Cheng CK, Luo JY, Lau CW, Chen ZY, Tian XY, Huang Y. Pharmacological basis and new insights of resveratrol action in the cardiovascular system. Br J Pharmacol. Mar 2020;177(6):1258-1277. doi:10.1111/bph.14801 https://www.ncbi.nlm.nih.gov/pubmed/31347157
- Imamura H, Yamaguchi T, Nagayama D, Saiki A, Shirai K, Tatsuno I. Resveratrol Ameliorates Arterial Stiffness Assessed by Cardio-Ankle Vascular Index in Patients With Type 2 Diabetes Mellitus. Int Heart J. Aug 3 2017;58(4):577-583. doi:10.1536/ihj.16-373 https://www.ncbi.nlm.nih.gov/pubmed/28701674
- Tome-Carneiro J, Gonzalvez M, Larrosa M, et al. Consumption of a grape extract supplement containing resveratrol decreases oxidized LDL and ApoB in patients undergoing primary prevention of cardiovascular disease: a triple-blind, 6-month follow-up, placebo-controlled, randomized trial. Mol Nutr Food Res. May 2012;56(5):810-21. doi:10.1002/mnfr.201100673 https://www.ncbi.nlm.nih.gov/pubmed/22648627
- Mahjabeen W, Khan DA, Mirza SA. Role of resveratrol supplementation in regulation of glucose hemostasis, inflammation and oxidative stress in patients with diabetes mellitus type 2: A randomized, placebo-controlled trial. Complement Ther Med. Jun 2022;66:102819. doi:10.1016/j.ctim.2022.102819 https://www.ncbi.nlm.nih.gov/pubmed/35240291
- Mohammadipoor N, Shafiee F, Rostami A, et al. Resveratrol supplementation efficiently improves endothelial health: A systematic review and meta-analysis of randomized controlled trials. Phytother Res. Jul 14 2022;doi:10.1002/ptr.7562
- Akbari M, Tamtaji OR, Lankarani KB, et al. The Effects of Resveratrol Supplementation on Endothelial Function and Blood Pressures Among Patients with Metabolic Syndrome and Related Disorders: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. High blood pressure & cardiovascular prevention : the official journal of the Italian Society of Hypertension. Aug 2019;26(4):305-319. doi:10.1007/s40292-019-00324-6 https://www.ncbi.nlm.nih.gov/pubmed/31264084
- Wong RH, Berry NM, Coates AM, et al. Chronic resveratrol consumption improves brachial flow-mediated dilatation in healthy obese adults. J Hypertens. Sep 2013;31(9):1819-27. doi:10.1097/HJH.0b013e328362b9d6 https://www.ncbi.nlm.nih.gov/pubmed/23743811
- Marques B, Trindade M, Aquino JCF, et al. Beneficial effects of acute trans-resveratrol supplementation in treated hypertensive patients with endothelial dysfunction. Clinical and experimental hypertension (New York, NY : 1993). 2018;40(3):218-223. doi:10.1080/10641963.2017.1288741 https://www.ncbi.nlm.nih.gov/pubmed/29431520
- Chekalina NI. Resveratrol has a positive effect on parameters of central hemodynamics and myocardial ischemia in patients with stable coronary heart disease. Wiadomosci lekarskie (Warsaw, Poland : 1960). 2017;70(2 pt 2):286-291. https://www.ncbi.nlm.nih.gov/pubmed/29059644
- Magyar K, Halmosi R, Palfi A, et al. Cardioprotection by resveratrol: A human clinical trial in patients with stable coronary artery disease. Clinical hemorheology and microcirculation. 2012;50(3):179-87. doi:10.3233/CH-2011-1424 https://www.ncbi.nlm.nih.gov/pubmed/22240353
- Garcia JP, Santana A, Baruqui DL, Suraci N. The Cardiovascular effects of chocolate. Reviews in cardiovascular medicine. Dec 30 2018;19(4):123-127. doi:10.31083/j.rcm.2018.04.3187 https://www.ncbi.nlm.nih.gov/pubmed/31064163
- Sun Y, Zimmermann D, De Castro CA, Actis-Goretta L. Dose-response relationship between cocoa flavanols and human endothelial function: a systematic review and meta-analysis of randomized trials. Food Funct. Oct 16 2019;10(10):6322-6330. doi:10.1039/c9fo01747j https://www.ncbi.nlm.nih.gov/pubmed/31524216
- Flammer AJ, Sudano I, Wolfrum M, et al. Cardiovascular effects of flavanol-rich chocolate in patients with heart failure. Eur Heart J. Sep 2012;33(17):2172-80. doi:10.1093/eurheartj/ehr448 https://www.ncbi.nlm.nih.gov/pubmed/22173910
- Bapir M, Untracht GR, Cooke D, et al. Cocoa flavanol consumption improves lower extremity endothelial function in healthy individuals and people with type 2 diabetes. Food Funct. Oct 17 2022;13(20):10439-10448. doi:10.1039/d2fo02017c https://www.ncbi.nlm.nih.gov/pubmed/36164983
- Grone M, Sansone R, Hoffken P, et al. Cocoa Flavanols Improve Endothelial Functional Integrity in Healthy Young and Elderly Subjects. J Agric Food Chem. Feb 19 2020;68(7):1871-1876. doi:10.1021/acs.jafc.9b02251 https://www.ncbi.nlm.nih.gov/pubmed/31294557
- West SG, McIntyre MD, Piotrowski MJ, et al. Effects of dark chocolate and cocoa consumption on endothelial function and arterial stiffness in overweight adults. Br J Nutr. Feb 2014;111(4):653-61. doi:10.1017/S0007114513002912 https://www.ncbi.nlm.nih.gov/pubmed/24274771
- Sesso HD, Manson JE, Aragaki AK, et al. Effect of cocoa flavanol supplementation for the prevention of cardiovascular disease events: the COcoa Supplement and Multivitamin Outcomes Study (COSMOS) randomized clinical trial. Am J Clin Nutr. Jun 7 2022;115(6):1490-1500. doi:10.1093/ajcn/nqac055 https://www.ncbi.nlm.nih.gov/pubmed/35294962
- Ramli NNS, Alkhaldy AA, Mhd Jalil AM. Effects of Caffeinated and Decaffeinated Coffee Consumption on Metabolic Syndrome Parameters: A Systematic Review and Meta-Analysis of Data from Randomised Controlled Trials. Medicina (Kaunas). Sep 11 2021;57(9)doi:10.3390/medicina57090957 https://www.ncbi.nlm.nih.gov/pubmed/34577880
- Pourmasoumi M, Hadi A, Marx W, Najafgholizadeh A, Kaur S, Sahebkar A. The Effect of Green Coffee Bean Extract on Cardiovascular Risk Factors: A Systematic Review and Meta-analysis. Adv Exp Med Biol. 2021;1328:323-345. doi:10.1007/978-3-030-73234-9_21 https://www.ncbi.nlm.nih.gov/pubmed/34981487
- Ding F, Ma B, Nazary-Vannani A, et al. The effects of green coffee bean extract supplementation on lipid profile in humans: A systematic review and meta-analysis of randomized controlled trials. Nutr Metab Cardiovasc Dis. Jan 3 2020;30(1):1-10. doi:10.1016/j.numecd.2019.10.002 https://www.ncbi.nlm.nih.gov/pubmed/31748178
- Asbaghi O, Sadeghian M, Nasiri M, et al. The effects of green coffee extract supplementation on glycemic indices and lipid profile in adults: a systematic review and dose-response meta-analysis of clinical trials. Nutr J. Jul 14 2020;19(1):71. doi:10.1186/s12937-020-00587-z https://www.ncbi.nlm.nih.gov/pubmed/32665012
- Han B, Nazary-Vannani A, Talaei S, et al. The effect of green coffee extract supplementation on blood pressure: A systematic review and meta-analysis of randomized controlled trials. Phytother Res. Nov 2019;33(11):2918-2926. doi:10.1002/ptr.6481 https://www.ncbi.nlm.nih.gov/pubmed/31429515
- Suzuki A, Nomura T, Jokura H, Kitamura N, Saiki A, Fujii A. Chlorogenic acid-enriched green coffee bean extract affects arterial stiffness assessed by the cardio-ankle vascular index in healthy men: a pilot study. Int J Food Sci Nutr. Nov 2019;70(7):901-908. doi:10.1080/09637486.2019.1585763 https://www.ncbi.nlm.nih.gov/pubmed/30907200
- Nie F, Liu L, Cui J, et al. Oligomeric Proanthocyanidins: An Updated Review of Their Natural Sources, Synthesis, and Potentials. Antioxidants (Basel). Apr 26 2023;12(5)doi:10.3390/antiox12051004 https://www.ncbi.nlm.nih.gov/pubmed/37237870
- Rodriguez-Perez C, Garcia-Villanova B, Guerra-Hernandez E, Verardo V. Grape Seeds Proanthocyanidins: An Overview of In Vivo Bioactivity in Animal Models. Nutrients. Oct 12 2019;11(10)doi:10.3390/nu11102435 https://www.ncbi.nlm.nih.gov/pubmed/31614852
- Bagchi D, Bagchi M, Stohs SJ, et al. Free radicals and grape seed proanthocyanidin extract: importance in human health and disease prevention. Toxicology. Aug 7 2000;148(2-3):187-97. doi:10.1016/s0300-483x(00)00210-9 https://www.ncbi.nlm.nih.gov/pubmed/10962138
- Cao AH, Wang J, Gao HQ, Zhang P, Qiu J. Beneficial clinical effects of grape seed proanthocyanidin extract on the progression of carotid atherosclerotic plaques. Journal of geriatric cardiology : JGC. Jul 2015;12(4):417-23. doi:10.11909/j.issn.1671-5411.2015.04.014 https://www.ncbi.nlm.nih.gov/pubmed/26345394
- Odai T, Terauchi M, Kato K, Hirose A, Miyasaka N. Effects of Grape Seed Proanthocyanidin Extract on Vascular Endothelial Function in Participants with Prehypertension: A Randomized, Double-Blind, Placebo-Controlled Study. Nutrients. Nov 20 2019;11(12)doi:10.3390/nu11122844 https://www.ncbi.nlm.nih.gov/pubmed/31757033
- Olechno E, Puscion-Jakubik A, Zujko ME. Chokeberry (A. melanocarpa (Michx.) Elliott)-A Natural Product for Metabolic Disorders? Nutrients. Jun 28 2022;14(13)doi:10.3390/nu14132688 https://www.ncbi.nlm.nih.gov/pubmed/35807867
- Kasprzak-Drozd K, Oniszczuk T, Soja J, et al. The Efficacy of Black Chokeberry Fruits against Cardiovascular Diseases. Int J Mol Sci. Jun 18 2021;22(12)doi:10.3390/ijms22126541 https://www.ncbi.nlm.nih.gov/pubmed/34207143
- Tasic N, Jakovljevic VLJ, Mitrovic M, et al. Black chokeberry Aronia melanocarpa extract reduces blood pressure, glycemia and lipid profile in patients with metabolic syndrome: a prospective controlled trial. Mol Cell Biochem. Jul 2021;476(7):2663-2673. doi:10.1007/s11010-021-04106-4 https://www.ncbi.nlm.nih.gov/pubmed/33666827
- Le Sayec M, Xu Y, Laiola M, et al. The effects of Aronia berry (poly)phenol supplementation on arterial function and the gut microbiome in middle aged men and women: Results from a randomized controlled trial. Clin Nutr. Nov 2022;41(11):2549-2561. doi:10.1016/j.clnu.2022.08.024 https://www.ncbi.nlm.nih.gov/pubmed/36228567
- Istas G, Wood E, Le Sayec M, et al. Effects of aronia berry (poly)phenols on vascular function and gut microbiota: a double-blind randomized controlled trial in adult men. Am J Clin Nutr. Aug 1 2019;110(2):316-329. doi:10.1093/ajcn/nqz075 https://www.ncbi.nlm.nih.gov/pubmed/31152545
- Hawkins J, Hires C, Baker C, Keenan L, Bush M. Daily supplementation with aronia melanocarpa (chokeberry) reduces blood pressure and cholesterol: a meta analysis of controlled clinical trials. J Diet Suppl. 2021;18(5):517-530. doi:10.1080/19390211.2020.1800887 https://www.ncbi.nlm.nih.gov/pubmed/32794414
- Sangild J, Faldborg A, Schousboe C, et al. Effects of Chokeberries (Aronia spp.) on Cytoprotective and Cardiometabolic Markers and Semen Quality in 109 Mildly Hypercholesterolemic Danish Men: A Prospective, Double-Blinded, Randomized, Crossover Trial. J Clin Med. Jan 3 2023;12(1)doi:10.3390/jcm12010373 https://www.ncbi.nlm.nih.gov/pubmed/36615174
- Pokimica B, Garcia-Conesa MT, Zec M, et al. Chokeberry Juice Containing Polyphenols Does Not Affect Cholesterol or Blood Pressure but Modifies the Composition of Plasma Phospholipids Fatty Acids in Individuals at Cardiovascular Risk. Nutrients. Apr 15 2019;11(4)doi:10.3390/nu11040850 https://www.ncbi.nlm.nih.gov/pubmed/30991718
- Mehmood A, Usman M, Patil P, Zhao L, Wang C. A review on management of cardiovascular diseases by olive polyphenols. Food science & nutrition. Sep 2020;8(9):4639-4655. doi:10.1002/fsn3.1668 https://www.ncbi.nlm.nih.gov/pubmed/32994927
- D'Angelo C, Franceschelli S, Quiles JL, Speranza L. Wide Biological Role of Hydroxytyrosol: Possible Therapeutic and Preventive Properties in Cardiovascular Diseases. Cells. Aug 21 2020;9(9)doi:10.3390/cells9091932 https://www.ncbi.nlm.nih.gov/pubmed/32825589
- Vijakumaran U, Shanmugam J, Heng JW, et al. Effects of Hydroxytyrosol in Endothelial Functioning: A Comprehensive Review. Molecules. Feb 16 2023;28(4)doi:10.3390/molecules28041861 https://www.ncbi.nlm.nih.gov/pubmed/36838850
- Noguera-Navarro C, Montoro-Garcia S, Orenes-Pinero E. Hydroxytyrosol: Its role in the prevention of cardiovascular diseases. Heliyon. Jan 2023;9(1):e12963. doi:10.1016/j.heliyon.2023.e12963 https://www.ncbi.nlm.nih.gov/pubmed/36704293
- Ikonomidis I, Katogiannis K, Chania C, et al. Association of hydroxytyrosol enriched olive oil with vascular function in chronic coronary disease. Eur J Clin Invest. Jul 2023;53(7):e13983. doi:10.1111/eci.13983 https://www.ncbi.nlm.nih.gov/pubmed/36912212
- Lockyer S, Rowland I, Spencer JPE, Yaqoob P, Stonehouse W. Impact of phenolic-rich olive leaf extract on blood pressure, plasma lipids and inflammatory markers: a randomised controlled trial. Eur J Nutr. Jun 2017;56(4):1421-1432. doi:10.1007/s00394-016-1188-y https://www.ncbi.nlm.nih.gov/pubmed/26951205
- Pla-Paga L, Companys J, Calderon-Perez L, et al. Effects of hesperidin consumption on cardiovascular risk biomarkers: a systematic review of animal studies and human randomized clinical trials. Nutr Rev. Dec 1 2019;77(12):845-864. doi:10.1093/nutrit/nuz036 https://www.ncbi.nlm.nih.gov/pubmed/31271436
- Mas-Capdevila A, Teichenne J, Domenech-Coca C, et al. Effect of Hesperidin on Cardiovascular Disease Risk Factors: The Role of Intestinal Microbiota on Hesperidin Bioavailability. Nutrients. May 20 2020;12(5)doi:10.3390/nu12051488 https://www.ncbi.nlm.nih.gov/pubmed/32443766
- Valls RM, Pedret A, Calderon-Perez L, et al. Effects of hesperidin in orange juice on blood and pulse pressures in mildly hypertensive individuals: a randomized controlled trial (Citrus study). Eur J Nutr. Apr 2021;60(3):1277-1288. doi:10.1007/s00394-020-02279-0 https://www.ncbi.nlm.nih.gov/pubmed/32661681
- Maruhashi T, Kajikawa M, Kishimoto S, et al. Diagnostic Criteria of Flow-Mediated Vasodilation for Normal Endothelial Function and Nitroglycerin-Induced Vasodilation for Normal Vascular Smooth Muscle Function of the Brachial Artery. J Am Heart Assoc. Jan 21 2020;9(2):e013915. doi:10.1161/JAHA.119.013915 https://www.ncbi.nlm.nih.gov/pubmed/31910779
- Salden BN, Troost FJ, de Groot E, et al. Randomized clinical trial on the efficacy of hesperidin 2S on validated cardiovascular biomarkers in healthy overweight individuals. Am J Clin Nutr. Dec 2016;104(6):1523-1533. doi:10.3945/ajcn.116.136960 https://www.ncbi.nlm.nih.gov/pubmed/27797708
- Morand C, Dubray C, Milenkovic D, et al. Hesperidin contributes to the vascular protective effects of orange juice: a randomized crossover study in healthy volunteers. Am J Clin Nutr. Jan 2011;93(1):73-80. doi:10.3945/ajcn.110.004945 https://www.ncbi.nlm.nih.gov/pubmed/21068346
- Rizza S, Muniyappa R, Iantorno M, et al. Citrus polyphenol hesperidin stimulates production of nitric oxide in endothelial cells while improving endothelial function and reducing inflammatory markers in patients with metabolic syndrome. J Clin Endocrinol Metab. May 2011;96(5):E782-92. doi:10.1210/jc.2010-2879 https://www.ncbi.nlm.nih.gov/pubmed/21346065
- Simpson T, Kure C, Stough C. Assessing the Efficacy and Mechanisms of Pycnogenol((R)) on Cognitive Aging From In Vitro Animal and Human Studies. Front Pharmacol. 2019;10:694. doi:10.3389/fphar.2019.00694 https://www.ncbi.nlm.nih.gov/pubmed/31333448
- Nattagh-Eshtivani E, Gheflati A, Barghchi H, et al. The role of Pycnogenol in the control of inflammation and oxidative stress in chronic diseases: Molecular aspects. Phytother Res. Jun 2022;36(6):2352-2374. doi:10.1002/ptr.7454 https://www.ncbi.nlm.nih.gov/pubmed/35583807
- Enseleit F, Sudano I, Periat D, et al. Effects of Pycnogenol on endothelial function in patients with stable coronary artery disease: a double-blind, randomized, placebo-controlled, cross-over study. Eur Heart J. Jul 2012;33(13):1589-97. doi:10.1093/eurheartj/ehr482 https://www.ncbi.nlm.nih.gov/pubmed/22240497
- Nishioka K, Hidaka T, Nakamura S, et al. Pycnogenol, French maritime pine bark extract, augments endothelium-dependent vasodilation in humans. Hypertension research : official journal of the Japanese Society of Hypertension. Sep 2007;30(9):775-80. doi:10.1291/hypres.30.775 https://www.ncbi.nlm.nih.gov/pubmed/18037769
- Tomou EM, Papakyriakopoulou P, Skaltsa H, Valsami G, Kadoglou NPE. Bio-Actives from Natural Products with Potential Cardioprotective Properties: Isolation, Identification, and Pharmacological Actions of Apigenin, Quercetin, and Silibinin. Molecules. Mar 5 2023;28(5)doi:10.3390/molecules28052387 https://www.ncbi.nlm.nih.gov/pubmed/36903630
- Terao J. Potential Role of Quercetin Glycosides as Anti-Atherosclerotic Food-Derived Factors for Human Health. Antioxidants (Basel). Jan 23 2023;12(2)doi:10.3390/antiox12020258 https://www.ncbi.nlm.nih.gov/pubmed/36829817
- Papakyriakopoulou P, Velidakis N, Khattab E, Valsami G, Korakianitis I, Kadoglou NP. Potential Pharmaceutical Applications of Quercetin in Cardiovascular Diseases. Pharmaceuticals (Basel). Aug 18 2022;15(8)doi:10.3390/ph15081019 https://www.ncbi.nlm.nih.gov/pubmed/36015169
- Brull V, Burak C, Stoffel-Wagner B, et al. Effects of a quercetin-rich onion skin extract on 24 h ambulatory blood pressure and endothelial function in overweight-to-obese patients with (pre-)hypertension: a randomised double-blinded placebo-controlled cross-over trial. Br J Nutr. Oct 28 2015;114(8):1263-77. doi:10.1017/S0007114515002950 https://www.ncbi.nlm.nih.gov/pubmed/26328470
- Egert S, Bosy-Westphal A, Seiberl J, et al. Quercetin reduces systolic blood pressure and plasma oxidised low-density lipoprotein concentrations in overweight subjects with a high-cardiovascular disease risk phenotype: a double-blinded, placebo-controlled cross-over study. Br J Nutr. Oct 2009;102(7):1065-74. doi:10.1017/S0007114509359127 https://www.ncbi.nlm.nih.gov/pubmed/19402938
- Hanna M, Jaqua E, Nguyen V, Clay J. B Vitamins: Functions and Uses in Medicine. Perm J. Jun 29 2022;26(2):89-97. doi:10.7812/TPP/21.204 https://www.ncbi.nlm.nih.gov/pubmed/35933667
- Zhang B, Dong H, Xu Y, Xu D, Sun H, Han L. Associations of dietary folate, vitamin B6 and B12 intake with cardiovascular outcomes in 115664 participants: a large UK population-based cohort. Eur J Clin Nutr. Mar 2023;77(3):299-307. doi:10.1038/s41430-022-01206-2 https://www.ncbi.nlm.nih.gov/pubmed/36100703
- Bo Y, Xu H, Zhang H, et al. Intakes of Folate, Vitamin B6, and Vitamin B12 in Relation to All-Cause and Cause-Specific Mortality: A National Population-Based Cohort. Nutrients. May 27 2022;14(11)doi:10.3390/nu14112253 https://www.ncbi.nlm.nih.gov/pubmed/35684053
- Talikoti P, Bobby Z, Hamide A. Supplementation of Water-Soluble Vitamins Reduces Hyperhomocysteinemia, Insulin Resistance, and High-Sensitivity C-reactive Protein in Prehypertension Subjects. Cureus. Jan 2023;15(1):e33481. doi:10.7759/cureus.33481 https://www.ncbi.nlm.nih.gov/pubmed/36751256
- Menzel D, Haller H, Wilhelm M, Robenek H. L-Arginine and B vitamins improve endothelial function in subjects with mild to moderate blood pressure elevation. journal article. Eur J Nutr. Mar 2018;57(2):557-568. doi:10.1007/s00394-016-1342-6 https://www.ncbi.nlm.nih.gov/pubmed/27817128
- Higdon J. Vitamin E. Oregon State University. Linus Pauling Institute. Micronutrient Information Center. Data on file.
- Han J, Zhao C, Cai J, Liang Y. Comparative efficacy of vitamin supplements on prevention of major cardiovascular disease: Systematic review with network meta-analysis. Complement Ther Clin Pract. May 2020;39:101142. doi:10.1016/j.ctcp.2020.101142 https://www.ncbi.nlm.nih.gov/pubmed/32379630
- Schwingshackl L, Boeing H, Stelmach-Mardas M, et al. Dietary Supplements and Risk of Cause-Specific Death, Cardiovascular Disease, and Cancer: A Systematic Review and Meta-Analysis of Primary Prevention Trials. Adv Nutr. Jan 2017;8(1):27-39. doi:10.3945/an.116.013516 https://www.ncbi.nlm.nih.gov/pubmed/28096125
- Ashor AW, Siervo M, Lara J, Oggioni C, Afshar S, Mathers JC. Effect of vitamin C and vitamin E supplementation on endothelial function: a systematic review and meta-analysis of randomised controlled trials. Br J Nutr. Apr 28 2015;113(8):1182-94. doi:10.1017/S0007114515000227 https://www.ncbi.nlm.nih.gov/pubmed/25919436
- Mathur P, Ding Z, Saldeen T, Mehta JL. Tocopherols in the Prevention and Treatment of Atherosclerosis and Related Cardiovascular Disease. Clin Cardiol. Sep 2015;38(9):570-6. doi:10.1002/clc.22422 https://www.ncbi.nlm.nih.gov/pubmed/26272221
- Hariri E, Kassis N, Iskandar JP, et al. Vitamin K(2)-a neglected player in cardiovascular health: a narrative review. Open Heart. Nov 2021;8(2)doi:10.1136/openhrt-2021-001715 https://www.ncbi.nlm.nih.gov/pubmed/34785587
- Poterucha TJ, Goldhaber SZ. Warfarin and Vascular Calcification. Am J Med. Jun 2016;129(6):635 e1-4. doi:10.1016/j.amjmed.2015.11.032 https://www.ncbi.nlm.nih.gov/pubmed/26714212
- Kosciuszek ND, Kalta D, Singh M, Savinova OV. Vitamin K antagonists and cardiovascular calcification: A systematic review and meta-analysis. Systematic Review. Front Cardiovasc Med. 2022-August-19 2022;9:938567. doi:10.3389/fcvm.2022.938567 https://www.ncbi.nlm.nih.gov/pubmed/36061545
- Han KH, O'Neill WC. Increased Peripheral Arterial Calcification in Patients Receiving Warfarin. J Am Heart Assoc. Jan 25 2016;5(1)doi:10.1161/jaha.115.002665 http://jaha.ahajournals.org/content/ahaoa/5/1/e002665.full.pdf
- Chatrou ML, Winckers K, Hackeng TM, Reutelingsperger CP, Schurgers LJ. Vascular calcification: the price to pay for anticoagulation therapy with vitamin K-antagonists. Blood Rev. Jul 2012;26(4):155-66. doi:10.1016/j.blre.2012.03.002 https://www.ncbi.nlm.nih.gov/pubmed/22520397
- Eelderink C, Kremer D, Riphagen IJ, et al. Effect of vitamin K supplementation on serum calcification propensity and arterial stiffness in vitamin K-deficient kidney transplant recipients: A double-blind, randomized, placebo-controlled clinical trial. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. Apr 2023;23(4):520-530. doi:10.1016/j.ajt.2022.12.015 https://www.ncbi.nlm.nih.gov/pubmed/36695702
- Brandenburg VM, Reinartz S, Kaesler N, et al. Slower Progress of Aortic Valve Calcification With Vitamin K Supplementation: Results From a Prospective Interventional Proof-of-Concept Study. Circulation. May 23 2017;135(21):2081-2083. doi:10.1161/CIRCULATIONAHA.116.027011 https://www.ncbi.nlm.nih.gov/pubmed/28533322
- Shea MK, O'Donnell CJ, Hoffmann U, et al. Vitamin K supplementation and progression of coronary artery calcium in older men and women. Am J Clin Nutr. Jun 2009;89(6):1799-807. doi:10.3945/ajcn.2008.27338 https://www.ncbi.nlm.nih.gov/pubmed/19386744
- Chen HG, Sheng LT, Zhang YB, et al. Association of vitamin K with cardiovascular events and all-cause mortality: a systematic review and meta-analysis. Eur J Nutr. Sep 2019;58(6):2191-2205. doi:10.1007/s00394-019-01998-3 https://www.ncbi.nlm.nih.gov/pubmed/31119401
- Shea MK, Barger K, Booth SL, et al. Vitamin K status, cardiovascular disease, and all-cause mortality: a participant-level meta-analysis of 3 US cohorts. Am J Clin Nutr. Jun 1 2020;111(6):1170-1177. doi:10.1093/ajcn/nqaa082 https://www.ncbi.nlm.nih.gov/pubmed/32359159
- Schultz CJ, Dalgaard F, Bellinge JW, et al. Dietary Vitamin K(1) Intake and Incident Aortic Valve Stenosis. Arterioscler Thromb Vasc Biol. Feb 2024;44(2):513-521. doi:10.1161/ATVBAHA.123.320271 https://www.ncbi.nlm.nih.gov/pubmed/38152887
- Vlasschaert C, Goss CJ, Pilkey NG, McKeown S, Holden RM. Vitamin K Supplementation for the Prevention of Cardiovascular Disease: Where Is the Evidence? A Systematic Review of Controlled Trials. Nutrients. Sep 23 2020;12(10)doi:10.3390/nu12102909 https://www.ncbi.nlm.nih.gov/pubmed/32977548
- Diederichsen ACP, Lindholt JS, Moller S, et al. Vitamin K2 and D in Patients With Aortic Valve Calcification: A Randomized Double-Blinded Clinical Trial. Circulation. May 3 2022;145(18):1387-1397. doi:10.1161/CIRCULATIONAHA.121.057008 https://www.ncbi.nlm.nih.gov/pubmed/35465686
- Hasific S, Oevrehus KA, Lindholt JS, et al. Effects of Vitamin K2 and D Supplementation on Coronary Artery Disease in Men: A RCT. JACC Adv. Nov 2023;2(9):100643. doi:10.1016/j.jacadv.2023.100643 https://www.ncbi.nlm.nih.gov/pubmed/38938724
- Higdon J. Vitamin C. Oregon State University. Linus Pauling Institute. Micronutrient Information Center. Data on file.
- Ashor AW, Lara J, Mathers JC, Siervo M. Effect of vitamin C on endothelial function in health and disease: a systematic review and meta-analysis of randomised controlled trials. Atherosclerosis. Jul 2014;235(1):9-20. doi:10.1016/j.atherosclerosis.2014.04.004 https://www.ncbi.nlm.nih.gov/pubmed/24792921
- Moser MA, Chun OK. Vitamin C and Heart Health: A Review Based on Findings from Epidemiologic Studies. Int J Mol Sci. Aug 12 2016;17(8)doi:10.3390/ijms17081328 https://www.ncbi.nlm.nih.gov/pubmed/27529239
- Desouza C, Chatterjee R, Vickery EM, et al. The effect of vitamin D supplementation on cardiovascular risk in patients with prediabetes: A secondary analysis of the D2d study. Journal of diabetes and its complications. Aug 2022;36(8):108230. doi:10.1016/j.jdiacomp.2022.108230 https://www.ncbi.nlm.nih.gov/pubmed/35753926
- Virtanen JK, Nurmi T, Aro A, et al. Vitamin D supplementation and prevention of cardiovascular disease and cancer in the Finnish Vitamin D Trial: a randomized controlled trial. Am J Clin Nutr. May 1 2022;115(5):1300-1310. doi:10.1093/ajcn/nqab419 https://www.ncbi.nlm.nih.gov/pubmed/34982819
- Bischoff-Ferrari HA, Vellas B, Rizzoli R, et al. Effect of Vitamin D Supplementation, Omega-3 Fatty Acid Supplementation, or a Strength-Training Exercise Program on Clinical Outcomes in Older Adults: The DO-HEALTH Randomized Clinical Trial. JAMA. Nov 10 2020;324(18):1855-1868. doi:10.1001/jama.2020.16909 https://www.ncbi.nlm.nih.gov/pubmed/33170239
- Rist PM, Buring JE, Cook NR, Manson JE, Rexrode KM. Effect of vitamin D and/or omega-3 fatty acid supplementation on stroke outcomes: A randomized trial. European journal of neurology : the official journal of the European Federation of Neurological Societies. Mar 2021;28(3):809-815. doi:10.1111/ene.14623 https://www.ncbi.nlm.nih.gov/pubmed/33131164
- Barbagallo M, Veronese N, Dominguez LJ. Magnesium in Aging, Health and Diseases. Nutrients. Jan 30 2021;13(2)doi:10.3390/nu13020463 https://www.ncbi.nlm.nih.gov/pubmed/33573164
- Kostov K, Halacheva L. Role of Magnesium Deficiency in Promoting Atherosclerosis, Endothelial Dysfunction, and Arterial Stiffening as Risk Factors for Hypertension. Int J Mol Sci. Jun 11 2018;19(6)doi:10.3390/ijms19061724 https://www.ncbi.nlm.nih.gov/pubmed/29891771
- Rooney MR, Alonso A, Folsom AR, et al. Serum magnesium and the incidence of coronary artery disease over a median 27 years of follow-up in the Atherosclerosis Risk in Communities (ARIC) Study and a meta-analysis. Am J Clin Nutr. Jan 1 2020;111(1):52-60. doi:10.1093/ajcn/nqz256 https://www.ncbi.nlm.nih.gov/pubmed/31622458
- Sun X, Zhuang X, Huo M, et al. Serum magnesium and the prevalence of peripheral artery disease: The Atherosclerosis Risk in Communities (ARIC) study. Atherosclerosis. Mar 2019;282:196-201. doi:10.1016/j.atherosclerosis.2018.12.004 https://www.ncbi.nlm.nih.gov/pubmed/30658844
- Zhao L, Hu M, Yang L, et al. Quantitative Association Between Serum/Dietary Magnesium and Cardiovascular Disease/Coronary Heart Disease Risk: A Dose-Response Meta-analysis of Prospective Cohort Studies. Journal of cardiovascular pharmacology. Dec 2019;74(6):516-527. doi:10.1097/FJC.0000000000000739 https://www.ncbi.nlm.nih.gov/pubmed/31815866
- Cambray S, Ibarz M, Bermudez-Lopez M, et al. Magnesium Levels Modify the Effect of Lipid Parameters on Carotid Intima Media Thickness. Nutrients. Aug 28 2020;12(9)doi:10.3390/nu12092631 https://www.ncbi.nlm.nih.gov/pubmed/32872319
- Farshidi H, Sobhani AR, Eslami M, et al. Magnesium Sulfate Administration in Moderate Coronary Artery Disease Patients Improves Atherosclerotic Risk Factors: A Double-Blind Clinical Trial Study. Journal of cardiovascular pharmacology. Sep 2020;76(3):321-328. doi:10.1097/FJC.0000000000000874 https://www.ncbi.nlm.nih.gov/pubmed/32618829
- Sobhani AR, Farshidi H, Azarkish F, et al. Magnesium Sulfate Improves Some Risk Factors for Atherosclerosis in Patients Suffering from One or Two Coronary Artery Diseases: A Double-blind Clinical Trial Study. Clinical pharmacology : advances and applications. 2020;12:159-169. doi:10.2147/CPAA.S261264 https://www.ncbi.nlm.nih.gov/pubmed/33061673
- Mohebi F, Ostadhadi S, Vaziri MS, et al. The effect of magnesium sulfate on gene expression and serum level of inflammatory cytokines in coronary artery disease patients. Inflammopharmacol. Oct 2023;31(5):2421-2430. doi:10.1007/s10787-023-01328-4 https://www.ncbi.nlm.nih.gov/pubmed/37665448
- Darooghegi Mofrad M, Djafarian K, Mozaffari H, Shab-Bidar S. Effect of magnesium supplementation on endothelial function: A systematic review and meta-analysis of randomized controlled trials. Atherosclerosis. Jun 2018;273:98-105. doi:10.1016/j.atherosclerosis.2018.04.020 https://www.ncbi.nlm.nih.gov/pubmed/29709832
- Schutten JC, Joris PJ, Groendijk I, et al. Effects of Magnesium Citrate, Magnesium Oxide, and Magnesium Sulfate Supplementation on Arterial Stiffness: A Randomized, Double-Blind, Placebo-Controlled Intervention Trial. J Am Heart Assoc. Mar 15 2022;11(6):e021783. doi:10.1161/JAHA.121.021783 https://www.ncbi.nlm.nih.gov/pubmed/35253448




