Life Extension Magazine®
For the past seven years, Life Extension has published extensive articles about chronic inflammation and the numerous diseases it causes, such as cancer, atherosclerosis, arthritis, dementia, and more. In these articles, we showed how aging people over-express a molecule called nuclear factor-kappa beta, which then ignites a lethal inflammatory cascade throughout the body. An abundance of new scientific studies has validated the multiple pathological effects inflicted by nuclear factor-kappa beta. Fortunately, scientists have discovered methods to safely suppress this insidious chronic inflammation-inducing agent. Aging humans are thus able to protect against a major cause of age-related disease. In this article, we enlighten Life Extension members about what nuclear factor-kappa beta is and what can be done to suppress it. Understanding the relationship between nuclear factor-kappa beta (NFkB) and inflammation is critical to maintaining your health and longevity. Over the last several years, scientists have gained new insights into how NFkB functions in the body. As a result, we are on the verge of finding ways to overcome our genetic predisposition toward degenerative conditions such as cancer, heart disease, arthritis, and even asthma. Simply put, NFkB is a protein that acts as a switch to turn inflammation on and off in the body. Scientists describe NFkB as a “smoke sensor” that detects dangerous threats like free radicals and infectious agents. In response to these threats, NFkB “turns on” the genes that produce inflammation. As we age, NFkB expression in the body increases, provoking widespread chronic inflammation and setting the stage for diseases ranging from atherosclerosis and diabetes to Alzheimer’s. The knowledge of this simple fact should motivate us to counteract NFkB’s deleterious effects and thus guard against many of the diseases commonly associated with aging. As we have reported over the last several years, inflammation is the key initiating factor in major degenerative diseases. In fact, some scientists estimate that inflammation underlies up to 98% of the diseases afflicting humans, including a vast array of seemingly different conditions such as cancer, heart disease, diabetes, and neurodegenerative disorders.1 NFkB is an instigating factor that unleashes inflammatory responses in chronic disease conditions. For example, NFkB can signal our cells to continue to multiply long past their normal life span, which can promote cancer. Furthermore, NFkB can further spark the smoldering inflammation that damages joint tissues, thereby provoking crippling arthritic conditions. NFkB likewise plays a role in spurring inflammation in the nervous system, which can set the stage for the onset of various neurological disorders. Scientists believe that NFkB-induced inflammation in the airways may play a role in asthma.
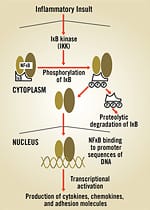
The identification of NFkB as a critical “switch” that “turns on” inflammation has profound implications for both preventing and treating some of today’s deadliest diseases. Clearly, NFkB is something we need to control if our goal is to lead a long and healthy life. Fortunately, ongoing research continues to uncover a wealth of natural remedies that suppress NFkB’s activity in the body. These remedies provide the foundation for safe, effective nutritional strategies to quell NFkB and disease-provoking inflammation, thus providing a formidable defense against a vast array of deadly diseases and against aging itself. Interacting with DNA: How NFkB WorksPresent in the interior portion (cytoplasm) of every cell, NFkB is normally bound to inhibitory proteins that keep it in an inactive state. When cells are exposed to infectious invaders or stressors such as free radicals or environmental toxins (like cigarette smoke), NFkB is activated. NFkB then travels to the cells’ command center, known as the nucleus, where it binds with DNA to turn certain genes on or off. By interacting with more than 400 different genes, NFkB can thus activate the body’s blueprints for inflammation.1 These gene products are used to coordinate further inflammatory and immune responses in the body. NFkB and Cancer DevelopmentOne of NFkB’s most lethal functions is inducing cancer in our bodies. Scientists are finding that, in addition to its central role in producing inflammation, NFkB plays an equally prominent and related role in the development of cancer.
NFkB acts in each of the main phases of cancer development, which are known as initiation, promotion, and progression. NFkB “switches on” genes that allow cells to become initiated, and once initiated, to have their growth promoted, and once promoted, to progress and invade healthy tissue.8 Successful cancers evade powerful repair and control mechanisms at each of the three distinct stages of cancer development.8 Since NFkB is involved in each of the three stages, it is critically important that we understand NFkB’s actions in our bodies and what we can do to better control them. The NFkB system has emerged as the central actor in the link between inflammation and cancer. NFkB affects both malignant and non-malignant tumor cells. In malignant cells, it turns on genes that create resistance to apoptotic cell death and DNA damage, in effect promoting cancer development by rendering cells capable of reproducing, even when they are exposed to pharmaceutical anti-cancer agents. In non-malignant tumor cells, NFkB turns on genes that produce factors to stimulate blood vessel formation, in support of rapid tumor enlargement and progression. Finally, byproducts produced by NFkB stimulation can also damage DNA, thereby contributing to the very earliest stages of tumor initiation.8 | |||||
How Inhibiting NFkB Helps Fight CancerRecent discoveries about NFkB confirm the deadly link between inflammation and cancer. It is well known that nutrients and drugs that reduce inflammation also help fight cancer.8,9 Some anti-inflammatory drugs, however, carry cardiovascular risk.10,11 Scientists hope that therapies that block NFkB may provide safe, effective action against both inflammation and cancer.
The ubiquitous presence of NFkB throughout the inflammation-cancer cycle suggests that the next breakthroughs in cancer treatment will likely center on the inhibition of NFkB and its actions. As scientists learn more about NFkB and the complex systems that regulate it, they also learn more about the wide array of substances that can inhibit its dangerous actions. For example, the anti-inflammatory drug ibuprofen inhibits not only the COX-2 enzyme but also NFkB,12 and has a well-established safety record. This drug, as well as many natural inhibitors of NFkB, will therefore play an important role in controlling the inflammatory components of tumor formation and growth. Because the NFkB factors are active in both the cancerous cells and inflammatory cells in tumors, nutrients or drugs that can inhibit NFkB show tremendous promise as anti-cancer or cancer-preventive agents.8 Scientists believe that the combination of NFkB inhibition with drugs or cytokines that induce cancer cell death has great promise in fighting cancer.13 Because the NFkB system is also involved in producing healthy immune responses, there are concerns about its long-term inhibition. While NFkB seems to be most profoundly involved in cancer at the stages of promotion and progression,8,14 it may be possible to use inhibitors for relatively short periods. Another potential use for such inhibitors would be in combination with chemotherapy or radiation treatments, as a means of controlling the associated inflammation and enhancing the effects of those treatments.8
Nutrients That Inhibit NFkBThe search is on for safe, effective inhibitors of NFkB. One of the most exciting features of the explosion of NFkB research is that it sheds new light on the mechanisms of many familiar nutrients.
Health-conscious people are quite familiar with how antioxidants, vitamins, minerals, and essential nutrients such as omega-3 fatty acids can maintain health and prevent disease. It is becoming increasingly clear that many such compounds exert some of their beneficial effects through interactions with the NFkB system. Although the precise mechanisms vary, all of these agents work by inhibiting NFkB activation, thus preventing the expression of genes involved in inflammation and cancer development. Here we summarize familiar nutrients whose NFkB-related actions are now coming to light. AntioxidantsAntioxidants are known to reduce inflammation and cancer risk. The identification of NFkB as the common link to both processes may serve to explain how these substances operate. Vitamins E and C have been shown to reduce inflammatory cytokine production that is a consequence of NFkB activation.20 N-acetylcysteine inhibits NFkB, which is likely the mechanism by which it confers its health-promoting effects.21 S-adenosyl-methionine (SAMe) exerts some of its powerful anti-inflammatory effects by reducing NFkB activation.22 The potent antioxidant lipoic acid binds to and inhibits NFkB in the cell’s nucleus.23 Zinc may also exert its antioxidant effect by reducing NFkB activation.24 Essential Fatty Acids and Other LipidsThe omega-3 fatty acids are also known to reduce inflammation and decrease the production of inflammatory cytokines. Evidence is emerging that these effects occur due to inhibition of NFkB activity by eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA), and other essential fatty acids in this class.25-27 EPA and DHA protect the eye’s retinal cells from oxidative damage. Moreover, these fatty acids may impair the overgrowth of blood vessel cells that occurs in several retinal diseases, by reducing the production of inflammatory cytokines, vascular growth factors, and adhesion molecules, all via the common pathway of NFkB inhibition.28 Isoflavones and PhytoestrogensSoy isoflavones and other plant flavonoids are well-established modulators of the immune system’s inflammatory responses. These phytoestrogens (plant-derived, estrogen-like molecules) are known to help reduce the risk of certain hormone-dependent cancers, as well as the risk and severity of osteoporosis.29 Researchers have shown that the isoflavone-induced inhibition of NFkB is the mechanism by which isoflavones reduce the invasiveness of breast cancer and increase programmed cell death in various human cancer cell lines.30-32 Evidence also indicates that isoflavones may act by the same mechanism to inhibit bone loss in osteoporosis.33 Some researchers have speculated that one of the reasons women live longer than men is related to the favorable effects of estrogen on up-regulating antioxidant genes often suppressed by NFkB, suggesting that the phytoestrogens might have similar effects in promoting longevity.34 From Garden to Medicine ChestHerbs and spices from around the world have long been sought for their pleasing flavors and healing qualities. Even today, these plant extracts are valued worldwide for promoting health and fighting disease. Scientists are discovering that many of these natural agents act through the universal mechanism of inhibiting the over-expression of NFkB.
Curcumin is a compound found in a number of South Asian spices, most prominently in turmeric, a component of curry seasoning. Curcumin has well-established antioxidant and anti-inflammatory effects.35,36 The extent to which curcumin exerts these effects by inhibiting NFkB is becoming increasingly clear.37 Curcumin acts directly within the cell’s nucleus and also acts on substances that activate NFkB. For example, it binds iron and copper in brain tissue, reducing the activation of NFkB that is associated with the production of amyloid beta proteins in Alzheimer’s disease.35 Strong evidence suggests that curcumin may fight the following inflammatory diseases:
| |||||||
Licorice root extracts are among the oldest remedies in Chinese medicine, and have long been used for their anti-inflammatory, anti-viral, anti-ulcer, and cancer-preventive properties.76,77 More recently, scientists discovered that a major component of licorice inhibited NFkB and protected rat liver cells from alcohol toxicity.78 Another licorice extract inhibited NFkB activation and decreased production of a pro-inflammatory cytokine in human colon cells that had been exposed to an inflammatory challenge.79 These results elegantly demonstrate how NFkB inhibition can interrupt the inflammatory cycle by which cytokines stimulate the production of still more cytokines. Glabridin, another licorice root extract, produces similar anti-inflammatory effects by inhibiting NFkB.80 Capsaicin, the main ingredient in red pepper, has both anti-inflammatory and anti-cancer effects.81-83 Red pepper compounds have long been used to manage inflammatory joint conditions.37 Capsaicin inhibits the induction of two inflammation-provoking enzymes in stimulated macrophage immune cells.82 This effect is attributable to its inhibition of NFkB activation.83 Capsaicin also induces cell death in many cancers by modulating NFkB.81 Like curcumin, capsaicin inhibits the growth of adult T-cell leukemia cells by impairing NFkB activation.84 Capsaicin further impairs cancer progression by reducing levels of vascular endothelial growth factor, thus depriving growing cancers of nutrients.85 Clove extract (eugenol) inhibits NFkB-mediated expression of inflammatory cytokines.86,87 Like capsaicin, eugenol inhibits NFkB activation in stimulated macro-phage immune cells,87 reducing their synthesis of COX-2 and inflammatory cytokines.86 Oil of cloves has been used in dental care for centuries, and eugenol is now widely used to promote healing and prevent excessive inflammation after root canal surgery.88,89 Ginger extracts exert anti-inflammatory activity and stimulate cancer cell death by inhibiting NFkB.90-92 Ginger reduces expression of the key inflammatory enzymes COX-1 and COX-2.93 Topical application of ginger extract inhibits skin inflammation in a mouse model92 by inhibiting NFkB.91 A ginger extract was shown to enhance tumor cell death and down-regulate production of tumor invasion factors by preventing activation of NFkB.90 Basil and rosemary extracts, which contain ursolic acid, reduce cancer cell proliferation and tumor progression through NFkB inhibition.94-96 By inactivating NFkB, ursolic acid prevents initiated cells from reproducing and also triggers tumor cell death.95 This compound further down-regulates molecules that are required for tumor invasion and metastasis.96 Ursolic acid works through its effects on NFkB to induce resting macrophage immune cells, and thus to participate in tumor cell destruction in the early stages of cancer.97 Ursolic acid derivatives that inhibit NFkB have been shown to suppress pro-inflammatory enzyme expression in mouse models of inflammation.98 This effect has been associated with reduced cardiac fibrosis (scar tissue) in the heart tissue of diabetic mice.94 Garlic has now been shown to exert its anti-inflammatory and immunomodulatory effects by inhibiting NFkB.37,99 Garlic extracts lowered NFkB activity by up to 41% in human blood and kidney cells that had been exposed to an inflammation-provoking challenge, thus reducing the expression of certain cytokines.100 These effects may be linked to the observation that a garlic compound inhibits damage to endothelial cells lining blood vessels and reduces atherosclerotic changes.101 Garlic’s inhibition of NFkB leads to reduced production of chemicals that cause lipid peroxidation, and this could provide further protection from atherosclerosis.102 NFkB inhibition is credited for garlic’s ability to protect liver cells from auto-immune damage in an animal model,103 as well as induce cell death in leukemia.104
Pomegranate fruit extract protects cells against the effects of ultraviolet B radiation by inhibiting ultraviolet light-stimulated NFkB activation.105 Pomegranate fruit extract also prevented chemically induced skin cancers in mice through NFkB-mediated effects on both cancer initiation and promotion.106 Blockade of NFkB by pomegranate fruit extract has shown promise in osteoarthritis by inhibiting the production of protein-digesting enzymes and inflammatory cytokines.107 Pomegranate wine reduced the activation of NFkB in vascular endothelial cells by inflammatory mediators or biomechanical stresses,108 thus protecting against atherosclerosis.109 SummaryScientists have discovered that by controlling our DNA, nuclear factor-kappa beta (NFkB) plays a central role in determining our health and longevity. By integrating signals of inflammation, NFkB appears to be the common link between such diverse conditions as heart disease, cancer, and arthritis. Agents that control NFkB’s influence within the human body—such as omega-3 fatty acids, phytoestrogens, curcumin, garlic, licorice, ginger, rosemary, and pomegranate—hold great promise in fighting many diverse diseases and in promoting long and healthy lives. Julius G. Goepp, MD, is a pediatrician with additional certification in pediatric emergency medicine. He received his MD from the University of Maryland and is currently Senior Consultant at Lupine Creative Consulting, Inc., in Rochester, NY.
| |||||
| References | |||||
| 1. Ahn KS, Aggarwal BB. Transcription Factor NF-{kappa}B: A Sensor for Smoke and Stress Signals. Ann N Y Acad Sci. 2005 Nov;1056:218-33. 2. Orozco G, Sanchez E, Collado MD, et al. Analysis of the functional NFKB1 promoter polymorphism in rheumatoid arthritis and systemic lupus erythematosus. Tissue Antigens. 2005 Feb;65(2):183-6. 3. Sato Y, Kato J, Takimoto R, et al. Hepatitis C virus core protein promotes proliferation of human hepatoma cells through enhancement of transforming growth factor-{alpha} expression via activation of NF-{kappa}B. Gut. 2006 Mar 31;[Epub ahead of print]. 4. Di Sabatino A, Morera R, Ciccocioppo R, et al. Oral butyrate for mildly to moderately active Crohn’s disease. Alimen Pharmacol Ther. 2005 Nov 1;22(9):789-94. 5. Kawano S, Kubota T, Monden Y, et al. Blockade of NF-{kappa}B improves cardiac function and survival after myocardial infarction. Am J Physiol Heart Circ Physiol. 2006 Apr 21;[Epub ahead of print]. 6. Uzzo RG, Crispen PL, Golovine K, Makhov P, Horwitz EM, Kolenko VM. Diverse effects of zinc on NF-{kappa}B and AP-1 transcription factors: implications for prostate cancer progression. Carcinogenesis. 2006 Apr 10;[Epub ahead of print]. 7. Sriwijitikamol A, Christ-Roberts C, Berria R, et al. Reduced skeletal muscle inhibitor of kappaB content is associated with insulin resistance in subjects with type 2 diabetes: reversal by exercise training. Diabetes. 2006 Mar;55(3):760-7. 8. Karin M, Greten FR. NF-kappaB: linking inflammation and immunity to cancer development and progression. Nat Rev Immunol. 2005 Oct;5(10):749-59. 9. Steinbach G, Lynch PM, Phillips RK, et al. The effect of celecoxib, a cyclooxygenase-2 inhibitor, in familial adenomatous polyposis. N Engl J Med. 2000 Jun 29;342(26):1946-52. 10. Bresalier RS, Sandler RS, Quan H, et al. Cardiovascular events associated with rofecoxib in a colorectal adenoma chemoprevention trial. N Engl J Med. 2005 Mar 17;352(11):1092-102. 11. Solomon SD, McMurray JJ, Pfeffer MA, et al. Cardiovascular risk associated with celecoxib in a clinical trial for colorectal adenoma prevention. N Engl J Med. 2005 Mar 17;352(11):1071-80. 12. Karin M, Yamamoto Y, Wang QM. The IKK NF-kappa B system: a treasure trove for drug development. Nat Rev Drug Discov. 2004 Jan;3(1):17-26. 13. Li Q, Verma IM. NF-kappaB regulation in the immune system. Nat Rev Immunol. 2002 Oct;2(10):725-34. 14. Karin M, Cao Y, Greten FR, Li ZW. NF-kappaB in cancer: from innocent bystander to major culprit. Nat Rev Cancer. 2002 Apr;2(4):301-10. 15. Maeda S, Kamata H, Luo JL, Leffert H, Karin M. IKKbeta couples hepatocyte death to cytokine-driven compensatory proliferation that promotes chemical hepatocarcinogenesis. Cell. 2005 Jul 1;121(7):977-90. 16. Kamata H, Honda S, Maeda S, et al. Reactive oxygen species promote TNFalpha-induced death and sustained JNK activation by inhibiting MAP kinase phosphatases. Cell. 2005 Mar 11;120(5):649-61. 17. Vakkila J, Lotze MT. Inflammation and necrosis promote tumour growth. Nat Rev Immunol. 2004 Aug;4(8):641-8. 18. Becker C, Fantini MC, Schramm C, et al. TGF-beta suppresses tumor progression in colon cancer by inhibition of IL-6 trans-signaling. Immunity. 2004 Oct;21(4):491-501. 19. Greten FR, Eckmann L, Greten TF, et al. IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell. 2004 Aug 6;118(3):285-96. 20. Grimble RF. Effect of antioxidative vitamins on immune function with clinical applications. Int J Vitam Nutr Res. 1997;67(5):312-20. 21. Lee JY, Je JH, Jung KJ, Yu BP, Chung HY. Induction of endothelial iNOS by 4-hydroxyhexenal through NF-kappaB activation. Free Radic Biol Med. 2004 Aug 15;37(4):539-48. 22. Majano PL, Garcia-Monzon C, Garcia-Trevijano ER, et al. S-Adenosylmethionine modulates inducible nitric oxide synthase gene expression in rat liver and isolated hepatocytes. J Hepatol. 2001 Dec;35(6):692-9. 23. Lee HA, Hughes DA. Alpha-lipoic acid modulates NF-kappaB activity in human monocytic cells by direct interaction with DNA. Exp Gerontol. 2002 Jan;37(2-3):401-10. 24. Prasad AS, Bao B, Beck FW, Kucuk O, Sarkar FH. Antioxidant effect of zinc in humans. Free Radic Biol Med. 2004 Oct 15;37(8):1182-90. 25. Zhao G, Etherton TD, Martin KR, et al. Anti-inflammatory effects of polyunsaturated fatty acids in THP-1 cells. Biochem Biophys Res Commun. 2005 Oct 28;336(3):909-17. 26. Jia Y, Turek JJ. Altered NF-kappaB gene expression and collagen formation induced by polyunsaturated fatty acids. J Nutr Biochem. 2005 Aug;16(8):500-6. 27. Li H, Ruan XZ, Powis SH, et al. EPA and DHA reduce LPS-induced inflammation responses in HK-2 cells: evidence for a PPAR-gamma-dependent mechanism. Kidney Int. 2005 Mar;67(3):867-74. 28. SanGiovanni JP, Chew EY. The role of omega-3 long-chain polyunsaturated fatty acids in health and disease of the retina. Prog Retin Eye Res. 2005 Jan;24(1):87-138. 29. Dijsselbloem N, Vanden BW, De NA, Haegeman G. Soy isoflavone phyto-pharmaceuticals in interleukin-6 affections. Multi-purpose nutraceuticals at the crossroad of hormone replacement, anti-cancer and anti-inflammatory therapy. Biochem Pharmacol. 2004 Sep 15;68(6):1171-85. 30. Kang JS, Yoon YD, Han MH, et al. Estrogen receptor-independent inhibition of tumor necrosis factor-alpha gene expression by phytoestrogen equol is mediated by blocking nuclear factor-kappaB activation in mouse macrophages. Biochem Pharmacol. 2005 Dec 19;71(1-2):136-43. 31. Li Y, Ahmed F, Ali S, et al. Inactivation of nuclear factor kappaB by soy isoflavone genistein contributes to increased apoptosis induced by chemotherapeutic agents in human cancer cells. Cancer Res. 2005 Aug 1;65(15):6934-42. 32. Valachovicova T, Slivova V, Bergman H, Shuherk J, Sliva D. Soy isoflavones suppress invasiveness of breast cancer cells by the inhibition of NF-kappaB/AP-1-dependent and -independent pathways. Int J Oncol. 2004 Nov;25(5):1389-95. 33. Jimi E, Ghosh S. Role of nuclear factor-kappaB in the immune system and bone. Immunol Rev. 2005 Dec;208:80-7. 34. Vina J, Borras C, Gambini J, Sastre J, Pallardo FV. Why females live longer than males? Importance of the upregulation of longevity-associated genes by oestrogenic compounds. FEBS Lett. 2005 May 9;579(12):2541-5. 35. Baum L, Ng A. Curcumin interaction with copper and iron suggests one possible mechanism of action in Alzheimer’s disease animal models. J Alzheimers Dis. 2004 Aug;6(4):367-77. 36. Holt PR, Katz S, Kirshoff R. Curcumin therapy in inflammatory bowel disease: a pilot study. Dig Dis Sci. 2005 Nov;50(11):2191-3. 37. Aggarwal BB, Shishodia S. Suppression of the nuclear factor-kappaB activation pathway by spice-derived phytochemicals: reasoning for seasoning. Ann NY Acad Sci. 2004 Dec;1030:434-41. 38. Jian YT, Mai GF, Wang JD, et al. Preventive and therapeutic effects of NF-kappaB inhibitor curcumin in rats colitis induced by trinitrobenzene sulfonic acid. World J Gastroenterol. 2005 Mar 28;11(12):1747-52. 39. Salh B, Assi K, Templeman V, et al. Curcumin attenuates DNB-induced murine colitis. Am J Physiol Gastrointest Liver Physiol. 2003 Jul;285(1):G235-43. 40. Nanji AA, Jokelainen K, Tipoe GL, et al. Curcumin prevents alcohol-induced liver disease in rats by inhibiting the expression of NF-kappa B-dependent genes. Am J Physiol Gastrointest Liver Physiol. 2003 Feb;284(2):G321-7. 41. Leclercq IA, Farrell GC, Sempoux C, dela PA, Horsmans Y. Curcumin inhibits NF-kappaB activation and reduces the severity of experimental steatohepatitis in mice. J Hepatol. 2004 Dec;41(6):926-34. 42. Cole GM, Morihara T, Lim GP, et al. NSAID and Antioxidant Prevention of Alzheimer’s Disease: Lessons from In Vitro and Animal Models. Ann NY Acad Sci. 2004 Dec;1035:68-84. 43. Kang G, Kong PJ, Yuh YJ, et al. Curcumin suppresses lipopolysaccharide-induced cyclooxygenase-2 expression by inhibiting activator protein 1 and nuclear factor kappab bindings in BV2 microglial cells. J Pharmacol Sci. 2004 Mar;94(3):325-8. 44. Witek-Zawada B, Koj A. Regulation of expression of stromyelysin-1 by proinflammatory cytokines in mouse brain astrocytes. J Physiol Pharmacol. 2003 Dec;54(4):489-96. 45. Yang F, Lim GP, Begum AN, et al. Curcumin inhibits formation of amyloid beta oligomers and fibrils, binds plaques, and reduces amyloid in vivo. J Biol Chem. 2005 Feb 18;280(7):5892-901. 46. Giri RK, Rajagopal V, Kalra VK. Curcumin, the active constituent of turmeric, inhibits amyloid peptide-induced cytochemokine gene expression and CCR5-mediated chemotaxis of THP-1 monocytes by modulating early growth response-1 transcription factor. J Neurochem. 2004 Dec;91(5):1199-210. 47. Shakibaei M, Schulze-Tanzil G, John T, Mobasheri A. Curcumin protects human chondrocytes from IL-l1beta-induced inhibition of collagen type II and beta1-integrin expression and activation of caspase-3: an immunomorphological study. Ann Anat. 2005 Nov;187(5-6):487-97. 48. Banerjee M, Tripathi LM, Srivastava VM, Puri A, Shukla R. Modulation of inflammatory mediators by ibuprofen and curcumin treatment during chronic inflammation in rat. Immunopharmacol Immunotoxicol. 2003 May;25(2):213-24. 49. Lev-Ari S, Strier L, Kazanov D, et al. Curcumin synergistically potentiates the growth-inhibitory and pro-apoptotic effects of celecoxib in osteoarthritis synovial adherent cells. Rheumatology (Oxford). 2006 Feb;45(2):171-7. 50. Duvoix A, Blasius R, Delhalle S, et al. Chemopreventive and therapeutic effects of curcumin. Cancer Lett. 2005 Jun 8;223(2):181-90. 51. Aggarwal BB, Shishodia S, Takada Y, et al. Curcumin suppresses the paclitaxel-induced nuclear factor-kappaB pathway in breast cancer cells and inhibits lung metastasis of human breast cancer in nude mice. Clin Cancer Res. 2005 Oct 15;11(20):7490-8. 52. Thomas RK, Sos ML, Zander T, et al. Inhibition of nuclear translocation of nuclear factor-kappaB despite lack of functional IkappaBalpha protein overcomes multiple defects in apoptosis signaling in human B-cell malignancies. Clin Cancer Res. 2005 Nov 15;11(22):8186-94. 53. Aggarwal S, Ichikawa H, Takada Y, et al. Curcumin (diferuloylmethane) down-regulates expression of cell proliferation and antiapoptotic and metastatic gene products through suppression of IkappaBalpha kinase and Akt activation. Mol Pharmacol. 2006 Jan;69(1):195-206. 54. Kim K, Ryu K, Ko Y, Park C. Effects of nuclear factor-kappaB inhibitors and its implication on natural killer T-cell lymphoma cells. Br J Haematol. 2005 Oct;131(1):59-66. 55. Baliga MS, Katiyar SK. Chemoprevention of photocarcinogenesis by selected dietary botanicals. Photochem Photobiol Sci. 2006 Feb;5(2):243-53. 56. Bush JA, Cheung KJ, Jr., Li G. Curcumin induces apoptosis in human melanoma cells through a Fas receptor/caspase-8 pathway independent of p53. Exp Cell Res. 2001 Dec 10;271(2):305-14. 57. LoTempio MM, Veena MS, Steele HL, et al. Curcumin suppresses growth of head and neck squamous cell carcinoma. Clin Cancer Res. 2005 Oct 1;11(19 Pt 1):6994-7002. 58. Deeb D, Jiang H, Gao X, et al. Curcumin sensitizes prostate cancer cells to tumor necrosis factor-related apoptosis-inducing ligand/Apo2L by inhibiting nuclear factor-kappaB through suppression of IkappaBalpha phosphorylation. Mol Cancer Ther. 2004 Jul;3(7):803-12. 59. Kumar AP, Garcia GE, Ghosh R, et al. 4-Hydroxy-3-methoxybenzoic acid methyl ester: a curcumin derivative targets Akt/NF kappa B cell survival signaling pathway: potential for prostate cancer management. Neoplasia. 2003 May;5(3):255-66. 60. Hong JH, Ahn KS, Bae E, Jeon SS, Choi HY. The effects of curcumin on the invasiveness of prostate cancer in vitro and in vivo. Prostate Cancer Prostatic Dis. 2006 Jan 3. 61. Ramachandran C, Rodriguez S, Ramachandran R, et al. Expression profiles of apoptotic genes induced by curcumin in human breast cancer and mammary epithelial cell lines. Anticancer Res. 2005 Sep;25(5):3293-302. 62. Divya CS, Pillai MR. Antitumor action of curcumin in human papillomavirus associated cells involves downregulation of viral oncogenes, prevention of NFkB and AP-1 translocation, and modulation of apoptosis. Mol Carcinog. 2006 May;45(5):320-32. 63. Plummer SM, Holloway KA, Manson MM, et al. Inhibition of cyclo-oxygenase 2 expression in colon cells by the chemopreventive agent curcumin involves inhibition of NF-kappaB activation via the NIK/IKK signalling complex. Oncogene. 1999 Oct 28;18(44):6013-20. 64. Garcea G, Berry DP, Jones DJ, et al. Consumption of the putative chemopreventive agent curcumin by cancer patients: assessment of curcumin levels in the colorectum and their pharmacodynamic consequences. Cancer Epidemiol Biomarkers Prev. 2005 Jan;14(1):120-5. 65. Chen A, Xu J, Johnson AC. Curcumin inhibits human colon cancer cell growth by suppressing gene expression of epidermal growth factor receptor through reducing the activity of the transcription factor Egr-1. Oncogene. 2006 Jan 12;25(2):278-87. 66. Jung EM, Lim JH, Lee TJ, et al. Curcumin sensitizes tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced apoptosis through reactive oxygen species-mediated upregulation of death receptor 5 (DR5). Carcinogenesis. 2005 Nov;26(11):1905-13. 67. Lev-Ari S, Strier L, Kazanov D, et al. Celecoxib and curcumin synergistically inhibit the growth of colorectal cancer cells. Clin Cancer Res. 2005 Sep 15;11(18):6738-44. 68. Shishodia S, Potdar P, Gairola CG, Aggarwal BB. Curcumin (diferuloylmethane) down-regulates cigarette smoke-induced NF-kappaB activation through inhibition of IkappaBalpha kinase in human lung epithelial cells: correlation with suppression of COX-2, MMP-9 and cyclin D1. Carcinogenesis. 2003 Jul;24(7):1269-79. 69. Lee J, Im YH, Jung HH, et al. Curcumin inhibits interferon-alpha induced NF-kappaB and COX-2 in human A549 non-small cell lung cancer cells. Biochem Biophys Res Commun. 2005 Aug 26;334(2):313-8. 70. Radhakrishna PG, Srivastava AS, Hassanein TI, Chauhan DP, Carrier E. Induction of apoptosis in human lung cancer cells by curcumin. Cancer Lett. 2004 May 28;208(2):163-70. 71. Bharti AC, Donato N, Singh S, Aggarwal BB. Curcumin (diferuloylmethane) down-regulates the constitutive activation of nuclear factor-kappa B and IkappaBalpha kinase in human multiple myeloma cells, leading to suppression of proliferation and induction of apoptosis. Blood. 2003 Feb 1;101(3):1053-62. 72. Han SS, Keum YS, Seo HJ, Surh YJ. Curcumin suppresses activation of NF-kappaB and AP-1 induced by phorbol ester in cultured human promyelocytic leukemia cells. J Biochem Mol Biol. 2002 May 31;35(3):337-42. 73. Tomita M, Kawakami H, Uchihara JN, et al. Curcumin (diferuloylmethane) inhibits constitutive active NF-kappaB, leading to suppression of cell growth of human T-cell leukemia virus type I-infected T-cell lines and primary adult T-cell leukemia cells. Int J Cancer. 2006 Feb 1;118(3):765-72. 74. Cheng AL, Hsu CH, Lin JK, et al. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res. 2001 Jul;21(4B):2895-900. 75. Sharma RA, Euden SA, Platton SL, et al. Phase I clinical trial of oral curcumin: biomarkers of systemic activity and compliance. Clin Cancer Res. 2004 Oct 15;10(20):6847-54. 76. Wang ZY, Nixon DW. Licorice and cancer. Nutr Cancer. 2001;39(1):1-11. 77. Shibata S. A drug over the millennia: pharmacognosy, chemistry, and pharmacology of licorice. Yakugaku Zasshi. 2000 Oct;120(10):849-62. 78. Wang JY, Guo JS, Li H, Liu SL, Zern MA. Inhibitory effect of glycyrrhizin on NF-kappaB binding activity in CCl4- plus ethanol-induced liver cirrhosis in rats. Liver. 1998 Jun;18(3):180-5. 79. Kang OH, Kim JA, Choi YA, et al. Inhibition of interleukin-8 production in the human colonic epithelial cell line HT-29 by 18 beta-glycyrrhetinic acid. Int J Mol Med. 2005 Jun;15(6):981-5. 80. Kang JS, Yoon YD, Cho IJ, et al. Glabridin, an isoflavan from licorice root, inhibits inducible nitric-oxide synthase expression and improves survival of mice in experimental model of septic shock. J Pharmacol Exp Ther. 2005 Mar;312(3):1187-94. 81. Lee YS, Kang YS, Lee JS, Nicolova S, Kim JA. Involvement of NADPH oxidase-mediated generation of reactive oxygen species in the apototic cell death by capsaicin in HepG2 human hepatoma cells. Free Radic Res. 2004 Apr;38(4):405-12. 82. Chen CW, Lee ST, Wu WT, et al. Signal transduction for inhibition of inducible nitric oxide synthase and cyclooxygenase-2 induction by capsaicin and related analogs in macrophages. Br J Pharmacol. 2003 Nov;140(6):1077-87. 83. Kim CS, Kawada T, Kim BS, et al. Capsaicin exhibits anti-inflammatory property by inhibiting IkB-a degradation in LPS-stimulated peritoneal macrophages. Cell Signal. 2003 Mar;15(3):299-306. 84. Zhang J, Nagasaki M, Tanaka Y, Morikawa S. Capsaicin inhibits growth of adult T-cell leukemia cells. Leuk Res. 2003 Mar;27(3):275-83. 85. Patel PS, Yang S, Li A, Varney ML, Singh RK. Capsaicin regulates vascular endothelial cell growth factor expression by modulation of hypoxia inducing factor-1alpha in human malignant melanoma cells. J Cancer Res Clin Oncol. 2002 Sep;128(9):461-8. 86. Murakami Y, Shoji M, Hirata A, et al. Dehydrodiisoeugenol, an isoeugenol dimer, inhibits lipopolysaccharide-stimulated nuclear factor kappa B activation and cyclooxygenase-2 expression in macrophages. Arch Biochem Biophys. 2005 Feb 15;434(2):326-32. 87. Murakami Y, Shoji M, Hanazawa S, Tanaka S, Fujisawa S. Preventive effect of bis-eugenol, a eugenol ortho dimer, on lipopolysaccharide-stimulated nuclear factor kappa B activation and inflammatory cytokine expression in macrophages. Biochem Pharmacol. 2003 Sep 15;66(6):1061-6. 88. Ozalp N, Saroglu I, Sonmez H. Evaluation of various root canal filling materials in primary molar pulpectomies: an in vivo study. Am J Dent. 2005 Dec;18(6):347-50. 89. Damle SG, Nadkarni UM. Calcium hydroxide and zinc oxide eugenol as root canal filling materials in primary molars: a comparative study. Aust Endod J. 2005 Dec;31(3):114-9. 90. Takada Y, Murakami A, Aggarwal BB. Zerumbone abolishes NF-kappaB and IkappaBalpha kinase activation leading to suppression of antiapoptotic and metastatic gene expression, upregulation of apoptosis, and downregulation of invasion. Oncogene. 2005 Oct 20;24(46):6957-69. 91. Kim SO, Kundu JK, Shin YK, et al. [6]-Gingerol inhibits COX-2 expression by blocking the activation of p38 MAP kinase and NF-kappaB in phorbol ester-stimulated mouse skin. Oncogene. 2005 Apr 7;24(15):2558-67. 92. Kim SO, Chun KS, Kundu JK, Surh YJ. Inhibitory effects of [6]-gingerol on PMA-induced COX-2 expression and activation of NF-kappaB and p38 MAPK in mouse skin. Biofactors. 2004;21(1-4):27-31. 93. Grzanna R, Lindmark L, Frondoza CG. Ginger—an herbal medicinal product with broad anti-inflammatory actions. J Med Food. 2005;8(2):125-32. 94. Huang TH, Yang Q, Harada M, et al. Pomegranate flower extract diminishes cardiac fibrosis in Zucker diabetic fatty rats: modulation of cardiac endothelin-1 and nuclear factor-kappaB pathways. J Cardiovasc Pharmacol. 2005 Dec;46(6):856-62. 95. Hsu YL, Kuo PL, Lin CC. Proliferative inhibition, cell-cycle dysregulation, and induction of apoptosis by ursolic acid in human non-small cell lung cancer A549 cells. Life Sci. 2004 Sep 24;75(19):2303-16. 96. Shishodia S, Majumdar S, Banerjee S, Aggarwal BB. Ursolic acid inhibits nuclear factor-kappaB activation induced by carcinogenic agents through suppression of IkappaBalpha kinase and p65 phosphorylation: correlation with down-regulation of cyclooxygenase 2, matrix metalloproteinase 9, and cyclin D1. Cancer Res. 2003 Aug 1;63(15):4375-83. 97. You HJ, Choi CY, Kim JY, et al. Ursolic acid enhances nitric oxide and tumor necrosis factor-alpha production via nuclear factor-kappaB activation in the resting macrophages. FEBS Lett. 2001 Dec 7;509(2):156-60. 98. Suh N, Honda T, Finlay HJ, et al. Novel triterpenoids suppress inducible nitric oxide synthase (iNOS) and inducible cyclooxygenase (COX-2) in mouse macrophages. Cancer Res. 1998 Feb 15;58(4):717-23. 99. Geng Z, Rong Y, Lau BH. S-allyl cysteine inhibits activation of nuclear factor kappa B in human T cells. Free Radic Biol Med. 1997;23(2):345-50. 100. Keiss HP, Dirsch VM, Hartung T, et al. Garlic (Allium sativum L.) modulates cytokine expression in lipopolysaccharide-activated human blood thereby inhibiting NF-kappaB activity. J Nutr. 2003 Jul;133(7):2171-5. 101. Ho SE, Ide N, Lau BH. S-allyl cysteine reduces oxidant load in cells involved in the atherogenic process. Phytomedicine. 2001 Jan;8(1):39-46. 102. Ide N, Lau BH. Garlic compounds minimize intracellular oxidative stress and inhibit nuclear factor-kappa b activation. J Nutr. 2001 Mar;131(3s):1020S-6S. 103. Bruck R, Aeed H, Brazovsky E, Noor T, Hershkoviz R. Allicin, the active component of garlic, prevents immune-mediated, concanavalin A-induced hepatic injury in mice. Liver Int. 2005 Jun;25(3):613-21. 104. Dirsch VM, Antlsperger DS, Hentze H, Vollmar AM. Ajoene, an experimental anti-leukemic drug: mechanism of cell death. Leukemia. 2002 Jan;16(1):74-83. 105. Afaq F, Malik A, Syed D, et al. Pomegranate fruit extract modulates UV-B-mediated phosphorylation of mitogen-activated protein kinases and activation of nuclear factor kappa B in normal human epidermal keratinocytes paragraph sign. Photochem Photobiol. 2005 Jan;81(1):38-45. 106. Afaq F, Saleem M, Krueger CG, Reed JD, Mukhtar H. Anthocyanin- and hydrolyzable tannin-rich pomegranate fruit extract modulates MAPK and NF-kappaB pathways and inhibits skin tumorigenesis in CD-1 mice. Int J Cancer. 2005 Jan 20;113(3):423-33. 107. Ahmed S, Wang N, Hafeez BB, Cheruvu VK, Haqqi TM. Punica granatum L. extract inhibits IL-1beta-induced expression of matrix metalloproteinases by inhibiting the activation of MAP kinases and NF-kappaB in human chondrocytes in vitro. J Nutr. 2005 Sep;135(9):2096-102. 108. Schubert SY, Neeman I, Resnick N. A novel mechanism for the inhibition of NF-kappaB activation in vascular endothelial cells by natural antioxidants. FASEB J. 2002 Dec;16(14):1931-3. 109. Tzima E, Irani-Tehrani M, Kiosses WB, et al. A mechanosensory complex that mediates the endothelial cell response to fluid shear stress. Nature. 2005 Sep 15;437(7057):426-31. |