Life Extension Magazine®

My editorial in the May 2013 issue of this publication generated quite a bit of feedback and critique.
Some Life Extension® members said it should be a mandatory part of physician education. Others raised concerns about the use of the PSA blood test as a screening tool, why I suggest Avodart® for certain men, and why drugs were mentioned since there are nutrients that function via similar mechanisms.
The most impressive critique came from Patrick C. Walsh, MD, who may be the most renowned prostate cancer expert in the world. Dr. Walsh was involved in identifying the genetic characteristic of hereditary prostate cancer and pioneered “nerve-sparing” surgery. I have urged hundreds of prostate cancer patients to travel to Johns Hopkins to have Patrick Walsh perform their surgery, as I consider him the finest in the world.

So when Dr. Walsh writes us, I pay attention, and Life Extension members should be informed that there are credentialed individuals that are against using drugs in the class of Avodart® for cancer prevention purposes.
Shortly after my editorial was published, the American Urological Association issued revised guidelines for PSA screening. They now say PSA screening should be mostly considered only for men aged 55-69.1 We vehemently disagree with this new recommendation and chastise this group for not emphasizing the need to devise safer and more efficient ways of performing prostate diagnostics.
To emphasize the seriousness of all this, the chart on this page shows the spiraling incidence of prostate cancer that occurs as men age. Autopsy results reveal that 85% of men have atypical cells in their prostate glands and 1 in 4 has cancer.2 While many men with atypical lesions or even malignant cells in their prostate do not ever progress to clinical disease, aging men cannot ignore this problem.
The public still accepts absurdly short life spans. We at Life Extension do not and that is just one reason why our position on prostate cancer differs from the mainstream.
There is something to be said about attending live lectures as opposed to staying glued to our computer/TV screens. A good speaker can make an impact that you may forever remember.

I’ll never forget a lecture I attended in 1977 at a South Florida condominium social hall. The place was packed with retirees. The lecturer was over age 80 and passionately urged all men to visit a urologist once a year for a digital rectal exam. He began by reading a long list of the names of the many members of his retirement community who had suffered agonizing deaths from metastatic prostate cancer.
The lecturer understood that a digital rectal exam would not detect all prostate cancers, but he knew it could save lives. If the PSA blood test had been available at that time, I can only imagine how feverish this benevolent speaker would have been in advocating PSA tests to his fellow men.
Move forward 35 years and the federal government and some mainstream medical groups are recommending against PSA screening, which is more reliable than digital rectal exams, though both ideally should be done annually.1,3
What Makes Prostate Cancer Different?
Prostate cancer is unusual in that it has a blood marker called prostate-specific antigen (PSA) that can facilitate early detection, thereby enabling therapies to be employed before cancer spreads to regional lymph nodes or distant metastases occur.4
With the advent and widespread use of PSA screening, an argument can be made based on a large human study that huge numbers of men could be spared agonizing deaths from metastatic prostate cancer.5,6 The earlier diagnosis of prostate cancer, however, must be put into context of the individual patient to ascertain which men need to be treated and which men are reasonable candidates for active surveillance or “watchful waiting.”
The journal European Urology published a study in 2013 conducted on nearly 35,000 men aged 55-69.5 This data came from the European Randomized Study of Screening for Prostate Cancer, a major, robust study examining the impact of PSA screening over a median period of 13 years on prostate cancer mortality. The eye-opening conclusion was that men who underwent repeated PSA screening were 51% less likely to die from prostate cancer than men who did not undergo screening. 5 If the statistics from this study are applied to the entire population of men aged 55-69 in the United States, PSA screening could potentially save over 80,000 lives in a 13-year period.6
The United States Preventive Services Task Force (USPSTF) published a report in 2012 recommending that men stop undergoing PSA screening.3
Life Extension disagreed with the USPSTF recommendation, particularly as it relates to our members to whom we are steadfastly committed. We know that in the absence of PSA screening, prostate cancer will once again be diagnosed at an advanced stage, when there is painful bulky disease and only a small chance of curative therapy.
The widespread use of PSA testing beginning in 1987 enabled doctors to identify prostate cancer at a greatly reduced stage of disease.7 If the dictum of the USPSTF is followed, a major advance in medicine will be erased.
The Staggering Statistics
Here is what the American Cancer Society says about prostate cancer in the United States:8
- About 238,590 new cases of prostate cancer will be diagnosed in 2013.
- About 29,720 men will die of prostate cancer in 2013.
- About 1 man in 6 will be diagnosed with prostate cancer during his lifetime.
- The average age at diagnosis is 67.
- Prostate cancer is the second leading cause of cancer death among American men.
- About 1 man in 36 will die from prostate cancer.
If prostate cancer were an infectious illness, there would be widespread panic. To put this in perspective, HIV infected less than 50,000 Americans in 2011.9
In 2013, the United States Preventive Services Task Force urged all Americans to undergo routine HIV screening.10

There are valid reasons for HIV screening, but almost five times more Americans are diagnosed with prostate cancer each year compared to HIV.8,9 The same government-funded Task Force that suggests universal HIV screening does not want aging men to benefit from early detection of prostate cancer. They maintain that the treatment is worse than the disease. They confuse the message conveyed by the PSA with the judgment and actions of physicians who too often are programmed toward invasive and expensive therapies.
Do we toss out the baby with the bath water, so to speak, because physicians are not taking the time, or possibly do not have the expertise to advise patients soundly? The actions of the USPSTF and the American Urological Association should be to fix the deficiency of the physician with strict guidelines, just as was done in the 1980s to alter the routine use of the radical mastectomy performed in almost every woman diagnosed with breast cancer.11
The United States Preventive Services Task Force (USPSTF) prefers aging men wallow in ignorance concerning their prostate health, which within the next decade will send death rates spiraling upwards. The USPSTF clearly wants aging men to bury their heads in the sand and not concern themselves about prostate cancer.
The hard statistics showing more than 238,000 newly diagnosed prostate cancer cases annually proves otherwise.8 While the USPTF recommendations will save government health programs billions of dollars in the short term, there will be catastrophic long term costs to pay when record numbers of men who could have been cured instead develop metastatic disease.
Why Life Extension Members Are Different
There are factors that influence mainstream recommendations that do not pertain to Life Extension members. The typical American male over age 60 is remarkably unhealthy, often suffering multiple underlying maladies relating to metabolic syndrome and other pathologies called “co-morbidities.”12 This is indicative of a state of disease in the biologic environment of the patient.
A frank diagnosis (or indication) of prostate cancer should act as an early warning that something is amiss in the patient’s overall health and that further attention is warranted to various systems. Thus a diagnosis of prostate cancer need not be equated with invasive procedures such as radical prostatectomy, radiation therapy, cryosurgery, high intensity focus ultrasound, or androgen deprivation therapy, but with a call to the patient and physician to be alert to pathologic states that if corrected can stabilize or repair some or all of the systems that are amiss.
One reason the USPSTF believes that PSA screening should be halted is that so many men are already in such poor health they are likely to die of some other cause before prostate cancer becomes clinically relevant.3

Cancer Deaths Since Advent
of PSA Screening
This is the opposite of Life Extension members, who go to extraordinary efforts to slow aging and protect against degenerative disease. It would be irrational for healthy Life Extension members to stop PSA screening merely because their age group on average is in such poor overall health.
Few doctors today have comprehensive programs designed to reverse multiple underlying factors that lead to clinically-diagnosed prostate cancer. The typical aging person does not know about lifestyle changes, drugs, and nutrients that may keep an indolent cancer confined to the prostate gland.
Life Extension members have long been armed with this information and have access to health advisors to help guide them to more effective ways of working with their physician to improve their odds of keeping low-grade prostate cancer, or indications of low-grade prostate cancer (such as rising PSA), under control. This issue of Life Extension magazine is dedicated to reminding members and alerting the public about these novel approaches to disease prevention.
Most urologists believe when PSA reaches a certain level that their only choice is to perform needle biopsies. They often overlook existing tests, such as testing and properly analyzing blood results of free PSA percentage, PSA density, and PSA velocity, along with other diagnostics such as PCA3 urinary test and advanced non-invasive techniques that can provide additional insight that may reduce the need for invasive procedures.13-17 Urology patients are not always made aware of these non-invasive choices, and especially of the importance of measuring the PSA rise over time (PSA velocity) to help ascertain if prostate biopsy is warranted.
What clearly separates Life Extension members from the general public, however, are the aggressive steps we take to achieve meaningful extensions of our healthy life spans. Those advising against PSA screening are largely “writing-off” men over age 70.
Life Extension male members need to ensure their prostate health is assessed and maintained at an optimal level for the many decades of extended life they expect.
American Urological Association Capitulates

When the United States Preventive Services Task Force suggested that aging men stop PSA screening altogether, the American Urological Association disagreed. About a year later, the American Urological Association issued revised guidelines that will sharply reduce the number of PSA screenings performed.1,20,21 And other professional groups have issued similar opinions.22
The latest recommendation from the American Urological Association (AUA) is for men over age 70 to avoid PSA screening.1 The AUA is essentially saying that once you move past age 70, your life span is too short to bother with.
The American Urological Association is also writing off men aged 40-54 for prostate screening because of the relative low incidence of cancer in this group compared to men over 54.1 This is a tragedy as it condemns younger men who do develop prostate cancer to probable death. Earlier diagnosis provides a huge advantage when attempting curative therapy. Just ask Prostate Cancer Foundation Chairman Michael Milken, who insisted on a PSA test at age 46 and discovered he had prostate cancer in time to benefit from curative therapy.23
On the flip side are famous people like Frank Zappa, Telly Savalas, Bill Bixby, and other younger men who likely could have identified their prostate cancer earlier had they undergone PSA screening.24 These men probably had rising PSA levels long before metastatic disease manifested.
Overlooking More Efficient Procedures

In recommending more limited PSA-screening, the American Urological Association is tacitly admitting that conventional diagnostic and early treatment of prostate cancer is so inadequate, or performed so incompetently, that it’s better to wait for full-blown metastatic disease to manifest. Once advanced stage prostate cancer develops, however, treatments are seldom curative.
Instead of looking at physicians who are diagnosing and treating early stage prostate cancer using less invasive procedures and then emulating these skilled artists, the American Urological Association has apparently caved in to accepting and promoting mediocrity within their profession.
A big problem is that most urologists are not properly assessing PSA results, nor are they efficiently implementing further diagnostic and treatment protocols. And on the other end of the spectrum are the many men who are promptly sent for ultrasound-guided biopsies after one PSA elevation. And again, to add insult to injury, the biopsies are often not ones targeted to abnormalities within the prostate but merely targeting the prostate as a gland.
It is one issue to biopsy an ultrasound lesion that may represent the needle in the haystack, but it’s another issue, and a sad one at that, when it is the haystack that is the target. You know that this is the case when a man has had 2, 3, or 4 prostate biopsies showing no cancer cells, and then he is referred, finally, to a competent physician who uses excellent ultrasound equipment to directly target suspicious lesions within the prostate gland.
In these cases, it seems the diagnosis is magically made; but it’s not magic, it is just an issue of a far higher degree of competence. All men are not equal in talent and all equipment is not of the same quality. The unfortunate outcome is that too many aging men are being subjected to needless and incompetently administered invasive procedures that sometimes result in unnecessary suffering and premature death.
Instead of recommending that medical professionals upgrade their evaluation and treatment protocols to deliver state-of-the-art technology, the United States Preventive Services Task Force suggests that aging men not undergo PSA screening at all, while the American Urological Association limits its recommendation for PSA screening mostly to men aged 55-69.1
The media treats these authoritarian groups as being virtually infallible.
Prostate Cancer Not an Isolated Disease

A common mistake made by doctors and patients is thinking that prostate cancer manifests in isolation from other pathological events occurring as a person ages. This is not the case.
Research shows that other serious pathological conditions are frequently seen in prostate cancer patients.25 These factors involved in prostate malignancy can adversely impact other parts of the body.26
For example, Life Extension has shown one way prostate cancer and coronary atherosclerosis are related is that they are both influenced by the breakdown of bone.27 As an aging man develops osteoporosis, excess calcium released into the blood contributes to arterial calcification.27 What’s lost in the bone ends up in the coronary arteries and other major vessels of the body.27,28
These atherosclerotic lesions are not vascular “calcifications” but bone growth or osteogenesis.28-30 Bone breakdown also releases growth factors into the blood that promote the proliferation of what may have been indolent prostate cancer cells.31 Therefore, it should come as no surprise that nutrients that prevent bone loss such as vitamin K2 also inhibit vascular calcification.32,33
PSA screening thus provides an important clue of a man’s overall health, with the advantage of identifying problems early enough to take effective corrective actions. That’s a LOT of benefit for assessing one’s prostate gland once a year utilizing PSA blood testing.
Where’s the Accountability?
The level of medical competency directly affects the quality and quantity of the lives of others, yet there is not enough monitoring of patient outcomes.
When it comes to treating prostate disease, there needs to be a reporting of serious side effects such as incontinence, impotence, and major blood loss or urethral strictures after a urologist performs a radical prostatectomy.
This kind of accountability is relatively non-existent in today’s bureaucratic medical environment, though the Internet may eventually enable patients to assess the degree of medical competency of a physician they entrust their life to.
What Makes Cancer Cells Propagate?
When designing prevention and treatment strategies, Life Extension focuses on underlying mechanisms of disease that are fueled by specific biological factors in the body. This is not perfect science however because you can block one factor involved in tumor development, and cancer cells will use other growth-promoting vehicles to progress.
What we seek to do is stay two steps ahead of the cancer by cutting off its many growth promoters and pathways used to escape eradication. For instance, we know that dihydrotestosterone (DHT) promotes prostate cell growth (proliferation).34 This growth affects both benign prostate cells as well as cancerous ones. In the context of a man with prostate cancer, a serial rise in PSA is circumstantial evidence that the tumor cell population is increasing. Such an increase in PSA is not only of importance insofar as prompting investigations to rule out prostate cancer. We have evidence that PSA breaks down natural barriers that keep isolated tumor cells confined to regions within the prostate gland. Remember that PSA is aserine protease, an enzyme that breaks down proteins.35 One such containment protein degraded by PSA is the extra-cellular matrix, i.e., the natural barrier that may confine cancer cells within the prostate gland.
But suppressing DHT alone is not a total solution. There are other prostate tumor growth promoters such as insulin,estrogen, prolactin, transforming growth factor beta (TGF-1 and TGF-2), and vascular endothelial growth factor (VEGF) that also should be brought under control.36-42 Fortunately, many of the nutrients Foundation members already take can help suppress growth factors used by prostate cancer cells (and other cancers) to proliferate.43-52
There are other mechanisms involved in the evolution of a prostate tumor such as 5-lipooxygenase (5-LOX)53-55 and cyclooxygenase-2 (COX-2)56 that can be markedly improved by dietary changes, along with curcumin,57,58 fish oil,59-61 boswellia,62 aspirin,63 Zyflamend®,64-68 and prescription COX-2 inhibitors like Celebrex®.69,70
Genetic factors involved in prostate cancer initiation and promotion may be favorably modulated by taking relatively high doses of vitamin D.71,72 Hormonal influences like prolactin and insulin can benefit from using prolactin-suppressing drugs like cabergoline (Dostinex®)73 or Lisuride74 and the insulin-suppressing drug metformin.75-77
The overriding goal in reversing any cancer is to induce favorable changes in the genes that regulate cell proliferation and apoptosis (cell destruction). We know that nutrients like curcumin,78-80 genistein,81-84 fish oil,85,86 and vitamin D87,88 favorably affect genes involved in carcinogenesis, as do drugs like aspirin,89,90 metformin,91-93 finasteride (Proscar®),94 and dutasteride (Avodart®).95
THE WHOLISTIC NATURE OF HEALTH IN RELATION TO PROSTATE CANCER.

As we learn more about specific health issues we see evidence of the interconnectivity of all key processes involved in mind and body functions. This should come as no surprise since this phenomenon characterizes all living entities, from the atom to the universe.
Importance of Food Choices

What one eats (and doesn’t eat) makes a huge impact on whether prostate cancer ever develops.102,103
Healthier eating patterns also improve the odds of treatment success.104,105
A rising PSA level or prostate cancer diagnosis can be the signal that it’s time to switch what you eat more towards a Mediterranean diet that focuses on fish instead of red meat, whole vegetables instead of glucose-spiking starches/sugars, foods cooked at lower temperatures, and reduced intake of omega-6 fats.106-108
Those who pioneered aggressive dietary changes to help treat cancer were decades ahead of their time. While it’s unlikely that aggressive dietary alterations will cure clinically diagnosed prostate cancer, there are strong mechanistic values to consuming foods/beverages that suppress prostate cancer proliferation (like cruciferous vegetables109-111 and green tea112,113) as opposed to continuing to eat foods that have been related to higher prostate cancer risk such as red meat,114-116 starches and sugars,117,118 excess dairy,115,119-121 and excess omega-6 fats that contribute to a high omega-6: omega-3 ratio.122,123
Vitamin D Decreases Gleason Tumor Score
If a needle biopsy of the prostate detects a malignancy, it will be graded with a Gleason score number as follows:
Under 7 (low-grade): Slow growing and not likely to be aggressive.96 Low-grade prostate cancers are seldom the cause of death in men over age 70, especially those that are in poor health.97 Low-grade are the majority of prostate tumors found and the ones where “watchful waiting” is often employed in lieu of radical procedures.97,98
Over 7 (high-grade): Fast growing, aggressive tumors that require intervention such as radical prostatectomy, radiation, androgen ablation, etc.96 High-grade prostate tumors make up less than 15%* of newly diagnosed prostate cancers.99
*Caveat: Errors in the pathology lab can result in lethal mistakes, such as issuing a low Gleason score to a high-grade tumor. These errors are discovered when a radical prostatectomy is performed and it is found to have a Gleason score of 8-10 as opposed to a 6 Gleason score found in the biopsied specimen.100
A study published in 2012 evaluated a group of men with early-stage prostate cancer who received a 4,000 IU vitamin D3 supplement each day for a year.101
Mean 25-hydroxyvitamin D blood levels at baseline were 32.8 ng/mL and increased to 66.2 ng/mL after vitamin D supplementation.101
After one year, 55% of the men showed a decrease in tumor sensitive biopsies or a decrease in the Gleason tumor score. An additional 11% showed no change (meaning the cancer had not progressed).
The study also showed that over time, supplementation with vitamin D3 led to a decrease in the number of positive cores taken during prostate biopsies. This is in stark contrast to the untreated control group who experienced an increase in the number of positive cores on repeat biopsies.101
Only 34% of men taking vitamin D progressed compared to 63% of the control group. This represents a 46% reduction in the number of men who moved to advancing disease, indicating powerful effects of taking 4,000 IU/day of vitamin D for one year.
The men in this study had not received any other treatment than vitamin D and all were in an active surveillance program that carefully measured disease progression or regression.
This study showed that just one intervention (4,000 IU/day/vitamin D) was able to reverse the clinical course of disease in a significant percentage of these prostate cancer patients.
This study helps validate the importance of PSA screening. Had these men not known they had early-stage prostate cancer, they would not have known to take vitamin D, and their disease would have likely progressed until symptoms such as bone pain manifested.
The first article in this month’s issue titled, “Impact of Diet on Prostate Cancer Risk and Mortality” describes foods that promote prostate cancer and which ones protect against it. We explain how consuming the wrong foods can fuel prostate cancer growth, while following healthy dietary choices can reduce the risk that you will develop clinically diagnosed prostate cancer.
Some men instinctively start eating healthier as they mature, but it took a higher PSA reading (1.4 ng/mL) ten years ago for me to alter my diet in a healthier direction. My diet is not perfect, but it’s a huge improvement over what I consumed in my younger years. My last PSA test came in at 0.4 ng/mL…a 71% decrease in a ten-year period (PSA levels normally rise with age).
If I had not had my PSA checked annually, I may have continued making poor dietary choices and may have developed prostate cancer by now. My father was diagnosed with it around age 75. He consumed a typical diet for his era, with a daily intake of red meat and high glycemic starches like potatoes, while never touching a vegetable or fruit. He set himself up perfectly to encourage prostate cancer growth and mutation.
Even for those who aren’t sure if they are making the proper food choices, laboratory tests like the Omega Score® test (a fatty acid profile) enable one to evaluate their diet and supplement program and make changes to optimize health. You are what you eat and what you assimilate does have a bearing on your health.
Five Stages of Prostate Cancer Progression
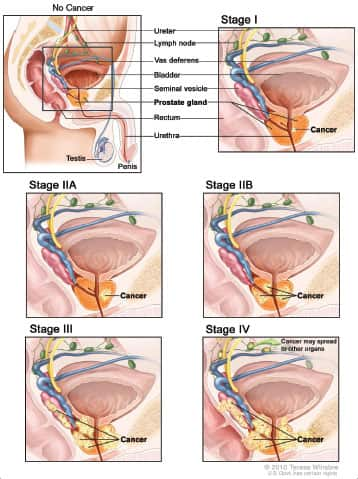 |
As prostate cancer progresses from Stage I to Stage IV, the cancer cells grow within the prostate, through the outer layer of the prostate into nearby tissue, and then to lymph nodes or other parts of the body.
A More Rational Approach

Most prostate tumors are very sensitive to their internal environment or what we prefer to call their “biological milieu.” We know this because when androgen-deprivation therapy is properly administered, PSA levels can drop to near zero and prostate cancer cells die through the process of programmed cell death, a.k.a. apoptosis.124,125
However, it is not uncommon for prostate cancers to eventually find other growth factors to fuel their continued proliferation and the anti-proliferative and pro-apoptotic effects of androgen-deprivation therapy wear off, as evidenced by a continuously rising PSA that was once brought down to below 0.05 ng/mL by adequately suppressing testosterone.36-42,125
When a diagnosis of prostate cancer occurs in the setting of a rising PSA in the lower range (below 4 ng/dL ideally), Life Extension views this as an opportunity for early intervention that might result in one’s body regaining control over tumor expansion.
We know that the drug Avodart® (dutasteride) lowers PSA levels by inhibiting the formation of dihydrotestosterone (DHT).126 Avodart® and its less potent cousin Proscar® (finasteride) are 5ARIs (5-alpha reductase inhibitors).127 5-alpha reductase is the enzyme that converts testosterone to DHT.127 The effect of DHT on prostate cancer cell growth is five times greater than that of testosterone.128 By blocking DHT, drugs like Avodart® and Proscar® provide a unique opportunity to suppress tumor growth. At the same time, comprehensive adjunct protocols can be initiated that are designed to deprive tumor cells of growth factors or fuels, further inhibiting cancer growth and/or invasion.

For example, a recent study found that men taking finasteride for prostate cancer prevention were far more likely to benefit if they had lower estrogen levels prior to initiation of treatment with finasteride.129 This study clearly showed high concentrations of estrogen to be associated with increased cancer risk. So much so that the elevated estrogen neutralized the prostate cancer prevention impact of finasteride. Life Extension has repeatedly warned aging men about the critical need of achieving estrogen balance. One reason was our continued observation of high estrogen levels in newly diagnosed prostate cancer patients. Men can easily suppress elevated estrogen levels with aromatase-inhibiting therapies.130
So in response to a rising PSA and/or other indicators of prostate disease, men have a range of diagnostic options to assess whether there is underlying malignancy and if there is, what may be helping to fuel it (such as elevated DHT or estrogen).
If non-invasive diagnostics indicate malignancy, a color Doppler ultrasound-guided biopsy can indicate whether it may be high-grade (Gleason score over 7 that requires treatment) or low-grade (Gleason score under 7 that may be controlled with comprehensive surveillance/intervention).
Some Life Extension members choose to attack a rising PSA as if there is already low-grade prostate cancer present, especially if they suffer urinary symptoms relating to benign prostate hyperplasia (enlargement). In consultation with their doctor, they may choose to take 0.5 mg of Avodart® daily (though it may not need to be taken every day) and simultaneously introduce an arsenal of mechanistic approaches to restrain benign and/or tumor cell propagation and induce benign and/or tumor cell apoptosis.
The use of Avodart® or finasteride can shrink prostate gland volume by 25% thus relieving benign symptoms, improve the accuracy of a needle biopsy if this diagnostic procedure is needed, and deprive tumor cells of one growth promoter, i.e. DHT.131,132
Genetic Tests for Men Undergoing Prostate Biopsy
About half of US men diagnosed with prostate cancer are classified as low-risk by use of conventional measures such as Gleason score (a form of tumor grading), the prostate-specific antigen test (PSA), and a physical exam.135 Nonetheless, nearly 90% of these low-risk patients will choose to undergo immediate aggressive treatment such as radical prostatectomy or radiation even though there is less than a 3% chance of deadly progression.135
A new test called Oncotype DX is now available to physicians and their patients. It measures the level of expression of 17 genes across four biological pathways to predict prostate cancer aggressiveness.135
Test results are reported as a Genomic Prostate Score (GPS) ranging from 0 to 100; this score is assessed along with other clinical factors to clarify a man’s risk prior to treatment intervention.135 This multi-gene test can be used in conjunction with the needle biopsy sample taken before the prostate is removed, thereby providing the opportunity for low risk patients to avoid invasive treatments. According to the principal investigator of the validation study, “Individual biological information from the Oncotype DX prostate cancer test almost tripled the number of patients who can more confidently consider active surveillance and avoid unnecessary treatment and its potential side effects.”135
The advantage of this test for those who choose the comprehensive surveillance program utilized by Life Extension members (which involves the use of several drugs, targeted nutrients, and adherence to healthy dietary patterns) is to provide greater assurance the right course of action is being followed.
For information about the Oncotype DX test, log on to www.oncotypedx.com.
Prolaris® is another genomic test developed to aid physicians in predicting prostate cancer aggressiveness in conjunction with clinical parameters such as Gleason score and PSA.136
Prolaris® measures prostate cancer tumor biology at the molecular level. By measuring and analyzing the level of expression of genes directly involved with cancer replication, Prolaris may be able to more accurately predict disease progression.136
Prolaris® is a tool designed to measure the aggressiveness of a patient’s cancers to better predict and stratify an individual’s relative risk of disease progression within ten years.136 It may enable physicians to better define a treatment/monitoring strategy for their patients.
Prolaris® claims to be significantly more prognostic than currently used variables and provides unique additional information that can be combined with other clinical factors in an attempt to make a more accurate prediction of a patient’s cancer aggressiveness and therefore disease progression.136
Prolaris® has been shown to predict clinical progression in four different clinical cohorts, in both pre and post-treatment scenarios.136
In the treatment of prostate cancer, Prolaris® is prognostic at the point of diagnosis and in the post-surgery setting.136
At diagnosis, Prolaris® can help to identify patients with less aggressive cancer who may be candidates for active surveillance. In addition, Prolaris® can define patients who appear clinically low-risk but have a more aggressive disease that requires more aggressive treatment.
Prolaris® testing is also well suited for use in post-prostatectomy patients that have higher risk features after surgery to better estimate their risk of disease recurrence and therefore adjust the level of monitoring or add additional therapy.
For more information about Prolaris®, log on to the company website: www.myriad.com

How Life Extension Differs From the Mainstream
A common approach to dealing with biopsied-confirmed low-grade prostate cancer is called “watchful waiting.” Under this scenario, PSA tests are performed at reasonable intervals and treatment decisions based on indicators of disease progression (or regression).
In the presence of persistently rising PSA and other markers, the patient and their doctor discuss wide ranges of treatment options ranging from surgical removal of prostate gland, different forms of radiation, cryoablation, and/or androgen ablation to temporarily reduce PSA and buy more time. All of these treatment modalities have side effects to consider.
Instead of merely “watching” a PSA rise until risky therapies are required, we at Life Extension view a low-grade prostate cancer (or even a biopsy that reveals no cancer) as an opportunity to intervene aggressively with a multitude of non-toxic approaches that benefit one’s overall health. Success or failure is measured by monthly PSA testing, along with other tests to ensure that other growth factors like insulin, estrogen, DHT, and prolactin are being adequately suppressed.
To clarify the point about a no cancer diagnosis, the accuracy of typical initial needle biopsies today is only around 75%.134 So if your urologist tells you he has good news, i.e., the biopsy showed no tumor cells in your prostate gland, there may be a 25% chance you do have tumor cells, thus making the kinds of comprehensive intervention that benefits your entire body a rational choice.
So rather than “watchfully wait,” as your underlying disease may progress, we suggest comprehensive intervention. The objective is to take away every route that enable tumor cells to propagate and escape confinement within the prostate gland.
For those who require a prostate biopsy, there are new (and expensive) genetic tests (described above) that may more accurately predict which tumors are aggressive and likely to metastasize and those that are so indolent that only minimal changes may be needed to keep control over them. If these genetic tests prove themselves in the clinical setting (outside the bias of company sponsored clinical trials), intelligently using the results of these tests can spare many men from needless treatments and provide information about genetic mutations to target in prostate cells may enable better long-term control.
Enhanced Diagnostic Procedures
What patients should understand is the diagnosis of prostate cancer per ultrasound-guided biopsies is also related to the skill of the physician performing the procedure, as well as the nature of the ultrasound (gray-scale versus color Doppler). CDU (color Doppler ultrasound) also indicates the degree of vascularity (angiogenesis) of the cancer, which if present is a factor associated with tumor aggressiveness. The more vascular the cancer the more aggressive it is. Dietary approaches, supplements, and medications to reduce angiogenesis should be considered in the arsenal of how we prevent the emergence or evolution of clinically significant prostate cancer.
An additional emerging area that may allow a better understanding of clinically significant prostate cancer and clarify the issue of risk of high-grade prostate cancer with 5-alpha reductase inhibitor drugs like Avodart® and Proscar® involves replacing the transrectal ultrasound of the prostate (TRUSP) with MRI utilizing parameters such as DWI (diffusion weighted imaging) and the associated grading of DWI using the Apparent Diffusion Coefficient (ADC). Studies indicate a much higher specificity for the diagnosis of prostate cancer than TRUSP when DWI and ADC are used together.154,155
Our Enlarging Prostate Glands
Aging results in a proliferation of prostate cells that is technically referred to as benign prostatic hyperplasia (BPH).137 The graphic on page 18 depicts an advanced case of BPH with a constricted urethra that would impede or block urine flow.
Illustrations on page 20 show a normal prostate gland compared to an extreme case of BPH.
Symptoms associated with BPH include frequent urination and urinary hesitancy that can be especially troublesome at night.137 In severe cases obstruction of urine flow requires insertion of a catheter into the bladder via the penile urethra.
A major culprit involved in the benign over-proliferation of prostate cells is dihydrotestosterone (DHT).138 Drugs such as Avodart® (dutasteride) or Proscar® (finasteride) reduce DHT levels and shrink the size of an enlarged prostate gland, which reduces BPH symptoms.139 These drugs also lower PSA levels by almost 50%, which may reflect the mechanism(s) that explain why men taking these drugs have reduced overall prostate cancer risk.140-142 In two large studies, men taking Avodart® or Proscar® had about a 24% reduced risk of prostate cancer.143,144

Men should know that testosterone is not the culprit behind prostate problems. Numerous studies suggest that youthful levels of testosterone do not increase prostate cancer risk.145-150 What happens in the aging man’s body, however, is that testosterone converts to estrogen and DHT, and these two testosterone metabolites have been shown to be involved in benign and malignant prostate disease. Fortunately, there are low-cost methods available to suppress DHT and estrogen in aging men, while maintaining youthful ranges of free testosterone.
Recall that PSA is not just a marker of prostate cancer, but functions as a tumor promoter by degrading barrier structures in the prostate gland that may contain isolated tumor cells.
What troubles Dr. Walsh and some other experts is that some of the men taking Avodart® or finasteride who do contract prostate cancer have been shown in two studies to develop more aggressive forms of the disease. They are so concerned that they warn men not to use these drugs for the purpose of prostate cancer prevention, as does the FDA.

On the flip side are proponents of these drugs who point out that Avodart® as well as Proscar® (finasteride) reduce prostate gland volume by such a degree that the ability to identify high-grade tumors via prostate biopsy is improved. So it does not appear that Avodart® or Proscar® causes more high-grade tumors. Instead, these drugs facilitate earlier detection of such cancers, which is another reason to consider taking them.
A frustration with needle biopsies is that they miss as many as 20-30% of prostate cancers.134,151,152 The larger one’s prostate gland, the easier it is to have the biopsy miss those sites that are malignant. The illustration on page 4 depicts a 12-core biopsy to show why a larger prostate gland makes it more difficult to detect malignant cells. So an advantage of shrinking one’s prostate gland using drugs like Avodart® or Proscar® is that if a needle biopsy is required, it may more accurately detect underlying malignancy.153
As you’ll read in the article in this issue titled The Avodart®-Proscar® Debate, there is compelling evidence that these drugs may reduce high-grade prostate cancer risk.
Another virtue to using 5-alpha reductase inhibitors (like Avodart® or Proscar®) is that in the presence of prostate cancer, PSA levels don’t decrease as much after these drugs are initiated.140-142
Physicians using 5-alpha reductase inhibitors should take into account the PSA-lowering effect of these agents by doubling the PSA lab value.156 Given that PSA decreases less in the presence of prostate cancer, the doubling of PSA will result in a higher value of PSA and will trigger the need for diagnostic investigations sooner.
What doctors have observed is that drugs like Avodart® or finasteride suppress PSA levels more effectively in men with benign prostate enlargement or low-grade prostate cancer. When PSA levels drop then start raising again, this indicates that the 5-alpha reductase inhibitor is reducing low-grade cells of questionable clinical significance but is not affecting higher grade malignancies.131 This finding is another plus for using a 5-alpha reductase inhibitor as it can increase the sensitivity of the PSA test to reveal which men need aggressive diagnostics such as needle biopsies.
Why We Suggest Certain Drugs
When it comes to combatting cancer, Life Extension long ago learned that the initial treatment regimen should be aggressive enough to deprive tumor cells of an opportunity to mutate into forms that are resistant to future therapies. If we know of a relatively side effect-free drug that works via a single or multiple mechanisms to impede tumor survival, we’re going to include it in our comprehensive surveillance program.
Let’s talk first about metformin. It was used in England in 1958 but did not make it into the United States until 1995—37 years later!157 I am familiar with metformin because the FDA tried to have me incarcerated for recommending it as an anti-aging drug long before it was “approved” to treat type II diabetes.

What’s been happening over the last ten years is an explosion of published studies that consistently show that metformin reduces the risks of certain tumors and may be an effective cancer treatment.158-165
People ask me all the time, how can an anti-diabetic drug work so well against cancer? The encouraging news is that metformin functions via multiple mechanisms to create a less favorable environment for tumor progression.166-175 We know that insulin (and glucose) increase the risk of many tumors.176 This is of particular concern to obese men with prostate tumors. Metformin lowers blood glucose and insulin levels. The sidebar on the next page reveals the multiple anti-cancer mechanism of metformin.
There are nutrients that can have similar effects such as standardized green coffee extract.177 We nonetheless suggest that a man with an elevated or rising PSA should ask his doctor to consider prescribing metformin. The starting dose can be 500 mg of extended release (Metformin ER) taken with breakfast each day. Under the supervision of the patient’s local medical doctor, the dose can be increased to 500 mg ER taken at breakfast and at dinner. (Dose ranges for non-extended release metformin are 250 - 850 mg taken before no more than three meals a day.) Metformin is an inexpensive generic drug and can be taken along with nutrients (like green coffee extract) that similarly function to reduce glucose/insulin.
Metformin does more than slash tumor-promoting glucose/insulin levels. It also acts directly on cancer cells to induce apoptosis and/or inhibit proliferation.91 Metformin does this conserving the process by which food is converted to energy.169-172 Healthy cells react to metformin by adjusting their functions to use less energy. A cancer cell, on the other hand, that is forced to minimize energy consumption is less able to exhibit aggressive metastatic or proliferative behavior.178 In other scenarios, the energy stress caused by metformin is sufficient to cause cancer cell death.
The National Cancer Institute is sponsoring a clinical study where metformin will be tested to see if it can slow the progression of prostate cancer in men undergoing active surveillance (watchful waiting) with low-grade tumors.179 We hope the study design includes the measurement of 2-hour post-prandial (2 hours after meals) blood glucose levels as well as glycosylated hemoglobin (HbA1c) to ascertain that optimal dosing of study subjects has been achieved.
Anti-Cancer Actions of Metformin
Numerous studies show the anti-diabetic drug metformin can slow growth of existing cancers and decrease risk of developing new cancers. Some studies show metformin may protect against prostate cancer and aid in treatment. Here are some of its anti-cancer mechanisms:
- Metformin reduces levels of glucose, insulin, and insulin-like growth factors that fuel tumor growth.166-169
- Metformin activates a powerful molecule called AMPK
(adenosine monophosphate-activated protein kinase) that subjects cancer cells to unique metabolic stresses not experienced by healthy tissues. (Activated AMPK promotes death [apoptosis] of malignant cells and prevents their development.)169,170 - Metformin independently inhibits mTOR (mammalian target of rapamycin) that regulates cell growth, energy metabolism, cell motility, cell survival, and protein synthesis.171,172
- Metformin mimics the benefits of a hormone called adiponectin in activating AMPK-dependent growth inhibition in prostate cancer cells.173
- Metformin blocks cancer cell reproductive cycles by decreasing levels of a growth-promoting protein called cyclin D1.174
- Metformin increases production of a protein (p27) that inhibits the cell division cycle.174
- Metformin suppresses vascular endothelial growth factor (VEGF) thereby cutting off the blood supply to tumors.175
At a cancer conference earlier this year, the results of a study were reported of 22 men (median age 64, median PSA 6 ng/mL) with confirmed prostate cancer that were given 500 mg of metformin three times a day 41 days prior to surgery (prostatectomy). In response to metformin the men showed the expected reductions in glucose and insulin growth factor-1 (IGF-1) blood levels, along with abdominal fat loss.180 What got the researchers excited was that compared to biopsied specimens, the surgically removed prostate glands showed a 32% reduction in a marker of cell proliferation (Ki-67) and a favorable alteration in a pathway tumor cells use to proliferate out of control (via mTOR).181
Knowledgeable members point out that curcumin interferes with these tumor growth pathways via similar mechanisms, which we at Life Extension have long been familiar with.182 My argument for recommending metformin is that it should produce potent additive effects to curcumin. Moreover, we still don’t know what the upper dose limits are for metformin and/or curcumin for cancer treatment, so taking both may have some obvious advantages.
Furthermore, because metformin is a drug, it tends to get more attention from researchers, perhaps because it is easier to obtain funding for drug studies. A European study published this year showed that metformin was effective against advanced castration-resistant prostate cancer. The doctors who conducted this study concluded:
To our knowledge, our results are the first clinical data to indicate that metformin use may improve PSA-recurrence free survival, distant metastasis-free survival, prostate cancer specific mortality, overall survival and reduce the development of castration resistant prostate cancer in prostate cancer patients. Further validation of metformin’s potential benefits is warranted.183
Daily Use of Aspirin May Decrease Prostate Risks
Researchers studied 2,447 men over 12 years, examining them every other year. After adjusting for age, diabetes, hypertension, and other factors, they found that men who took a daily aspirin or another NSAID (like ibuprofen) reduced their risk of moderate or severe urinary symptoms by 27% and lowered their risk of an enlarged prostate by 47%. Even more intriguing was the finding that men who consumed aspirin or another NSAID were 48% less likely to have an elevated level of prostate-specific antigen (PSA).187
Aspirin inhibits the cyclooxygenase (COX-1 and COX-2) enzymes, which are also involved in the arachidonic acid inflammatory pathway.188,189 COX-2 in particular is known to promote the proliferation of prostate cancer cells.56
Interestingly, men who are on androgen deprivation therapy to treat prostate cancer often show rising insulin levels that can stimulate tumor growth.167,184 By taking metformin, some of the side effects of androgen deprivation therapy can be mitigated, as was shown in this newly published European study.
So while nutrients like curcumin and green coffee extract and others may share functions that are similar to metformin, we cannot ignore the strong data showing specific benefits to low-cost metformin.
Another hormone that prostate tumors use to escape eradication is prolactin,39 and this can easily be suppressed by taking 0.25 mg to 0.5 mg of cabergoline (Dosintex®) two to three times weekly.185
Aspirin functions in multiple ways to interfere with prostate cancer propagation and metastasis and it may induce genetic changes that facilitate apoptosis.186 There is too much data about the potential role of aspirin as an adjuvant cancer treatment for men with rising PSAs not to use it.
What if PSA Screen Detects a Potential Problem?
If an annual PSA screen reveals a potential problem, a man has an early opportunity to:
- Review state-of-the-art studies to establish his status regarding the presence of prostate cancer.
- Confirm the diagnosis and get a Gleason score reading by an expert in prostate cancer pathology.
- Utilize published nomograms and neural nets to present the patient probabilities of organ-confined prostate cancer, capsular penetration, or disease progression to seminal vesicles and/or lymph nodes.
- Obtain refined laboratory studies and imaging studies to confirm or refute the above.
- Sit down with a physician that is least biased on a particular procedure and discuss the pros and cons of all therapies, including active surveillance.
- Investigate and discuss all co-related illnesses that might have gone unrecognized but that play a role in stimulating prostate cancer growth.
Treat Yourself As If You Already Have Prostate Cancer
This article is supposed to be about prostate cancer prevention, and here I am talking about therapies overlooked by most doctors that may facilitate enhanced treatment outcomes.
The reason we can’t ignore treatments is that aging men should accept the reality that in all likelihood there are malignant cells in their prostate glands now. This makes it easier to consistently follow prevention programs that can reduce the risk that clinically diagnosed disease will ever manifest. It also keeps one on the lookout for non-toxic treatments that may also have preventative benefits.
As I have related in the past, when my PSA reading came back at 1.4 ng/mL in year 2003, I treated it as if I had early stage prostate cancer by adopting healthier dietary choices and taking every nutrient and drug that had shown efficacy in prostate cancer prevention. Ten years later my PSA is 0.4 ng/mL.
I will remain on an aggressive prostate cancer treatment regimen and in the process reduce my risk for virtually every other age-related disease.
The articles in this month’s issue provide comprehensive approaches for the prevention of prostate cancer, including a comprehensive overview demonstrating the prostate cancer prevention benefits in response to Avodart® and finasteride. Men with any type of prostate malignancy may also benefit, as the programs we advocate for prevention may also facilitate better overall treatment.
For longer life,
William Faloon
Don’t Accept Archaic Diagnostics
The highly variable skills of the urologist performing TRUSP (transrectal ultrasound guided needle biopsy of the prostate) is of great concern when a biopsy is needed.
Too often the urologist uses the TRUSP to target the prostate gland per se, rather than abnormal areas within the prostate. Rarely do we see a dedicated TRUSP report that mentions all of the important findings that can and should be related by the urologist e.g., gland volume, PSA density, status of the capsule and seminal vesicles, as well as location of hypoechoic and hyperechoic lesions within the prostate. Using the TRUSP to target the prostate gland, and not the various lesions within the gland is akin to diluting a vintage wine with ice cubes. (For illustration and a description of a model TRUSP report, see Appendix F of the book A Primer on Prostate Cancer by Strum and Pogliano available from Life Extension Media by calling 1-800-544-4440 or logging on to www.lifeextension.com
The varying quality of the ultrasound device and whether it is a standard gray-scale ultrasound or a color Doppler ultrasound is also significant. Color Doppler ultrasound, for instance, discloses pathologic states of increased blood vessel growth (angiogenesis) that is associated with more clinically aggressive prostate cancer, which is often of a higher Gleason score.18
MRI (magnetic resonance imaging) using DWI (diffusion weighted imaging) will also add to understanding the risk a particular patient with prostate cancer faces. That’s because when color Doppler ultrasound is combined with MRI-DWI, a predictive value regarding the level of aggressiveness of the prostate cancer may be established.19
In this manner, selecting only those men whose prostate cancers are most likely to be “bad actors” and who need invasive therapy can be accomplished, while sparing those men with cancers of low grade, which are often amenable to changes in lifestyle, diet, and use of supplements.
References
- Available at: http://www.auanet.org/advnews/press_releases/article.cfm?articleno=290. Accessed August 22, 2013.
- Billis A. Latent carcinoma and atypical lesions of prostate. An autopsy study. Urology. 1986 Oct;28(4):324-9.
- Moyer VA, U.S. Preventive Services Task Force. Screening for prostate cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2012 Jul 17;157(2):120-34.
- Catalona WJ, Richie JP, Ahmann FR, et al. Comparison of digital rectal examination and serum prostate specific antigen in the early detection of prostate cancer: results of a multicenter clinical trial of 6,630 men. J Urol. 1994 May;151(5):1283-90.
- Bokhorst LP, Bangma CH, van Leenders GJ, et al. Prostate-specific antigen-based prostate cancer screening: Reduction of prostate cancer mortality after correction for nonattendance and contamination in the Rotterdam Section of the European Randomized Study of Screening for Prostate Cancer. Eur Urol. Aug 11 2013.
- Available at http://www.census.gov/population/age/data/2011comp.html. Accessed September 23,2013.
- Available at: http://www.cancer.gov/cancertopics/factsheet/detection/psa. Accessed September 24, 2013.
- Available at: http://www.cancer.org/cancer/prostatecancer/detailedguide/prostate-cancer-key-statistics. Accessed Aug 26, 2013.
- Available at: http://www.cdc.gov/hiv/statistics/basics/ataglance.html. Accessed August 27, 2013.
- Available at: http://www.uspreventiveservicestaskforce.org/uspstf13/hiv/hivfinalrs.htm. Accessed September 2013.
- Ghossain A, Ghossain MA. History of mastectomy before and after Halsted. J Med Liban. 2009 Apr-Jun;57(2):65-71.
- Ervin RB. Prevalence of metabolic syndrome among adults 20 years of age and over, by sex, age, race and ethnicity, and body mass index: United States, 2003-2006. Natl Health Stat Report. 2009 May 5;(13):1-7.
- Vessella RL, Lange PH, Partin AW, et al. Probability of prostate cancer detection based on results of a multicenter study using the AxSYM free PSA and total PSA assays. Urology. 2000 Jun;55(6):909-14.
- Lieberman S. Can percent free prostate-specific antigen reduce the need for prostate biopsy? Eff Clin Pract. 1999 Nov-Dec;2(6):266-71.
- Stephan C, Stroebel G, Heinau M, et al. The ratio of prostate-specific antigen (PSA) to prostate volume (PSA density) as a parameter to improve the detection of prostate carcinoma in PSA values in the range of < 4 ng/mL. Cancer. 2005 Sep 1;104(5):993-1003.
- Loeb S, Carter HB. Point: Impact of prostate-specific antigen velocity on management decisions and recommendations. J Natl Compr Canc Netw. 2013 Mar 1;11(3):281-5.
- Hessels D, Schalken JA. The use of PCA3 in the diagnosis of prostate cancer. Nat Rev Urol. 2009 May;6(5):255-61.
- Strohmeyer D, Frauscher F, Klauser A, et al. Contrast-enhanced transrectal color doppler ultrasonography (TRCDUS) for assessment of angiogenesis in prostate cancer. Anticancer Res. 2001 Jul-Aug;21(4B):2907-13.
- Ibrahiem EI, Mohsen T, Nabeeh AM, Osman Y, Hekal IA, Abou El-Ghar M. DWI-MRI: single, informative, and noninvasive technique for prostate cancer diagnosis. ScientificWorldJournal. 2012;2012:973450.
- Pollack CE, Platz EA, Bhavsar NA, et al. Primary care providers’ perspectives on discontinuing prostate cancer screening. Cancer. 2012 Nov 15;118(22):5518-24.
- Allard CB, Dason S, Lusis J, Kapoor A. Prostate cancer screening: Attitudes and practices of family physicians in Ontario. Can Urol Assoc J. 2012 6(3):188-93.
- Qaseem A, Barry MJ, Denberg TD, et al. Screening for prostate cancer: a guidance statement from the Clinical Guidelines Committee of the American College of Physicians. Ann Intern Med. 2013 May 21;158(10):761-9.
- Available at: http://www.mikemilken.com/pcfbook_chapter1.pdf. Accessed September 25, 2013.
- Available at: http://www.pccnc.org/awareness. Accessed September 25, 2013.
- Post PN, Kil PJ, Hendrikx AJ, Janssen-Heijnen ML, Crommelin MA, Coebergh JW. Comorbidity in patients with prostate cancer and its relevance to treatment choice. BJU Int. 1999 Oct;84(6):652-6.
- Howcroft TK, Campisi J, Louis GB, et al. The role of inflammation in age-related disease. Aging (Albany NY). 2013 Jan;5(1):84-93.
- Available at: https://www.lifeextension.com/magazine/2009/1/vitamin-k-protection-against-arterial-calcification-bone-loss-cancer-aging. Accessed September 25, 2013.
- Available at: http://content.onlinejacc.org/article.aspx?articleid=1136110. Accessed September 25, 2013.
- Available at: http://circres.ahajournals.org/content/84/2/250.full. Accessed September 25, 2013.
- Abedin M, Tintut Y, Demer LL. Vascular calcification: mechanisms and clinical ramifications. Arterioscler Thromb Vasc Biol. 2004 Jul;24(7):1161-70.
- Available at: http://media.curetoday.com/downloads/documents/bonehealth_pg_rev_b.pdf. Accessed August 30, 2013.
- Beulens JW, Bots ML, Atsma F, et al. High dietary menaquinone intake is associated with reduced coronary calcification. Atherosclerosis. 2009 Apr;203(2):489-93.
- Fodor D, Albu A, Poantă L, Porojan M. Vitamin K and vascular calcifications. Acta Physiol Hung. 2010 Sep;97(3):256-66.
- Wen J, Zhao Y, Li J, et al. Suppression of DHT-induced paracrine stimulation of endothelial cell growth by estrogens via prostate cancer cells. Prostate. 2013 Jul;73(10):1069-81.
- Pezzato E, Sartor L, Dell’Aica I, et al. Prostate carcinoma and green tea: PSA-triggered basement membrane degradation and MMP-2 activation are inhibited by (-) epigallocatechin-3-gallate. Int J Cancer. 2004 Dec 10;112(5):787-92.
- Cox ME, Gleave ME, Zakikhani M, et al. Insulin receptor expression by human prostate cancers. Prostate. 2009 Jan 1;69(1):33-40.
- Singh PB, Matanhelia SS, Martin FL. A potential paradox in prostate adenocarcinoma progression: oestrogen as the initiating driver. Eur J Cancer. 2008 May;44(7):928-36.
- Giton F, de la Taille A, Allory Y, et al. Estrone sulfate (E1S), a prognosis marker for tumor aggressiveness in prostate cancer (PCa). J Steroid Biochem Mol Biol. 2008 Mar;109(1-2):158-67.
- Dagvadorj A, Collins S, Jomain JB, et al. Autocrine prolactin promotes prostate cancer cell growth via Janus kinase-2-signal transducer and activator of transcription-5a/b signaling pathway. Endocrinology. 2007 Jul;148(7):3089-101.
- Tu WH, Thomas TZ, Masumori N, et al. The loss of TGF-beta signaling promotes prostate cancer metastasis. Neoplasia. 2003 May-Jun;5(3):267-77.
- Ling MT, Lau TC, Zhou C, et al. Overexpression of Id-1 in prostate cancer cells promotes angiogenesis through the activation of vascular endothelial growth factor (VEGF). Carcinogenesis. 2005 Oct;26(10):1668-76.
- Häggström S, Bergh A, Damber JE. Vascular endothelial growth factor content in metastasizing and nonmetastasizing Dunning prostatic adenocarcinoma. Prostate. 2000 Sep 15;45(1):42-50.
- Meyer F, Galan P, Douville P, et al. Antioxidant vitamin and mineral supplementation and prostate cancer prevention in the SU.VI.MAX trial. Int J Cancer. 2005 Aug 20;116(2):182-6.
- Ripple MO, Henry WF, Schwarze SR, Wilding G, Weindruch R. Effect of antioxidants on androgen-induced AP-1 and NF-kappaB DNA-binding activity in prostate carcinoma cells. J Natl Cancer Inst. 1999 Jul 21;91(14):1227-32.
- Yan L, Spitznagel EL. Soy consumption and prostate cancer risk in men: a revisit of a meta-analysis. Am J Clin Nutr. 2009 Apr;89(4):1155-63.
- Hussain M, Banerjee M, Sarkar FH, et al. Soy isoflavones in the treatment of prostate cancer. Nutr Cancer. 2003 47(2):111-7.
- Giovannucci E, Ascherio A, Rimm EB, Stampfer MJ, Colditz GA, Willett WC. Intake of carotenoids and retinol in relation to risk of prostate cancer. J Natl Cancer Inst. 1995 Dec 6;87(23):1767-76.
- McLarty J, Bigelow RL, Smith M, Elmajian D, Ankem M, Cardelli JA. Tea polyphenols decrease serum levels of prostate-specific antigen, hepatocyte growth factor, and vascular endothelial growth factor in prostate cancer patients and inhibit production of hepatocyte growth factor and vascular endothelial growth factor in vitro. Cancer Prev Res (Phila). 2009 Jul;2(7):673-82.
- Liang JY, Liu YY, Zou J, Franklin RB, Costello LC, Feng P. Inhibitory effect of zinc on human prostatic carcinoma cell growth. Prostate. 1999 Aug 1;40(3):200-7.
- Singh RP, Raina K, Sharma G, Agarwal, R. Silibinin inhibits established prostate tumor growth, progression, invasion, and metastasis and suppresses tumor angiogenesis and epithelial-mesenchymal transition in transgenic adenocarcinoma of the mouse prostate model mice. Clin Cancer Res. 2008 Dec 1;14(23):7773-80.
- Smith S, Sepkovic D, Bradlow HL, Auborn KJ. 3,3’-Diindolylmethane and genistein decrease the adverse effects of estrogen in LNCaP and PC-3 prostate cancer cells. J Nutr. 2008 Dec;138(12):2379-85.
- Xing N, Chen Y, Mitchell SH, Young CY. Quercetin inhibits the expression and function of the androgen receptor in LNCaP prostate cancer cells. Carcinogenesis. 2001 Mar;22(3):409-14.
- Gupta S, Srivastava M, Ahmad N, et al. Lipoxygenase-5 is overexpressed in prostate adenocarcinoma. Cancer. 2001 Feb 15;91(4):737-43.
- Matsuyama M, Yoshimura R, Mitsuhashi M, et al. Expression of lipoxygenase in human prostate cancer and growth reduction by its inhibitors. Int J Oncol. 2004 Apr;24(4):821-7.
- Ghosh J, Myers CE. Arachidonic acid stimulates prostate cancer cell growth: critical role of 5-lipoxygenase. Biochem Biophys Res Commun. 1997 Jun 18;235(2):418-23.
- Xu S, Gao JP, Zhou WQ. Cyclooxygenase-2 and cyclooxygenase-2 inhibitors in prostate cancer. Zhonghua Nan Ke Xue. 2008 Nov;14(11):1031-4.
- Bengmark S. Curcumin, an atoxic antioxidant and natural NFkappaB, cyclooxygenase-2, lipooxygenase, and inducible nitric oxide synthase inhibitor: a shield against acute and chronic diseases. JPEN J Parenter Enteral Nutr. 2006 Jan;30(1):45-51.
- Lantz RC, Chen GJ, Solyom AM, Jolad SD, Timmermann BN. The effect of turmeric extracts on inflammatory mediator production. Phytomedicine. 2005 Jun;12(6-7):445-52.
- Taccone-Gallucci M, Manca-di-Villahermosa S, Battistini L, et al. N-3 PUFAs reduce oxidative stress in ESRD patients on maintenance HD by inhibiting 5-lipoxygenase activity. Kidney Int. 2006 Apr;69(8):1450-4.
- Calder PC. N-3 polyunsaturated fatty acids and inflammation: from molecular biology to the clinic. Lipids. 2003 Apr;38(4):343-52.
- Norrish AE, Skeaff CM, Arribas GL, Sharpe SJ, Jackson RT. Prostate cancer risk and consumption of fish oils: a dietary biomarker-based case-control study. Br J Cancer. 1999 Dec;81(7):1238-42.
- Safayhi H, Sailer ER, Ammon HP. Mechanism of 5-lipoxygenase inhibition by acetyl-11-keto-beta-boswellic acid. Mol Pharmacol. 1995 Jun;47(6):1212-6.
- Salinas CA, Kwon EM, FitzGerald LM, et al. Use of aspirin and other nonsteroidal antiinflammatory medications in relation to prostate cancer risk. Am J Epidemiol. 2010 Sep 1;172(5):578-90.
- Bemis DL, Capodice JL, Anastasiadis AG, Katz AE, Buttyan R. Zyflamend, a unique herbal preparation with nonselective COX inhibitory activity, induces apoptosis of prostate cancer cells that lack COX-2 expression. Nutr Cancer. 2005 52(2):202-12.
- Yang P, Cartwright C, Chan D, Vijjeswarapu M, Ding J, Newman RA. Zyflamend-mediated inhibition of human prostate cancer PC3 cell proliferation: effects on 12-LOX and Rb protein phosphorylation. Cancer Biol Ther. 2007 6(2):228-36.
- Capodice JL, Gorroochurn P, Cammack AS, et al. Zyflamend in men with high-grade prostatic intraepithelial neoplasia: results of a phase I clinical trial. J Soc Integr Oncol. 2009 7(2):43-51.
- Huang EC, McEntee MF, Whelan J. Zyflamend, a combination of herbal extracts, attenuates tumor growth in murine xenograft models of prostate cancer. Nutr Cancer. 2012 64(5):749-60.
- Sandur SK, Ahn KS, Ichikawa H, et al. Zyflamend, a polyherbal preparation, inhibits invasion, suppresses osteoclastogenesis, and potentiates apoptosis through down-regulation of NF-kappa B activation and NF-kappa B-regulated gene products. Nutr Cancer. 2007 57(1):78-87.
- Harris RE. Cyclooxygenase-2 (cox-2) blockade in the chemoprevention of cancers of the colon, breast, prostate, and lung. Inflammopharmacology. 2009 Apr;17(2):55-67.
- Pruthi RS, Derksen JE, Moore D, et al. Phase II trial of celecoxib in prostate-specific antigen recurrent prostate cancer after definitive radiation therapy or radical prostatectomy. Clin Cancer Res. 2006 Apr 1;12(7 Pt 1):2172-7.
- Chen L, Davey Smith G, Evans DM, et al. Genetic variants in the vitamin d receptor are associated with advanced prostate cancer at diagnosis: findings from the prostate testing for cancer and treatment study and a systematic review. Cancer Epidemiol Biomarkers Prev. 2009 Nov;18(11):2874-81.
- Flanagan JN, Young MV, Persons KS, et al. Vitamin D metabolism in human prostate cells: implications for prostate cancer chemoprevention by vitamin D. Anticancer Res. 2006,Jul-Aug;26(4A):2567-72.
- Webster J, Piscitelli G, Polli A, et al. Dose-dependent suppression of serum prolactin by cabergoline in hyperprolactinaemia: a placebo controlled, double blind, multicentre study. Clin Endocrinol (Oxf). 1992 Dec;37(6):534-41.
- Bohnet HG, Hanker JP, Horowski R, Wickings EJ, Schneider HP. Suppression of prolactin secretion by lisuride throughout the menstrual cycle and in hyperprolactinaemic menstrual disorders. Acta Endocrinol (Copenh). 1979 Sep;92(1):8-19.
- Clements A, Gao B, Yeap SH, Wong MK, Ali SS, Gurney H. Metformin in prostate cancer: two for the price of one. Ann Oncol. 2011 Dec;22(12):2556-60.
- Hitron A, Adams V, Talbert J, Steinke D. The influence of antidiabetic medications on the development and progression of prostate cancer. Cancer Epidemiol. 2012 Aug;36(4):e243-50.
- Wright JL, Stanford JL. Metformin use and prostate cancer in Caucasian men: results from a population-based case-control study. Cancer Causes Control. 2009 Nov;20(9):1617-22.
- Teiten MH, Gaascht F, Eifes S, Dicato M, Diederich M. Chemopreventive potential of curcumin in prostate cancer. Genes Nutr. 2010 Mar;5(1):61-74.
- Shishodia S, Singh T, Chaturvedi MM. Modulation of transcription factors by curcumin. Adv Exp Med Biol. 2007 595:127-48.
- Reuter S, Gupta SC, Park B, Goel A, Aggarwal BB. Epigenetic changes induced by curcumin and other natural compounds. Genes Nutr. 2011 May;6(2):93-108.
- Chen Y, Zaman MS, Deng G, et al. MicroRNAs 221/222 and genistein-mediated regulation of ARHI tumor suppressor gene in prostate cancer. Cancer Prev Res (Phila). 2011 Jan;4(1):76-86.
- Lakshman M, Xu L, Ananthanarayanan V, et al. Dietary genistein inhibits metastasis of human prostate cancer in mice. Cancer Res. 2008 Mar 15;68(6):2024-32.
- Davis JN, Singh B, Bhuiyan M, Sarkar FH. Genistein-induced upregulation of p21WAF1, downregulation of cyclin B, and induction of apoptosis in prostate cancer cells. Nutr Cancer. 1998 32(3):123-31.
- Davis JN, Kucuk O, Sarkar FH. Genistein inhibits NF-kappa B activation in prostate cancer cells. Nutr Cancer. 1999 35(2):167-74.
- Berquin IM, Min Y, Wu R, et al. Modulation of prostate cancer genetic risk by omega-3 and omega-6 fatty acids. J Clin Invest. 2007 Jul;117(7):1866-75.
- Deckelbaum RJ, Worgall TS, Seo T. n-3 fatty acids and gene expression. Am J Clin Nutr. 2006 Jun;83(6 Suppl):1520S-1525S.
- Krishnan AV, Peehl DM, Feldman D. Inhibition of prostate cancer growth by vitamin D: Regulation of target gene expression. J Cell Biochem. 2003 Feb 1;88(2):363-71.
- Mantell DJ, Owens PE, Bundred NJ, Mawer EB, Canfield AE. 1 alpha,25-dihydroxyvitamin D(3) inhibits angiogenesis in vitro and in vivo. Circ Res. 2000 Aug 4;87(3):214-20.
- Yoo J, Lee YJ. Aspirin enhances tumor necrosis factor-related apoptosis-inducing ligand-mediated apoptosis in hormone-refractory prostate cancer cells through survivin down-regulation. Mol Pharmacol. 2007 Dec;72(6):1586-92.
- Kim KM, Song JJ, An JY, Kwon YT, Lee YJ. Pretreatment of acetylsalicylic acid promotes tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis by down-regulating BCL-2 gene expression. J Biol Chem. 2005 Dec 9;280(49):41047-56.
- Jalving M, Gietema JA, Lefrandt JD, et al. Metformin: taking away the candy for cancer? Eur J Cancer. 2010 Sep;46(13):2369-80.
- Avci CB, Harman E, Dodurga Y, Susluer SY, Gunduz C. Therapeutic potential of an anti-diabetic drug, metformin: alteration of miRNA expression in prostate cancer cells. Asian Pac J Cancer Prev. 2013;14(2):765-8.
- Isakovic A, Harhaji L, Stevanovic D, et al. Dual antiglioma action of metformin: cell cycle arrest and mitochondria-dependent apoptosis. Cell Mol Life Sci. 2007 May;64(10):1290-302.
- Luo J, Dunn TA, Ewing CM, Walsh PC, Isaacs WB. Decreased gene expression of steroid 5 alpha-reductase 2 in human prostate cancer: implications for finasteride therapy of prostate carcinoma. Prostate. 2003 Oct 1;57(2):134-9.
- Schmidt LJ, Regan KM, Anderson SK, Sun Z, Ballman KV, Tindall DJ. Effects of the 5 alpha-reductase inhibitor dutasteride on gene expression in prostate cancer xenografts. Prostate. 2009 Dec 1;69(16):1730-43.
- Available at: http://www.stjohnprovidence.org/innerpage.aspx?pageid=1446. Accessed September 26, 2013.
- Stangelberger A, Waldert M, Djavan B. Prostate cancer in elderly men. Rev Urol. 2008 Spring;10(2):111-9.
- Gofrit ON, Zorn KC, Taxy JB, etal. Predicting the risk of patients with biopsy Gleason score 6 to harbor a higher grade cancer. J Urol. 2007 Nov;178(5):1925-8.
- Bastian PJ, Boorjian SA, Bossi A, et al. High-risk prostate cancer: from definition to contemporary management. Eur Urol. 2012 Jun;61(6):1096-106.
- Carter HB, Partin AW, Walsh PC, et al. Gleason score 6 adenocarcinoma: should it be labeled as cancer? J Clin Oncol. 2012 Dec 10;30(35):4294-6.
- Marshall DT, Savage SJ, Garrett-Mayer E, et al. Vitamin D3 supplementation at 4000 international units per day for one year results in a decrease of positive cores at repeat biopsy in subjects with low-risk prostate cancer under active surveillance. J Clin Endocrinol Metab. 2012 Jul;97(7):2315-24.
- Miano L. Mediterranean diet, micronutrients and prostate carcinoma: a rationale approach to primary prevention of prostate cancer. Arch Ital Urol Androl. 2003 Sep;75(3):166-78.
- Itsiopoulos C, Hodge A, Kaimakamis M. Can the Mediterranean diet prevent prostate cancer? Mol Nutr Food Res. 2009 Feb;53(2):227-39.
- Ornish D, Magbanua MJ, Weidner G, et al. Changes in prostate gene expression in men undergoing an intensive nutrition and lifestyle intervention. Proc Natl Acad Sci U S A. 2008 Jun 17;105(24):8369-74.
- Kenfield SA, Chang ST, Chan JM. Diet and lifestyle interventions in active surveillance patients with favorable-risk prostate cancer. Curr Treat Options Oncol. 2007 Jun;8(3):173-96.
- Ferris-Tortajada J, Berbel-Tornero O, Garcia-Castell J, Ortega-Garcia JA, Lopez-Andreu JA. Dietetic factors associated with prostate cancer: protective effects of Mediterranean diet. Actas urologicas espanolas. 2012 Apr;36(4):239-245.
- Kenfield SA, Dupre N, Richman EL, et al. Mediterranean diet and prostate cancer risk and mortality in the Health Professionals Follow-up Study. Eur Urol. 2013 Aug 13.
- Sofi F, Cesari F, Abbate R, Gensini GF, Casini A. Adherence to Mediterranean diet and health status: meta-analysis. BMJ. 2008 Sep 11;337:a1344.
- Xiao D, Srivastava SK, Lew KL, et al. Allyl isothiocyanate, a constituent of cruciferous vegetables, inhibits proliferation of human prostate cancer cells by causing G2/M arrest and inducing apoptosis. Carcinogenesis. 2003 May;24(5):891-7.
- Garikapaty VP, Ashok BT, Chen YG, et al. Anti-carcinogenic and anti-metastatic properties of indole-3-carbinol in prostate cancer. Oncol Rep. 2005 Jan;13(1):89-93.
- Srivastava SK, Xiao D, Lew KL, et al. Allyl isothiocyanate, a constituent of cruciferous vegetables, inhibits growth of PC-3 human prostate cancer xenografts in vivo. Carcinogenesis. 2003 Oct;24(10):1665-70.
- Chuu CP, Chen RY, Kokontis JM, Hiipakka RA, Liao S. Suppression of androgen receptor signaling and prostate specific antigen expression by (-)-epigallocatechin-3-gallate in different progression stages of LNCaP prostate cancer cells. Cancer Lett. 2009 Mar 8;275(1):86-92.
- Thakur VS, Gupta K, Gupta S. Green tea polyphenols causes cell cycle arrest and apoptosis in prostate cancer cells by suppressing class I histone deacetylases. Carcinogenesis. 2012 Feb;33(2):377-84.
- Punnen S, Hardin J, Cheng I, Klein EA, Witte JS. Impact of meat consumption, preparation, and mutagens on aggressive prostate cancer. PLoS One. 2011 6(11):e27711.
- Michaud DS, Augustsson K, Rimm EB, Stampfer MJ, Willet WC, Giovannucci E. A prospective study on intake of animal products and risk of prostate cancer. Cancer Causes Control. 2001 Aug;12(6):557-67.
- Richman EL, Kenfield SA, Stampfer MJ, Giovannucci EL, Chan JM. Egg, red meat, and poultry intake and risk of lethal prostate cancer in the prostate-specific antigen-era: incidence and survival. Cancer Prev Res (Phila). 2011 Dec;4(12):2110-21. 19.
- Bidoli E, Talamini R, Bosetti C, et al. Macronutrients, fatty acids, cholesterol and prostate cancer risk. Ann Oncol. 2005 Jan;16(1):152-7.
- Freedland SJ, Aronson WJ. Dietary intervention strategies to modulate prostate cancer risk and prognosis. Curr Opin Urol. 2009 May;19(3):263-7.
- Song Y, Chavarro JE, Cao Y, et al. Whole milk intake is associated with prostate cancer-specific mortality among U.S. male physicians. J Nutr. 2013 Feb;143(2):189-96.
- Chan JM, Stampfer MJ, Ma J, Gann PH, Gaziano JM, Giovannucci E. Dairy products, calcium, and prostate cancer risk in the Physicians’ Health Study. Am J Clin Nutr. 2001 74(4):549-554.
- Gao X, LaValley MP, Tucker KL. Prospective studies of dairy product and calcium intakes and prostate cancer risk: a meta-analysis. J Natl Cancer Inst. 2005 Dec 7;97(23):1768-77.
- Williams CD, Whitley BM, Hoyo C, et al. A high ratio of dietary n-6/n-3 polyunsaturated fatty acids is associated with increased risk of prostate cancer. Nutr Res. 2011 Jan 31(1):1-8.
- Masko EM, Allott EH, Freedland SJ. The relationship between nutrition and prostate cancer: is more always better? Eur Urol. 2013 May;63(5):810-20.
- Grossmann ME, Huang H, Tindall DJ. Androgen receptor signaling in androgen-refractory prostate cancer. J Natl Cancer Inst. 2001 Nov 21;93(22):1687-97.
- Available at: http://cdn.intechopen.com/pdfs/24233/intech-paradigm_shift_in_the_concept_of_hormonal_milieu_of_prostate_cancer.pdf. Accessed September 30, 2013.
- Arena F. Specific antigen prostatic changes during treatment with finasteride or dutasteride for benign prostatic hyperplasia. Minerva Urol Nefrol. 2013 Sep;65(3):211-216.
- Available at: http://www.medscape.com/viewarticle/745454_4. Accessed September 30, 2013.
- Available at: http://www.urologyhealth.org/urology/index.cfm?article=149. Accessed September 30, 2013.
- Kristal AR, Till C, Tangen CM, et al. Associations of serum sex steroid hormone and 5α-androstane-3α,17β-diol glucuronide concentrations with prostate cancer risk among men treated with finasteride. Cancer Epidemiol Biomarkers Prev. 2012 Oct;21(10):1823-32.
- Ta N, Walle T. Aromatase inhibition by bioavailable methylated flavones. J Steroid Biochem Mol Biol. 2007 Oct;107(1-2):127-9.
- Cohen YC, Liu KS, Heyden NL, et al. Detection bias due to the effect of finasteride on prostate volume: a modeling approach for analysis of the Prostate Cancer Prevention Trial. J Natl Cancer Inst. 2007 Sep 19;99(18):1366-74.
- Nickel JC. Comparison of clinical trials with finasteride and dutasteride. Rev Urol. 2004;6 Suppl 9:S31-9.
- Yavuz BB, Ozkayar N, Halil M, et al. Free testosterone levels and implications on clinical outcomes in elderly men. Aging Clin Exp Res. 2008 Jun;20(3):201-6
- Taira AV, Merrick GS, Galbreath RW, et al. Performance of transperineal template-guided mapping biopsy in detecting prostate cancer in the initial and repeat biopsy setting. Prostate Cancer Prostatic Dis. 2010 Mar;13(1):71-7.
- Available at: http://files.shareholder.com/downloads/ghdx/2097290524x0x661617/85f38a0e-ccd5-47de-a348-8d0c2b5c03bd/ghdx_news_2013_5_8_general.pdf. Accessed September 29, 2013.
- Available at: http://www.myriad.com/products/prolaris. Accessed September 29, 2013.
- Gharaee-Kermani M, Macoska JA. Promising molecular targets and biomarkers for male BPH and LUTS. Curr Urol Rep. 2013 Aug 3.
- Clark RV, Hermann DJ, Cunningham GR, Wilson TH, Morrill BB, Hobbs S. Marked suppression of dihydrotestosterone in men with benign prostatic hyperplasia by dutasteride, a dual 5alpha-reductase inhibitor. J Clin Endocrinol Metab. 2004 May;89(5):2179-84.
- Schmidt LJ, Tindall DJ. Steroid 5 α-reductase inhibitors targeting BPH and prostate cancer. J Steroid Biochem Mol Biol. 2011 May;125(1-2):32-8.
- Kaplan SA, Ghafar MA, Volpe MA, Lam JS, Fromer D, Te AE. PSA response to finasteride challenge in men with a serum PSA greater than 4 ng/ml and previous negative prostate biopsy: preliminary study. Urology. 2002 Sep;60(3):464-8.
- Handel LN, Agarwal S, Schiff SF, Kelty PJ, Cohen SI. Can effect of finasteride on prostate-specific antigen be used to decrease repeat prostate biopsy? Urology. 2006 Dec;68(6):1220-3.
- Available at: http://www.medscape.com/viewarticle/734687. Accessed September 29, 2013.
- Thompson IM, Goodman PJ, Tangen CM, et al. The influence of finasteride on the development of prostate cancer. N Engl J Med. 2003 Jul 17;349(3):215-24.
- Andriole GL, Bostwick DG, Brawley OW, et al; REDUCE Study Group. Effect of dutasteride on the risk of prostate cancer. N Engl J Med. 2010 Apr 1;362(13):1192-202.
- Tan RS, Salazar JA. Risks of testosterone replacement therapy in ageing men. Expert Opin Drug Saf. 2004 Nov;3(6):599-606.
- Agarwal PK, Oefelein MG. Testosterone replacement therapy after primary treatment for prostate cancer. J Urol. 2005 Feb;173(2):533-6.
- Gooren L. Androgen deficiency in the aging male: benefits and risks of androgen supplementation. J Steroid Biochem Mol Biol. 2003 Jun;85(2-5):349-55.
- Morgentaler A. Testosterone replacement therapy and prostate cancer. Urol Clin North Am. 2007 Nov;34(4):555-63.
- Rhoden EL, Averbeck MA, Teloken PE. Androgen replacement in men under-going treatment for prostate cancer. J Sex Med. 2008 Sep;5(9):2202-8.
- Raynaud JP. Prostate cancer risk in testosterone-treated men. J Steroid Biochem Mol Biol. 2006 Dec;102(1-5):261-6.
- Rabbani F, Stroumbakis N, Kava BR, Cookson MS, Fair WR. Incidence and clinical significance of false-negative sextant prostate biopsies. J Urol. 1998 Apr;159(4):1247-50.
- Numao N, Kawakami S, Sakura M, et al. Characteristics and clinical significance of prostate cancers missed by initial transrectal 12-core biopsy. BJU Int. 2012 Mar;109(5):665-71.
- Kulkarni GS, Al-Azab R, Lockwood G, et al. Evidence for a biopsy derived grade artifact among larger prostate glands. J Urol. 2006 Feb;175(2):505-9.
- Shimamoto T, Ashida S, Yamasaki I, et al. The clinical value of 3 tesla diffusion-weighted magnetic resonance imaging in the diagnosis of prostate cancer. Hinyokika Kiyo. 2012 Mar;58(3):143-8.
- Ibrahiem EI, Mohsen T, Nabeeh AM, Osman Y, Hekal IA, Abou El-Ghar M. DWI-MRI: single, informative, and noninvasive technique for prostate cancer diagnosis. Scientific World Journal. 2012 973450.
- Andriole GL, Marberger M, Roehrborn CG. Clinical usefulness of serum prostate specific antigen for the detection of prostate cancer is preserved in men receiving the dual 5alpha-reductase inhibitor dutasteride. J Urol. 2006 May;175(5):1657-62.
- Dowling RJ, Goodwin PJ, Stambolic V. Understanding the benefit of metformin use in cancer treatment. BMC Med. 2011 Apr 6;9:33.
- Hirsch HA, Iliopoulos D, Tsichlis PN, Struhl K. Metformin selectively targets cancer stem cells, and acts together with chemotherapy to block tumor growth and prolong remission. Cancer Res. 2009 Oct 1;69(19):7507-11.
- Anisimov VN, Berstein LM, Egormin PA, et al. Effect of metformin on life span and on the development of spontaneous mammary tumors in HER-2/neu transgenic mice. Exp Gerontol. 2005 Aug-Sep;40(8-9):685-93.
- Vazquez-Martin A, Oliveras-Ferraros C, Del Barco S, Martin-Castillo B, Menendez JA. The anti-diabetic drug metformin suppresses self-renewal and proliferation of trastuzumab-resistant tumor-initiating breast cancer stem cells. Breast Cancer Res Treat. 2011 Apr;126(2):355-64.
- Tomimoto A, Endo H, Sugiyama M, et al. Metformin suppresses intestinal polyp growth in ApcMin/+ mice. Cancer Sci. 2008 Nov;99(11):2136-41.
- Gotlieb WH, Saumet J, Beauchamp MC, Gu J, Lau S, Pollak MN, Bruchim I. In vitro metformin anti-neoplastic activity in epithelial ovarian cancer. Gynecol Oncol. 2008 Aug;110(2):246-50.
- Cantrell LA, Zhou C, Mendivil A, Malloy KM, Gehrig PA, Bae-Jump VL. Metformin is a potent inhibitor of endometrial cancer cell proliferation--implications for a novel treatment strategy. Gynecol Oncol. 2010 Jan;116(1):92-8.
- Libby G, Donnelly LA, Donnan PT, Alessi DR, Morris AD, Evans JM. New users of metformin are at low risk of incident cancer: a cohort study among people with type 2 diabetes. Diabetes Care. 2009 Sep;32(9):1620-5.
- Memmott RM, Mercado JR, Maier CR, Kawabata S, Fox SD, Dennis PA. Metformin prevents tobacco carcinogen—induced lung tumorigenesis. Cancer Prev Res (Phila). 2010 Sep;3(9):1066-76.
- Evans JM, Donnelly LA, Emslie-Smith AM, Alessi DR, Morris AD. Metformin and reduced risk of cancer in diabetic patients. BMJ. 2005 Jun 4;330(7503):1304-5
- Currie CJ, Poole CD, Gale EA. The influence of glucose-lowering therapies on cancer risk in type 2 diabetes. Diabetologia. 2009 Sep;52(9):1766-77.
- Nagi DK, Yudkin JS. Effects of metformin on insulin resistance, risk factors for cardiovascular disease, and plasminogen activator inhibitor in NIDDM subjects. A study of two ethnic groups. Diabetes Care. 1993 16(4):621-29.
- Choi YK, Park KG. Metabolic roles of AMPK and metformin in cancer cells. Mol Cells. 2013 Jun 19.
- Luo Z, Zang M, Guo W. AMPK as a metabolic tumor suppressor: control of metabolism and cell growth. Future Oncol. 2010 Mar;6(3):457-70.
- Ben Sahra I, Regazzetti C, Robert G, et al. Metformin, independent of AMPK, induces mTOR inhibition and cell-cycle arrest through REDD1. Cancer Res. 2011 Jul 1;71(13):4366-72.
- Loubière C, Dirat B, Tanti JF, Bost F. New perspectives for metformin in cancer therapy. Ann Endocrinol (Paris). 2013 May;74(2):130-6.
- Zakikhani M, Dowling RJ, Sonenberg N, Pollak MN. The effects of adiponectin and metformin on prostate and colon neoplasia involve activation of AMP-activated protein kinase. Cancer Prev Res (Phila Pa). 2008 Oct;1(5):369-75.
- Ben Sahra I, Laurent K, Loubat A, et al. The antidiabetic drug metformin exerts an antitumoral effect in vitro and in vivo through a decrease of cyclin D1 level. Oncogene. 2008 Jun 5;27(25):3576-86.
- Ersoy C, Kiyici S, Budak F, et al. The effect of metformin treatment on VEGF and PAI-1 levels in obese type 2 diabetic patients. Diabetes Res Clin Pract. 2008 Jul;81(1):56-60.
- Parekh N, Lin Y, Vadiveloo M, Hayes RB, Lu-Yao GL. Metabolic dysregulation of the insulin-glucose axis and risk of obesity-related cancers in the Framingham Heart Study-Offspring Cohort (1971-2008). Cancer Epidemiol Biomarkers Prev. 2013 Sep 24.
- Ong KW, Hsu A, Tan BK. Anti-diabetic and anti-lipidemic effects of chlorogenic acid are mediated by ampk activation. Biochem Pharmacol. 2013 May 1;85(9):1341-51.
- Dunlap SM, Chiao LJ, Nogueira L, et al. Dietary energy balance modulates epithelial-to-mesenchymal transition and tumor progression in murine claudin-low and basal-like mammary tumor models. Cancer Prev Res (Phila). 2012 Jul;5(7):930-42.
- Available at: http://www.clinicaltrials.gov/ct2/show/nct01864096. Accessed October 1, 2013.
- Available at: http://www.abstractsonline.com/Plan/ViewAbstract.aspx?sKey=d14acbff-7602-45e9-a577-11db6325068e&cKey=a5dfd7e0-c8eb-4d99-814c-8423fdd9519c&mKey =%7b2D8C569E-B72C-4E7D-AB3B-070BEC7EB280%7d. Accessed October 1, 2013.
- Available at: http://journals.lww.com/oncology-times/blog/onlinefirst/pages/post.aspx?postid=451. Accessed October 10, 2013.
- Ravindran J, Prasad S, Aggarwal BB. Curcumin and cancer cells: how many ways can curry kill tumor cells selectively? AAPS J. 2009 Sep;11(3):495-510.
- Spratt DE, Zhang C, Zumsteg ZS, Pei X, Zhang Z, Zelefsky MJ. Metformin and prostate cancer: reduced development of castration-resistant disease and prostate cancer mortality. Eur Urol. 2013 Apr;63(4):709-16.
- Hvid T, Winding K, Rinnov A, et al. Endurance training improves insulin sensitivity and body composition in prostate cancer patients treated with androgen deprivation therapy. Endocr Relat Cancer. 2013 Aug 19;20(5):621-32.
- Available at: http://www.drugs.com/cdi/cabergoline.html. Accessed October 1, 2013.
- Langley RE, Burdett S, Tierney JF, Cafferty F, Parmar MK, Venning G. Aspirin and cancer: has aspirin been overlooked as an adjuvant therapy? Br J Cancer. 2011 Oct 11;105(8):1107-13.
- St Sauver JL, Jacobson DJ, McGree ME, Lieber MM, Jacobsen SJ. Protective association between nonsteroidal antiinflammatory drug use and measures of benign prostatic hyperplasia. Am J Epidemiol. 2006 Oct 15;164(8):760-8.
- Egan K, FitzGerald GA. Eicosanoids and the vascular endothelium. Handb Exp Pharmacol. 2006 176 (Pt 1):189-211.
- Wu KK. Aspirin and other cyclooxygenase inhibitors: new therapeutic insights. Semin Vasc Med. 2003 May;3(2):107-12.
- Available at: http://seer.cancer.gov/faststats/selections.php?run=runit&output=2&data=2&statistic=1&year=201302&race=1 &sex=2&age=1&series=cancer&cancer=66#Output. Accessed October 4, 2013.
- Available at: http://www.cancer.gov/cancertopics/factsheet/detection/psa. Accessed October 4, 2013.
- Available at: http://cancerpreventionresearch.aacrjournals.org/content/1/3/174.full. Accessed October 3, 2013.

