Life Extension Magazine®
Groundbreaking research funded by the Life Extension Foundation and conducted at BioMarker Pharmaceuticals in northern California is unraveling the factors responsible for aging and disease, as well as strategies and technologies that may one day help us live in good health for more than 100 years. In this article, we discuss dramatic breakthroughs in life-extension technologies such as gene expression and caloric restriction, along with steps you can begin taking today to ensure a long and disease-free life. The year is 1890 and you have just been born. You are expected to live a long, full life, raise a family, pursue a career, and leave a legacy behind before you die at the ripe old age of 45. That was the average life expectancy of Americans born at the close of nineteenth century, who thought they were living a natural, productive, and full life span. Today, based on 2006 estimates in the World Factbook, Brazilians will live to be 71.97 years old on average, Americans will live to be 77.85 years old, Canadians will live to 80.22 years, and Japanese to 81.25 years.1 Life expectancy in the most developed nations is expected to slowly increase and peak in the mid-80s, while some visionaries foresee an eventual life span of hundreds of years or longer. Like our ancestors of the late 1800s, many people today still believe that life expectancy can be predicted solely by looking at the current actuarial data, and that there really is not much we can do to change it.
What controls the rate at which we age, and which factors determine the potential maximum human life span? Biogerontologists are actively addressing these questions, using the most recent advances in science and technology. The Life Extension Foundation has played a significant role in funding and promoting biomedical aging research. By contrast, the National Institutes of Health invests less than 0.1% of its annual budget—which totaled $28 billion in 2006—in research on the biology of aging and its relation to age-related diseases. Recent developments in genetics, genomics, and biogerontology are providing clues as to which factors in our genes, diet, and environment determine how long we can live. These inquiries seek to understand how we can live longer and also improve the quality of our lives by removing the burden of chronic diseases associated with growing older. Longevity Genes in Yeast, Worms, Flies, and MiceScientists studying the genetic basis of aging know that certain genes—especially in scientific models such as yeast, worms, and flies—can have a profound influence on the maximum life span of those organisms. Interest is now shifting to the search for similar genes in higher organisms such as mice, which serve as a valuable pre-clinical animal model for drug-discovery research in humans. The number of genes that have been found to influence aging in model organisms is expanding, as shown in Table 1.2 The goal of scientists searching for longevity genes is to understand how these genes function, the roles they play within specific molecular pathways, how they control fundamental biological processes, and to what extent they or their functions are shared among different organisms. The key longevity genes, however, are those that provide information about why some species live longer than others, shedding light on factors that affect actual rates of aging. While scientists are just beginning to identify these genes, they believe that such genes are responsible for regulating complex developmental and degenerative processes, and that further study will provide clues for both life-span extension and chronic disease prevention.
The longevity genes so far identified in yeast, flies, worms, and mice are under intensive study to determine their biological functions and how these functions relate to extending life span. Important insights are emerging. For example, several genes that are shared among these very different species appear to function in the insulin-signaling pathway, offering a clue to the relationship between life span and the regulation of metabolism. Other genes appear to be related to caloric restriction, which has radically extended life span in mammals. Understanding the mechanisms that link fewer total calories with life-span extension is of central importance, and is an area that strongly interests the Life Extension Foundation.
Uncovering Longevity Genes in HumansWhile scientists cannot yet manipulate longevity genes in humans in the same ways they can in animals, several investigational approaches are under way to identify and understand the roles of these genes in humans. One approach involves the study of families that exhibit exceptional longevity. These investigations have shown that the offspring of centenarians (people who live to 100 years or older) are likely to inherit significantly better health, as measured by the prevalence of hypertension, diabetes, heart attack, and stroke.3 Longevity in these individuals appears to be highly correlated with relatively high levels of beneficial high-density lipoprotein (HDL) and low levels of harmful low-density lipoprotein (LDL), as well as with larger molecule sizes of both HDL and LDL. Those individuals who inherit a specific polymorphism (a variation of a certain gene) enjoy exceptional longevity, in addition to much better health and cognitive performance.4 This is the first example of linking a human gene mutation to an exceptional longevity phenotype (the characteristics of an organism, as determined by both genetic and environmental influences). The mutation in this case is in a gene involved in lipoprotein metabolism, known as CETP (cholesteryl ester transfer protein, plasma). It suggests that other such genes are likely to be discovered through similar studies of families. This kind of research is helping to identify which genotype (the genetic make-up of an organism) can lead to extended disease-free aging. The same research group recently identified another longevity-linked gene associated with lipoprotein levels and sizes. A polymorphism for the gene apolipo-protein C-III, or APOC3, is linked to a favorable lipoprotein profile, cardiovascular health, insulin sensitivity, and longevity.5 Just how many such genes influence longevity and how they contribute to your health and chances for a long life span remain unanswered questions. Aside from the genetic advantages with which some of us are born, the real question is: are there genes we all share that could be manipulated to help us live longer and healthier? For an answer to this question, we begin our inquiry at the level of the single-celled organism that makes bread dough rise. A Little Stress May Be a Good ThingIn the mid-1990s, a professor at the Massachusetts Institute of Technology discovered that a single gene in yeast, when present in multiple copies, caused the life span of the mother yeast cell to increase by about 30%. Life span in yeast can be measured by counting the number of times a mother cell divides to produce daughter cells before dying. This story grew more compelling when it was found that this gene “codes” for an enzyme that works directly on proteins surrounding DNA, and that extra copies of this gene, known as SIR2, also extended the life span of worms (the nematode C. elegans) by as much as 50%. The SIR2 gene and its mammalian homologue, SIRT1, are now the focus of intensive study regarding their connections and functions in relation to how organisms respond to stress.
MIT professor Leonard Guarente and his former post-doctoral fellow, David Sinclair, who is now at Harvard University, recently coauthored an article in Scientific American that describes the interesting history behind the SIR2/SIRT1 story.6 It turns out that the gene relatives of SIR2, called sirtuins, have evolved to connect genetics with aging, diet, and environmental stressors. These scientists now believe that genes involved in an organism’s ability to withstand a stressful environment can boost the body’s natural capacity to ward off the decline normally associated with aging. The organism’s response seems to be an increase in its capability for defense and repair. In essence, so-called longevity genes like SIR2 are survival genes, in that they enhance health status and extend life span. Mild stressors, such as caloric restriction, can activate the sirtuin pathways. The result is a coordinated shift in an organism’s metabolism, including improved DNA stability, increased repair of DNA damage, improved immune function, prolonged cell survival, and enhanced energy production. Life-Extending Benefits of ResveratrolA key finding that further elevates this story to potential relevance for humans is the discovery that certain molecules, called sirtuin activators, can “turn on” this pathway. One of these molecules, resveratrol, is found in red wine and grape extracts. Various plants produce resveratrol in response to stress. Resveratrol drew particular attention when scientists found that resveratrol-fed flies and worms both exhibited significant increases in life span. Survival studies of mice are currently under way, since the biological mechanisms that respond to compounds like resveratrol are believed to be conserved in mice as well as in humans. In addition to its effects on aging, resveratrol has also been effective in animal models of neurological disease. Resveratrol protects against cell death in worm and mouse models of Huntington’s disease, and protects against amyloid beta toxicity, which suggests a therapeutic potential for resveratrol and other sirtuin-activating compounds in Alzheimer’s disease.7,8 Recent studies funded by the Life Extension Foundation and conducted by BioMarker Pharmaceuticals in northern California have showed significant beneficial effects of grape extract with resveratrol. The biological consequences of increased life span and improvement of neurological disorders in an animal model have been dissected at the molecular level. Further investigations into the links between specific genes and cellular pathways are under way. Resveratrol and other sirtuin activators may be no more than a chapter in the book of longevity, but the rest of the story will require a greater understanding of all the molecular pathways, critical gene targets, and suitable intervention points necessary to manipulate something as complex as the mechanisms that control the rate at which we age. We at BioMarker Pharmaceuticals are also attempting to moderate the risk of acquiring a host of chronic diseases associated with the aging process. Our emphasis is on preventing illnesses associated with aging, not just treating diseases once they arise. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Caloric Restriction Extends Maximum Life SpanWhile the SIR2 story is a fascinating one, it is highly likely that other longevity genes operate in mice and other mammals, including humans. What we have learned so far is that restricting calorie intake (while maintaining good nutrition) extends life span in yeast, worms, flies, mice, rats, dogs, and probably monkeys. It is the only intervention proven to extend maximum life span in these species by slowing or reversing normal aging. What is needed now is to translate this research from animals to humans. Scientists are pushing forward by studying the effects of caloric restriction in humans and identifying changes in gene expression associated with the life span-extending effects of caloric restriction.
The National Institutes of Health is funding controlled clinical studies to determine whether the health benefits associated with caloric restriction apply to humans. These studies are beginning to generate important results showing that caloric restriction positively affects biomarkers of longevity while improving heart health and function. Although reducing calorie intake appears to benefit humans, it is unlikely that a severe calorie-restricted diet would be widely adopted. The Life Extension Foundation and its affiliates are attempting to develop a substitute for caloric restriction—that is, a therapeutic intervention that will produce the health and longevity effects of caloric restriction, without forgoing normal food intake. Results of Clinical Caloric-Restriction StudiesMore than 70 years after caloric restriction was shown to work in rodents, emerging scientific evidence suggests that caloric restriction produces health benefits in humans as well.9 In 2002, the National Institutes of Health began funding studies to determine whether the effects of caloric restriction apply to humans. Three centers were chosen to conduct these studies: the Washington University in St. Louis School of Medicine, the Pennington Biomedical Research Center in Baton Rouge, and the Jean Mayer USDA Human Nutrition Research Center on Aging at Tufts University in Boston. The first two groups have now published their results, and it is clear that if you eat less, you will be healthier. These results also support the Life Extension Foundation’s strategy to fund research to develop therapies that can induce the healthy effects produced by caloric restriction. The group at Washington University focused on caloric restriction’s effects on the heart. Their studies show that caloric restriction has a “powerful protective effect against atherosclerosis” and appears to prevent primary aging in the heart.10,11 In this study, a group of men and women, averaging 50 years of age, ate a calorie-restricted diet (1,112-1,958 calories per day) for an average of six years. They were compared to a control group that ate a typical American diet (1,976-3,537 calories per day). The calorie-restricted group showed no evidence of risk factors for atherosclerosis. Their total cholesterol and LDL levels were lower than those of the controls, as were their glucose, insulin, C-reactive protein (a marker for inflammation), and triglyceride levels. The artery-protective lipoprotein HDL was also higher in the calorie-restricted group than in the controls.10 In fact, these middle-aged adults had the same triglyceride levels normally found in the top fifth percentile of healthy 20-year-olds. The calorie-restricted group also had a 40% reduction in carotid arterial wall thickness compared to the controls, and no evidence of atherosclerotic plaque formation. The same research group also evaluated heart function in the calorie-restricted subjects. A decline in cardiac performance, as measured by diastolic function, is a primary marker of aging. The study found that in calorie-restricted individuals, diastolic function resembled that found in adults about 15 years younger in age. Because the subjects had been on the restricted diet for an average of six years, the researchers concluded that caloric restriction may even have a rejuvenating effect on human health.11 One lesson learned from this study is that even if you are not lucky enough to have inherited the “good” longevity genes that allow some individuals to live beyond 100 years, you can still implement strategies to ensure a longer, healthier life.
While the Washington University researchers studied people who had chosen a calorie-restricted diet themselves, the Pennington group has just published the first randomized, controlled clinical study of caloric restriction.12 Overweight but non-obese men and women (as determined by a body mass index of 25-30) who were fed a diet reduced in calories by 25% were compared to a control group for a period of six months. This study was designed to measure caloric restriction’s effects on biomarkers that have been associated with longevity, oxidative stress, and how individuals adapt to changes in metabolism. Although the study was intended as a pilot for a two-year study to begin later in 2006, its results to date have already been called “striking.” Like mice and monkeys, the calorie-restricted human subjects had decreased fasting insulin levels and body temperature, indicating improvements in two biomarkers of longevity. These results support the hypothesis that caloric restriction attenuates the human aging process. Calorie-restricted subjects also adapted their metabolism to the reduction in calories by decreasing the amount of energy they expended, an effect that extended beyond what could be explained by a loss of body weight. In addition, the calorie-restricted group exhibited reduced DNA damage. Reactive oxygen species, which are byproducts of energy metabolism, are known to attack DNA, lipids, and proteins. This reduction in DNA damage links the decreased oxidative stress induced by caloric restriction to the oxidative stress theory, a popular theory that connects damage caused by reactive oxygen species over time to the onset of diseases like cancer, as well as to aging itself. These studies provide evidence that caloric restriction works in humans as well as in animals, and offer clues as to how it might work. Now that accumulating evidence suggests that caloric restriction may slow aging and protect against age-related diseases in humans, does this mean that animal studies will have less to contribute? No. In fact, the opposite is true. Now that we have evidence that caloric restriction benefits humans, we can employ animal studies to investigate the intricate interrelationships between genes, molecular pathways involved in the pathology of chronic diseases and aging, and the physiological changes induced by caloric restriction. Using this strategy, the Life Extension Foundation will continue to support scientific research by BioMarker to find interventions that exert the same beneficial effects as caloric restriction. BioMarker’s Visionary MissionBioMarker’s mission is to understand how longevity genes that confer long life span and protection against age-related diseases are altered by interventions designed to mimic the effects of caloric restriction and other models of life-span extension, such as Ames dwarf mice. BioMarker employs gene-expression profiling—using DNA “chips” or microarrays—to observe how nearly 40,000 markers for all the genes that make up the basic blueprint for a mouse respond to caloric restriction or various candidate caloric-restriction mimetics—that is, agents that reproduce the beneficial effects of caloric restriction. The sophisticated instrumentation, technology, and current approaches that BioMarker employs were described in a previous article published in Life Extension (“Life Extension’s Visionary Plan to Conquer Aging and Death,” January 2006).13 Genes that respond to caloric restriction in various organs and tissues of an animal model (a long-lived hybrid mouse) are first identified in controlled studies. These patterns are then used to identify pharmaceutical compounds, molecules, foods, dietary supplements, and botanical extracts that reproduce, to a significant degree, the same gene-expression patterns produced by caloric restriction. Candidate caloric-restriction mimetics are then subjected to functional testing using various biochemical and biological assays. Final confirmation of caloric-restriction mimetics will require testing in humans, and BioMarker is already planning such studies. While studies are now demonstrating caloric restriction’s benefits for heart health in humans, BioMarker scientists recently identified how heart genes respond to caloric restriction in an animal model.14 This study showed that caloric restriction’s key beneficial effects act rapidly to change the way the heart functions, enhancing its contractility and energy production. Animals on lifelong caloric restriction have hearts that have reduced perivascular collagen deposition in the left ventricle and smaller cardiac muscle cells, resembling the hearts of much younger animals. Gene-expression studies have shown that eight weeks of caloric restriction reproduced nearly 20% of the total gene-expression changes found in long-term calorie-restricted animals. These genes are likely responsible for caloric restriction’s cardioprotective effects. Another important study recently showed that the animal model we use is capable of detecting compounds that exhibit caloric-restriction mimetic activity.15 A number of drugs commonly used to treat diabetes were screened using this model. We discovered that metformin, an off-patent drug that is a first-line therapy for overweight people with type II diabetes, reproduced gene-expression changes in the liver that are very similar to those produced by long-term caloric restriction. Eight weeks of metformin treatment produced gene-expression changes that were even more similar to those induced by long-term caloric restriction than did eight weeks of caloric restriction. These results validate our screening model and provide valuable information about how to identify and develop caloric-restriction mimetics in the future.
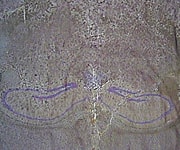
Combating Aging and Brain DiseaseNow that BioMarker scientists have developed a screening model for caloric-restriction mimetics and have identified key genes that are involved in the response to caloric restriction by the heart and liver, they are now looking into caloric restriction’s effects on brain health. Neurodegenerative diseases such as Alzheimer’s and Parkinson’s, as well as age-related cognitive decline, are all major targets of pharmaceutical industry research. Caloric restriction’s neuroprotective effects, as demonstrated in animal models, are profound and likely to be produced in humans as well. Scientists are studying the brain using the same approach used to evaluate caloric restriction-responsive genes in the heart and liver, but with a twist. Rather than subject the entire brain to gene-expression analysis, a new technology allows for examination of specific regions of the brain. The Arcturus Laser Capture Microdissection (LCM) and Laser Cutting System connects a microscope to a UV laser. Thin frozen sections of the brain can be viewed through the microscope, and the system can be programmed to “harvest” cells from specific brain regions. Our studies are focusing on the hippocampus, which is involved in spatial learning and memory, and is one of the first regions of the brain to suffer damage from Alzheimer’s disease. The LCM has been used to collect specific hippocampal neurons for RNA preparation (ribonucleic acid, which is involved in transmitting genetic expression) prior to subjecting the material to gene-expression profile analysis. Figures 1 and 2 show stained hippocampal neurons before and after harvesting with the LCM. BioMarker scientists hope to identify specific brain genes that respond to caloric restriction, and to use this information in developing caloric-restriction mimetics that will ward off disabling neurodegenerative diseases and enable our brains to remain healthy and youthful in advancing age. SummaryWe are living in a time of rapid technological advances. Longevity genes identified in yeast, worms, flies, and mice are directly relevant to humans. The powerful health and longevity benefits of caloric restriction in animals are now being demonstrated in humans. Scientists are actively pursuing intervention strategies based on the caloric-restriction model. The Life Extension Foundation has been a pioneer in funding research and charting the course for one of the most important scientific endeavors of all time: the search to understand how genes determine human life span, and how we can manipulate these genes to live longer, healthier lives. Humans seeking to slow aging and reduce degenerative disease risk may consider reducing food intake and ingesting 20-40 mg of resveratrol and 250-850 mg of metformin each day. These doses are based on studies conducted at BioMarker as well as analysis of the most current scientific literature. Caution: Those suffering from malnutrition should not attempt to restrict their calorie intake. Likewise, people who have medical conditions such as hypoglycemia, or liver or kidney disorders, should not take the prescription drug metformin. Always consult your physician before taking any prescription drug or nutritional supplement. | |||||||
| References | |||||||
| 1. Available at: www.cia.gov/cia/publications/ factbook/rankorder/2102rank.html. Accessed April 24, 2006. 2. Butler RN, Austad SN, Barzilai N, et al. Longevity genes: from primitive organisms to humans. J Gerontol A Biol Sci Med Sci. 2003 Jul;58(7):581-4. 3. Atzmon G, Schechter C, Greiner W, et al. Clinical phenotype of families with longevity. J Am Geriatr Soc. 2004 Feb;52(2):274-7. 4. Atzmon G, Rincon M, Rabizadeh P, Barzilai N. Biological evidence for inheritance of exceptional longevity. Mech Ageing Dev. 2005 Feb;126(2):341-5. 5. Atzmon G, Rincon M, Schechter CB, et al. Lipoprotein genotype and conserved pathway for exceptional longevity in humans. PLoS Biol. 2006 Apr;4(4):e113. 6. Sinclair DA, Guarente L. Unlocking the secrets of longevity genes. Sci Am. 2006 Mar;294(3):48-7. 7. Parker AJ, Arango M, Abderrahmane S, et al. Resveratrol rescues mutant polyglutamine cytotoxicity in nematode and mammalian neurons. Med Sci (Paris). 2005 May;21(5):556-7. 8. Chen J, Zhou Y, Mueller-Steiner S, et al. SIRT1 protects against microglia-dependent amyloid-beta toxicity through inhibiting NF-kappaB signaling. J Biol Chem. 2005 Dec 2;280(48):40364-74. 9. McCay CM, Crowell MF, Maynard LA. The effect of retarded growth upon the length of life span and upon the ultimate body size. 1935. Nutrition. 1989 May;5(3):155-71. 10. Fontana L, Meyer TE, Klein S, Holloszy JO. Long-term calorie restriction is highly effective in reducing the risk for atherosclerosis in humans. Proc Natl Acad Sci U S A. 2004 Apr 27;101(17):6659-63. 11. Meyer TE, Kovacs SJ, Ehsani AA, et al. Long-term caloric restriction ameliorates the decline in diastolic function in humans. J Am Coll Cardiol. 2006 Jan 17;47(2):398-402. 12. Heilbronn LK, de JL, Frisard MI, et al. Effect of 6-month calorie restriction on biomarkers of longevity, metabolic adaptation, and oxidative stress in overweight individuals: a randomized controlled trial. JAMA. 2006 Apr 5;295(13):1539-48. 13. Kent S. Life Extension’s visionary plan to conquer aging and death. Life Extension. January 2006:54-65. 14. Dhahbi JM, Tsuchiya T, Kim HJ, Mote PL, Spindler SR. Gene expression and physiologic responses of the heart to the initiation and withdrawal of caloric restriction. J Gerontol A Biol Sci Med Sci. 2006 Mar;61(3):218-31. 15. Dhahbi JM, Mote PL, Fahy GM, Spindler SR. Identification of potential caloric restriction mimetics by microarray profiling. Physiol Genomics. 2005 Nov 17;23(3):343-50. |