
Premenstrual Syndrome (PMS)
Premenstrual Syndrome (PMS)
Last Section Update: 12/2024
1 Overview
Summary and Quick Facts for Premenstrual Syndrome (PMS)
- Many women experience troublesome physical, emotional and cognitive symptoms during the two weeks before menstruation. These symptoms are called premenstrual syndrome, or PMS. A severe condition closely related to PMS, called premenstrual dysphoric disorder (PMDD), can dramatically reduce a woman’s quality of life.
- This protocol will help you understand the monthly hormonal changes that contribute to PMS. Learn about medical treatments that may reduce PMS symptoms, several dietary and lifestyle changes and supplements that can support a healthy cycle.
- Clinical trials have shown that chasteberry (Vitex agnus-castus) extract helped improve several PMS-related symptoms.
Millions of women experience troublesome physical, emotional, and cognitive symptoms during the two weeks before menstruation. These symptoms are called premenstrual syndrome, or PMS, and can interfere with day-to-day activities. A severe premenstrual condition closely related to PMS, called premenstrual dysphoric disorder (PMDD), can impact a woman’s life as much as major depressive disorder. Vitamin B6 and saffron, among other integrative interventions, can greatly help reduce these irritating and debilitating symptoms.
Common Signs and Symptoms of PMS
Physical:
- Bloating, weight gain
- Acne
- Breast tenderness
Psychological:
- Irritability
- Mood swings
- Fatigue
Behavioral:
- Food cravings
- Angry outbursts
- Decreased motivation
Possible Causes of PMS/PMDD
- Estrogen and progesterone are high when PMS occurs
- Deregulation of the hypothalamic-pituitary-adrenal axis
- Altered serotonin levels
Risk Factors for PMS/PMDD
- Smoking and alcohol consumption
- Obesity
- High-fat, high-sugar diet
- Traumatic stress or having post-traumatic stress disorder
Conventional Medical Treatments
- Medications for symptoms: Non-steroidal anti-inflammatory drugs (ibuprofen, naproxen), mefenamic acid, and spironolactone
- Medications to regulate mood: SSRIs, drugs to treat anxiety
- Medications to suppress ovulation and regulate hormones: oral contraceptive pills, gonadotropin-releasing hormone agonists
Note: Some newer contraceptive formulations contain the progestin drospirenone, which may increase the risk of life-threatening blood clots known as venous thromboembolism.
- Bioidentical hormones for hormone regulation
Dietary and Lifestyle Considerations
- Avoid high-sugar and high-salt foods, caffeine, and alcohol
- Exercise improved physical and psychological symptoms in women with PMS
- Stress management
Integrative Interventions
- Chasteberry: This has been found through many research studies to be “one of the most effective therapeutic options for PMS.”
- Vitamin B6: Vitamin B6 has been shown to improve symptoms of bloating, headache, breast pain, depression, and irritability.
- Magnesium: Research has found that supplementation with magnesium significantly reduced weight gain, bloating, and breast tenderness in women with PMS.
- Saffron: Supplementation with saffron reduces symptoms of depression and overall premenstrual symptom severity.
- Fish Oil: Compared with placebo, daily treatment with omega-3 fatty acids led to significant reductions in depression, anxiety, lack of concentration, bloating, nervousness, headaches, and breast tenderness.
2 Premenstrual Syndrome (PMS)
Millions of women, at some point in their lives, experience troublesome physical, emotional, and cognitive symptoms during the two weeks leading up to menstruation (Bhatia 2002; MGH 2013). When these symptoms interfere with day-to-day life, this is called premenstrual syndrome or PMS (Marjoribanks 2013; O'Brien 2011; Rapkin 2012; Alvero 2014). It is estimated that 8–20% of reproductive-aged women experience moderate-to-severe PMS (Rapkin 2009).
Premenstrual dysphoric disorder or PMDD is a premenstrual condition closely related to PMS that affects an estimated 3–8% of women (Rees 2014; Marjoribanks 2013; Steiner 2006). PMDD is usually characterized by severe psychological symptoms such as depression, anxiety, or persistent anger. PMDD is much more severe than PMS and can impact a woman’s life as much as major depressive disorder (Pearlstein 2008; Rapkin 2009; Epperson 2012).
Both PMS and PMDD occur in cycles. During the luteal phase of the menstrual cycle, which lasts from ovulation to the onset of menstruation, levels of the hormones estrogen and progesterone in a woman’s body change (Justice 1999; Ounis-Skali 2006). These hormonal fluctuations coincide with the onset of PMS symptoms. It is thought that changing hormone levels affect brain chemicals called neurotransmitters and neuropeptides, which help regulate mood (Freeman 2002). Direct actions of hormones, coupled with their effects on neurotransmitters, are believed to contribute to PMS and PMDD. The symptoms of PMS and PMDD usually go away when, or soon after, menstruation begins and return again during the next luteal phase (Rapkin 2012; Rees 2014; Rapkin 2014).
Dietary and lifestyle changes may be sufficient to resolve symptoms in mild cases of PMS. These include exercising; eating a healthy diet rich in vegetables, whole grains, and fruits; avoiding excess salt, sugar, alcohol, and caffeine; getting adequate sleep; managing stress; and not smoking. Over-the-counter pain relievers can help address physical symptoms such as cramps, pain, and headaches, but are not without side effects, especially if used long-term (OWH 2012; Alvero 2014). Cognitive behavioral therapy and other behavioral and self-help modalities may also reduce PMS and PMDD symptoms (Pearlstein 2008; Willacy 2012).
For women whose symptoms are not relieved by dietary and lifestyle modifications alone, several drugs are available to treat PMS and PMDD, though many women find several of them to be only partially effective or experience unwanted side effects (Kleinstauber 2012; Rapkin 2009; Marjoribanks 2013). These medications include oral contraceptives and gonadotropin-releasing hormone agonists, which block ovulation; and serotonin reuptake inhibitors and anxiolytics, which modify brain neurochemical metabolism (Mayo Clinic 2012).
Women who suffer from PMS or PMDD are not limited to conventional treatments, however. Many integrative interventions, including calcium and vitamin D, chasteberry extract, magnesium, St. John’s wort, and vitamin B6 have been shown to reduce symptoms of PMS in clinical studies (Bertone-Johnson 2005; Canning 2010; Yonkers 2008; Alvero 2014; OWH 2012). Moreover, given the hormonal basis of PMS and PMDD symptoms, women who experience either of these conditions should undergo a blood test to evaluate their sex hormone levels. Hormonal imbalance, which can be revealed by blood testing, may be an important underlying factor in PMS and PMDD for some women. Once a hormonal imbalance has been identified, some women may benefit from using bioidentical hormone replacement therapy to restore balance among their hormones (Dennerstein 1985; Fugh-Berman 2007; Holtorf 2009; Hudson 2013).
In this protocol you will learn about the possible causes of PMS and PMDD, and how to differentiate these two conditions. You will also learn how PMS and PMDD are typically treated and common side effects of conventional treatments. Emerging treatment modalities will be examined and a number of integrative interventions that may help relieve symptoms of PMS and PMDD will be described.
3 Background
The Menstrual Cycle
During the menstrual cycle, fluctuations in sex hormone levels prepare a woman’s reproductive system for pregnancy. A normal cycle generally ranges from 25–36 days. The first day of menstruation is considered day one of the cycle and is part of the follicular phase (Brzyski 2013). Key hormones involved in the menstrual cycle are luteinizing hormone (LH), follicle-stimulating hormone (FSH), estrogen, and progesterone. The fluctuation pattern of these hormones over the course of the menstrual cycle is depicted in Figure 1.
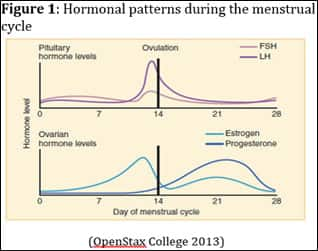 |
- Follicular phase. The follicular phase, which begins on the first day of menstrual bleeding, usually lasts 13–14 days (Hawkins 2008; Brzyski 2013).
- Ovulatory phase. The ovulatory phase usually lasts for 16–32 hours and occurs at approximately day 14 of the menstrual cycle (Hawkins 2008; Brzyski 2013; Ajala 2013).
- Luteal phase. The luteal phase usually lasts for approximately 14 days (Hawkins 2008; Brzyski 2013).
- Menstruation. The entire menstrual cycle resets itself on the first day of menstruation, and the process is repeated (Hawkins 2008; Brzyski 2013).
In order for a woman to be diagnosed with PMS, troublesome symptoms must occur during the luteal phase of the menstrual cycle, end soon after menstruation, and not recur until the next luteal phase.
4 Signs and Symptoms
The specific PMS or PMDD symptoms each woman experiences may differ. However, a woman will typically experience the same symptoms from one cycle to the next. Symptoms occur during the luteal phase of the menstrual cycle, usually peak approximately two days before menstruation, and are typically relieved by the start of menstruation or a few days thereafter. In some women, symptoms linger into the next cycle, but there must be a symptom-free period of time before the next ovulatory phase in order for the condition to qualify as PMS. PMS, by definition, cannot occur after menopause. Nevertheless, symptoms similar to those caused by PMS can occur during perimenopause or after the reproductive years, particularly if a woman is receiving combined estrogen-progestogen hormone replacement (Rapkin 2014; Yonkers 2008).
It has been reported that there are over 150 symptoms associated with PMS or PMDD (Alvero 2014). However, irritability, anxiety, depression, mood swings, bloating, abdominal discomfort, breast tenderness, headache, and fatigue are the most common symptoms. Many of these symptoms can be further aggravated by stress, alcohol or caffeine consumption, and smoking (Rapkin 2014; Alvero 2014).
Table 1: Common Physical, Psychological, and Behavioral Symptoms of PMS or PMDD*
Physical Symptoms |
|
Psychological Symptoms |
|
Behavioral Symptoms |
|
*Derived from (Alvero 2014; Yonkers 2008; Rapkin 2014)
5 Possible Causes
The exact cause of PMS and PMDD is unclear, but it is generally agreed that PMS and PMDD develop through a combination of cyclical changes in hormone levels and dysregulation of brain neurochemical activity. Interestingly, the relationship between hormones and neurochemicals appears to be bidirectional. Hormones activate brain neurochemical pathways, directly or indirectly, and are involved in the regulation of many essential brain functions; in turn, these neurochemicals appear to impact hormones (Rasgon 2001; Andreen 2009; Kuhl 2002; Finocchi 2011). Hormones also interact with the hypothalamic-pituitary-adrenal axis, which then impacts the stress hormone cortisol, another interaction that may contribute to premenstrual symptoms (Segebladh 2013; Girdler 2001).
Estrogen and Progesterone
Young women who have not started menstruation do not have a menstrual cycle, and thus do not experience PMS. Women who have undergone menopause also do not have a menstrual cycle or a luteal phase and thus do not experience PMS. This provides evidence that the hormones progesterone and estrogen contribute to PMS and PMDD. Furthermore, PMS and PMDD occur during the luteal phase of the menstrual cycle, when progesterone and estrogen levels are high (Rapkin 2014; Brzyski 2013). However, PMS symptoms do not appear to solely relate to hormone levels, as researchers have been unable to detect differences in hormone levels or patterns in women with PMS compared with control women (Rubinow 1988; Backstrom 1983; Rapkin 2012).
Another possibility that has received some attention from researchers is that hormone metabolites, or derivatives, may underlie some aspects of PMS and PMDD. For example, several studies have suggested that allopregnanolone, a progesterone metabolite, may be involved in PMS and PMDD (Reddy 2010; Andreen 2006; Backstrom 2011; Nillni 2011; Rapkin 1997; Monteleone 2000; Lombardi 2004; Bernardi 2004; Engin 2007; Klatzkin 2006).
Additionally, the relationship or “balance” between sex hormones in a woman’s body may play a discrete but influential role in PMS and PMDD. For example, the fluctuating balance between sex hormones during a woman’s menstrual cycle influences neurotransmitter systems in the brain, which in turn modulate mood and cognition. This is intriguing because altered activity or signaling of certain neurotransmitter systems is thought to contribute to some PMS symptoms (Steiner 2003; Studd 2011; Hudson 2013). Thus, helping establish balance between sex hormones may relieve PMS symptoms for some women (Studd 2011; Hudson 2013).
Hypothalamic-Pituitary-Adrenal Axis
The hypothalamic-pituitary-adrenal (HPA) axis comprises two brain regions—the hypothalamus and the pituitary gland—and the adrenal glands, which are found on top of each kidney. These three structures work in concert to regulate neurochemical and hormonal signals passed between the brain and adrenal glands, helping manage the response to stress through regulation of cortisol. This functional group is called the HPA axis (Smith 2006). Deregulation of the HPA axis in women with PMS or PMDD can affect cortisol and allopregnanolone levels, resulting in changes in mood and altered responses to stress (Klatzkin 2010; Segebladh 2013; Girdler 2007; Mortola 1989). A more detailed discussion about the HPA axis is available in the Stress Management protocol.
Serotonin
Serotonin, a neurotransmitter, is involved in the regulation of mood and response to stress, eating behavior, and circadian rhythm (sleep-wake cycle) (Caspi 2003; Parsey 2006; Hainer 2006; Monti 2011). Serotonin activity is partly controlled by ovarian sex hormone levels. Therefore, fluctuations in hormone levels during the menstrual cycle can alter serotonin levels and lead to changes in mood (Benmansour 2012; Rapkin 2012; Yonkers 2008). Some but not all studies have found evidence of a relationship between serotonin signaling and sex steroids in the context of premenstrual symptoms (Clayton 2006; Yen 2013; Dhingra 2007; Gingnell 2010; Magnay 2010; Magnay 2006; Yonkers 2008).
Other Potential Mechanisms
Prolactin is a hormone released by the pituitary gland to stimulate breast development and lactation (Liou 2012), while beta-endorphins are neurochemicals that control pain, reward, and addictive behaviors (Sprouse-Blum 2010; Roth-Deri 2008). Both may play a role in premenstrual symptoms. Women who have PMS may have high prolactin levels or low beta-endorphin levels, and this may contribute to some of the psychological and physical symptoms of PMS (van Die 2013; Cunningham 2009). There is also limited evidence to suggest that thyroid dysfunction may be more common in women who experience PMS (Girdler 1995; Schmidt 1993). Women who suffer from PMS should consider having blood testing to determine whether they have a thyroid hormone imbalance. More information about thyroid hormone testing and correcting thyroid hormone levels is available in the Hyperthyroidism and Hypothyroidism protocols.
6 Risk Factors
Smoking and Alcohol Consumption
A population study in over 3 000 women found that those who smoke had a more than two-fold higher risk of developing PMS, while those who began smoking before age 15 had a greater than 2.5-fold risk, compared with women who never smoked. Former smokers who smoked 25 or more cigarettes per day had a 1.8-fold higher risk of developing PMS relative to women who never smoked. Smoking more cigarettes over a longer period of time also increased PMS risk (Bertone-Johnson 2008).
A study in over 200 women found that those with PMS drank significantly more servings of alcohol per week, both pre- and postmenstrually, than did those without PMS. This same study found that women who had 10 or more alcoholic drinks per week in their postmenstrual phase were significantly more likely to have moderate to severe PMS. Other studies have also found a relationship between alcohol consumption and PMS (Rossignol 1991; Bryant 2006).
Obesity
Increasing body mass index (BMI) is associated with PMS. In one study, a BMI > 27.5 conferred a significantly higher risk of PMS than a BMI < 20. Higher BMI was significantly associated with symptoms of backache, swelling of extremities, and abdominal cramping (Bertone-Johnson 2010). A survey of 874 women found that those who were obese had a 2.8-fold higher risk of PMS (Masho 2005). Higher BMI has also been associated with PMDD (Yen 2010).
Diet
Two surveys found that a diet high in fat is associated with worse PMS symptoms (Goker 2014; Nagata 2004). There is also evidence that women with PMDD have an increased desire for high-fat and high-calorie foods during the luteal phase compared to the follicular phase (Reed 2008). Another survey found that consumption of foods and beverages high in sugar was associated with PMS (Rossignol 1991).
Psychological Risk Factors
Multiple studies have found that traumatic stress or having post-traumatic stress disorder increases a woman’s odds of developing PMDD (Pilver, Levy 2011; Wittchen 2003; Perkonigg 2004). There is also evidence that women who perceive discrimination during their lifetimes, including gender and race discrimination, are more likely to experience PMDD (Pilver, Desai 2011). One study found that women with PMS were more than three times as likely to report significant trauma in childhood compared with those without PMS (Bertone-Johnson 2014).
Lastly, it is important to note that women with psychiatric illnesses may experience exacerbation of their condition during the luteal phase of their menstrual cycle (Nillni 2011; Kim 2004; Miyaoka 2011; Cirillo 2012; Rees 2014). You can learn more about psychiatric conditions that are sometimes mistaken for PMS and PMDD in Life Extension’s Anxiety and Depression protocols.
7 Diagnosis
PMS and PMDD are diagnosed on the basis of a woman’s symptoms and medical history (Pinkerton 2013; Nillni 2011; Rees 2014). It is important that other gynecological conditions that may cause similar symptoms be distinguished from PMS and PMDD. Menopausal symptoms, endometriosis, dysmenorrhea (painful cramping during the menstrual period), and several other conditions may be mistaken for PMS or PMDD (Yonkers 2008; Rapkin 2014).
PMS and PMDD symptoms must be cyclical, in that a symptom-free interval occurs during the follicular phase. Patients with symptoms that do not remit during the follicular phase may have another disorder or condition with similar symptoms (Rees 2014; Alvero 2014). Practice guidelines recommend that women record their symptoms in a daily diary for at least two consecutive menstrual cycles to help establish a diagnosis (Nevatte 2013; Endicott 2006).
Tests may be performed to rule out other diseases or conditions that cause symptoms similar to those caused by PMS. For instance, thyroid function tests can rule out the possibility of thyroid disease (Rees 2014). Questionnaires may be used to screen for depression and generalized anxiety disorder (Alvero 2014). Luteinizing hormone (LH) and follicle-stimulating hormone (FSH) levels can help determine if symptoms may be related to menopause (BreastCancer.org 2013).
Physicians can diagnose PMS on the basis of standardized criteria from sources such as the International Statistical Classification of Diseases and Related Health Problems 10th edition (ICD-10), which uses the term “premenstrual tension syndrome,” or the American Congress of Obstetricians and Gynecologists (ACOG) guidelines. PMDD is diagnosed based on the guidelines from the Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5) (Rees 2014). The requirements and possible symptoms for each diagnostic tool are listed in Table 2.
Table 2: Symptoms and Symptom Requirements for the ICD-10, ACOG, and DSM-5 Diagnostic Tools for PMS and PMDD*
|
Diagnostic Tool |
Requirements |
Symptoms |
ICD-10 — PMS |
|
|
ACOG — PMS |
|
Affective symptoms:
Physical symptoms:
|
DSM-5 for PMDD |
|
|
*Derived from (Rees 2014; ACOG 2001)
8 Conventional Treatment
Treatment of PMS or PMDD depends in part on the type and severity of symptoms. Most mild cases of PMS can be treated with lifestyle changes (as described in the Dietary and Lifestyle Considerations section) or over-the-counter drugs to manage physical symptoms (Rapkin 2014), though lifestyle changes are recommended for all patients (Rees 2014). Over-the-counter non-steroidal anti-inflammatory drugs (NSAIDs), such as ibuprofen (Advil, Midol) and naproxen (Aleve), are commonly used to treat physical symptoms such as cramping and breast tenderness.
Mefenamic acid (Ponstel), a prescription NSAID commonly prescribed for menstrual pain, is also sometimes used in PMS. A small randomized controlled trial of mefenamic acid found it significantly reduced premenstrual physical symptoms and mood swings (Mira 1986; NLM 2012). Diuretics, such as spironolactone (Aldactone), may be prescribed to treat premenstrual swelling and bloating (Mayo Clinic 2012).
Standard conventional treatment of PMS or PMDD primarily relies on medications that modulate mood; synthetic hormones that interrupt ovulation; referral for cognitive behavioral therapy; or in rare and extremely severe cases, surgical removal of a woman’s ovaries.
Psychiatric Medications
Selective serotonin reuptake inhibitors. Selective serotonin reuptake inhibitors (SSRIs) are commonly used to treat mood disorders influenced by serotonin, such as anxiety and depression. SSRIs are considered the first-line pharmacologic treatment option for psychological and behavioral symptoms of PMS/PMDD, with improvements observed in 60–80% of women (Marjoribanks 2013). Among women with PMDD who respond to SSRI treatment, there is generally a greater than 50% improvement in symptoms. SSRIs may be effective when taken either continuously, or only during the luteal phase. Currently, fluoxetine (Prozac or Sarafem), sertraline (Zoloft), and paroxetine (Paxil) are the only SSRIs approved by the US Food and Drug Administration (FDA) for the treatment of PMDD (Rapkin 2013).
Side effects of SSRIs are fairly common, with those most frequently reported being nausea, fatigue or weakness, sleepiness, reduced libido, and sweating. These adverse effects have been shown to be dose-related (Marjoribanks 2013). Sexual dysfunction is another relatively common side effect of SSRIs, occurring in 80% of those who take these medications continuously over long periods of time (ARHP 2008). Another potential problem is SSRI discontinuation syndrome, in which patients experience withdrawal symptoms when stopping the medications. These include sensory and gastrointestinal symptoms, dizziness, lethargy, sleep disturbances, and psychological symptoms (Renoir 2013). Gradual withdrawal of SSRI medication appears to prevent some of these problems (Haddad 1998).
Anxiolytic drugs. Anxiolytic drugs are used to treat anxiety. Anxiolytics prescribed for PMS or PMDD include the benzodiazepine alprazolam (Xanax), buspirone (Buspar), and clonidine (Catapres) (Alvero 2014; Rees 2014; Rapkin 2014). Benzodiazepines such as alprazolam have potential to cause significant side effects including physical dependency, as well as withdrawal symptoms upon abrupt discontinuation. Therefore, alprazolam is only used if a patient fails to respond to other treatments, such as SSRI therapy (Freeman 2003).
Suppression of Ovulation
Since PMS/PMDD symptoms are attributed, at least in part, to fluctuations in sex hormones, some treatments work by interrupting ovulation:
Oral contraceptive pills. Oral contraceptive pills (OCPs) that contain a synthetic progesterone-like drug (a progestin) and an estrogen are commonly prescribed to treat symptoms of PMS; however, their effect may depend on the formulation (Rapkin 2012; Lopez 2012; ARHP 2008). Oral contraceptives work by inhibiting ovulation and preventing the changes in hormone levels that occur during the luteal phase (Nevatte 2013). Certain OCPs with 21 days of hormone tablets and 7 days of pills that contain no hormones appear to reduce the physical symptoms of PMS; however, they have limited impact on psychological symptoms (Rapkin 2012). Additionally, some of these OCPs still allow for normal hormonal fluctuations during the hormone-free week, and many women experience PMS-like symptoms during this period (Sulak 2000). Some relatively newer OCPs offer either a continuous hormone dosage without a drug-free interval or a reduced drug-free period, thus reducing the incidence of PMS-like symptoms during the drug-free period (ARHP 2008). An analysis of four studies that evaluated continuous hormone use with levonorgestrel (a progestin) and ethinyl estradiol tablets revealed overall improved symptoms in 30–81% of women (Freeman 2012).
Newer hormone formulations containing 24-day drospirenone (a progestin) and ethinyl estradiol with only a four-day drug-free interval significantly improve PMDD symptoms and are approved by the FDA to treat PMDD (Lopez 2012; ARHP 2008). It is uncertain if these formulations have any effect in women with less severe symptoms (Lopez 2012).
Potential Risks From Newer Hormone PreparationsRecent studies suggest some of the newer hormone formulations containing the progestin drospirenone, which is often combined with ethinyl estradiol, may increase the risk of life-threatening blood clots known as venous thromboembolism (FDA 2013). Venous thromboembolism includes deep vein thrombosis and pulmonary embolism (Rodriguez 2012). In approximately 6–32% of deep vein thrombosis cases, a clot can travel from the extremities to the lungs, forming a pulmonary embolism. It is estimated that approximately 300 000 people die from pulmonary embolism each year in the United States (Ozaki 2012). All OCPs are associated with a slightly increased risk of blood clot formation (Tchaikovski 2010; Stegeman 2013). However, some studies have demonstrated that contraceptives containing drospirenone and newer progestogens are associated with up to a 3.3-fold greater risk of venous thromboembolism compared with older formulations (Wu 2013; Parkin 2011; Jick 2011; Tricotel 2014). The FDA issued a position statement on April 4th, 2012 stating that OCPs containing drospirenone may be associated with a higher risk of blood clot formation than other progestin-containing pills (FDA 2013). It is important for patients to discuss their complete medical history with their physicians to determine if drospirenone-containing OCPs pose any unnecessary risks. Common brand names of drospirenone-containing OCPs are Beyaz, Gianvi, Loryna, Ocella, Safyral, Syeda, Yasmin, Yaz, and Zarah (FDA 2013). |
Gonadotropin-releasing hormone agonists. Gonadotropin-releasing hormone (GnRH) agonists interrupt the hormonal cycle and ovulation. This creates a state of medically-induced menopause, which, if continued long-term, carries a risk of osteoporosis and heart disease similar to that of natural menopause. Thus, the use of GnRH agonists for PMS or PMDD is limited to 3–6 month courses, and GnRH agonists are typically only used when other treatments such as SSRIs or OCPs have failed (Rees 2014; Kumar 2014; Magon 2011).
The low levels of circulating sex hormones caused by GnRH agonists can also cause menopausal symptoms such as hot flashes, vaginal dryness, and headaches. To alleviate their undesired effects and allow longer treatment periods, estrogen, progesterone, or both are commonly prescribed as “add back therapy”; however, this can reintroduce symptoms of PMS (Nevatte 2013). To minimize serious complications, hormone replacement therapy is recommended when GnRH treatment lasts longer than six months (Rees 2014; Rapkin 2013). Commonly prescribed GnRH agonists include leuprolide (Eligard, Lupron), buserelin (Suprefact), goserelin (Zoladex), and nafarelin (Synarel) (Freeman 2003).
Estradiol. High doses of estradiol in the form of a patch, gel, or intrauterine implant are sometimes used to suppress ovulation in order to treat PMS/PMDD (Nevatte 2013; Watson 1989; Smith 1995). A side effect of estradiol administration is increased risk of endometrial hyperplasia, which is non-cancerous overgrowth of cells lining the inside of the uterus (CRUK 2014). Endometrial hyperplasia may lead to endometrial cancer in some cases (Goncharenko 2013; Boruban 2008). Bioidentical progesterone may be used to reduce the risk of endometrial hyperplasia in women being treated with transdermal estradiol (PEPI Trial Writing Group 1996).
Bioidentical Hormones and PMSSome evidence suggests that hormonal imbalance may contribute to PMS symptoms (Studd 2011). Since sex hormones exert broad influence throughout the body, a relative excess or deficiency of one or more hormones may give rise to a wide spectrum of symptoms, such as those associated with PMS. Thus, ensuring hormonal balance may ease PMS symptoms for some women (Steiner 2003; Studd 2011). Diligent use of bioidentical hormone replacement therapy is a natural way to accomplish this. Bioidentical hormones are hormones that have the identical chemical structure as hormones produced by the human body. These include hormones approved by the FDA for treatment of menopausal symptoms, or hormones sold over-the-counter or compounded by a compounding pharmacy. Bioidentical estrogens include estriol, estradiol, and estrone. Bioidentical progesterones include oral micronized progesterone and progesterones sold over-the-counter or compounded in the form of gels and creams (Files 2011; Fugh-Berman 2007; Marsden 2010). FDA-approved bioidentical hormones have also been used to treat PMS and PMDD (Fugh-Berman 2007; Files 2011). Achieving hormonal balance through the use of bioidentical hormones may require different protocols for different women, since each woman’s body may not respond exactly the same way to specific hormone levels. A reasonable approach is for a woman who experiences bothersome PMS symptoms to undergo hormone blood testing about seven days before the cycle starts, which corresponds to day 21 of a typical 28 day cycle. (Day number 1 is the day that bleeding begins). This will allow an innovative physician to discern the levels of hormones in the woman’s body during her luteal phase, when PMS symptoms occur. If, for example, a woman is found to have a low level of progesterone relative to her level of estrogen, then her progesterone levels can be bolstered using transdermal bioidentical micronized progesterone. If this results in waning of the woman’s symptoms, the woman could then work with her doctor to continue progesterone therapy and follow up with a blood test on the 21st day of her next menstrual cycle to monitor the impact of the bioidentical progesterone on her hormone levels and PMS symptoms. Likewise, a similar approach could be undertaken with bioidentical estradiol, or possibly even testosterone, depending on blood test results. This approach is difficult to study in organized clinical trials involving many women, because each woman may require a different dose of bioidentical hormones or different hormones altogether. Unfortunately, this means that published scientific evidence in favor of treating PMS with bioidentical hormone therapy has been slow to accumulate. However, some evidence has been published. For example, a randomized controlled trial showed that oral bioidentical progesterone therapy resulted in an improvement in mood and some physical symptoms of PMS after one and two months of treatment (Dennerstein 1985; Fugh-Berman 2007). Also, several integrative physicians have reported clinical success using bioidentical hormones (Hudson 2013). Women interested in learning more about bioidentical hormones can refer to the Life Extension magazine article Bioidentical Hormones: Why are They Still Controversial? and to the Female Hormone Restoration protocol. |
Cognitive Behavioral Therapy
Cognitive behavioral therapy (CBT) may be an effective treatment for the psychological and behavioral symptoms of PMS (Busse 2009). CBT is commonly used to treat mood disorders, anxiety, and depression (Duckworth 2012). A review of randomized, controlled trials found that CBT significantly reduced anxiety and depression, with possible benefit for behavioral changes, in women with PMS (Busse 2009).
Bilateral Oophorectomy
For women with severe PMDD that does not respond to any other treatment, bilateral oophorectomy (surgical removal of the ovaries) with or without hysterectomy (surgical removal of the uterus) may be considered (Rapkin 2014; Rees 2014). Bilateral oophorectomy removes PMDD hormone-related symptomatology; however, due to the highly invasive nature of this procedure, it should only be considered when all other treatment options have failed (Alvero 2014). Removal of the ovaries induces menopause by eliminating the source of most sex steroid hormones (Cunningham 2009; AMS 2013).
9 Dietary and Lifestyle Considerations
Diet
Foods containing high amounts of simple sugars or salt may promote bloating and weight gain, and should be avoided (Alvero 2014). Some authors have theorized that consuming more complex carbohydrates during the luteal phase may increase serotonin levels and improve symptoms of PMS (Nevatte 2013). Women suffering from PMS should avoid caffeine and alcohol because they may exacerbate PMS (Rossignol 1990; Alvero 2014).
Exercise
Four small studies examined the impact of exercise on PMS signs and symptoms; all of them found benefit. Breast tenderness, fluid retention, stress, anxiety, depression, muscle stiffness, cramps, tension, and restlessness improved in these trials (Dennerstein 1985). In a study comparing aerobic exercise (60 minutes, 3 times a week for 8 weeks) to no exercise in women with PMS, women who exercised had significant reductions in physical and psychological symptoms of PMS (Samadi 2013).
Stress Management
Managing stress may help reduce PMS symptoms (Alvero 2014). Speaking with friends or writing in a journal may help some women cope with stress. Additionally, yoga, massage, or relaxation therapy may be useful (OWH 2012; ACOG 2001). Adequate rest and sleep may improve response to treatment (Alvero 2014; NIH 2007). A more thorough discussion about strategies to manage stress is available in the Stress Management protocol.
Acupuncture
Acupuncture is frequently used to treat symptoms of PMS in Asian countries. Two separate analyses of 8 and 10 randomized, controlled trials found that acupuncture may be an effective treatment option for the physical symptoms of PMS. In all the trials, acupuncture was more effective than no treatment; sham acupuncture; or a progestin, anxiolytic, or both for reducing PMS symptoms. In four trials symptoms were reduced more than 50% from baseline (Kim 2011; Jang 2014). Women reported symptom improvements in as few as two sessions, and efficacy was observed when treatment was performed in both the luteal and follicular phases (Jang 2014). However, it is important to note that many of these trials were poorly designed, with small numbers of subjects (Kim 2011; Jang 2014). Larger, well-designed trials are needed to confirm these findings.
10 Nutrients
Chasteberry (Vitex agnus-castus)
Chasteberry (Vitex agnus-castus) is a shrub found on riverbanks and shores of the Mediterranean region, Southern Europe, and Central Asia (Rani 2013). A rigorous review of published clinical trials found that seven out of eight trials deemed chasteberry superior to placebo for PMS treatment (van Die 2013). Results from a randomized, controlled trial showed that 91 women who took 20 mg chasteberry extract daily had more improvements in psychological and physical PMS symptoms than 87 women who took placebo. Symptoms of irritability, mood, anger, headaches, and breast fullness were reduced (Schellenberg 2001). A follow-up study found that women who took 20 mg chasteberry had greater improvements in symptoms than women who took 8 mg chasteberry extract. However, symptom improvement peaked at 20 mg, and no additional benefit was observed by increasing the dose to 30 mg (Schellenberg 2012).
Three randomized studies comparing chasteberry extract to placebo, and two open-label trials have shown similar benefits with 20–40 mg chasteberry extract or 40 drops of crude chasteberry extract. Marked improvement in aches and pains, anger and short temper, anxiety and nervousness, appetite and food cravings, backache, bloating, breast swelling or pain, crying spells, depression, extremity swelling, fatigue, irritability, lower abdominal cramping, mood, and restlessness were observed (Momoeda 2014; Zamani 2012; Ambrosini 2013; Ma, Lin, Chen, Wang 2010; Ma, Lin, Chen, Zhang 2010).
A trial of chasteberry extract for PMS in nursing students found a significant benefit, with nearly 70% of participants reporting complete resolution of their PMS symptoms by the end of the trial. The authors called chasteberry extract “one of the most effective therapeutic options for PMS” (Ibrahim 2012). A comparison of chasteberry extract with the SSRI fluoxetine in PMDD found that a similar percentage of participants responded to each, with chasteberry more effective for physical symptoms and fluoxetine more effective for psychological symptoms (Atmaca 2003).
Calcium and Vitamin D
Levels of calcium and vitamin D fluctuate throughout the menstrual cycle in all women, most likely because calcium and vitamin D metabolism are influenced by ovarian sex hormones (Thys-Jacobs 2000). However, one study found that compared with women who do not have PMDD, women with PMDD had lower ionized calcium levels during menstruation, lower urinary excretion of calcium during the late follicular phase and early luteal phase, and lower vitamin D levels during the luteal phase (Thys-Jacobs 2007).
A randomized controlled trial compared 1000 mg calcium carbonate daily for three months to placebo. During the calcium treatment, PMS symptom scores were significantly lower during both the luteal and menstrual phases. Seventy-three percent of women reported fewer symptoms during calcium treatment. Negative mood, bloating, and menstrual pain were all significantly relieved by calcium (Thys-Jacobs 1989).
A large, multi-center trial compared treatment with 1200 mg calcium carbonate daily to placebo for moderate-to-severe PMS. The women in this study took calcium or placebo for three menstrual cycles. The women who took calcium had significantly lower average PMS symptom scores in the second and third months of treatment. They also had an overall 48% reduction in total symptom score by the third cycle. Negative mood, bloating, food cravings, and pain were all significantly reduced by the third menstrual cycle in the calcium group (Thys-Jacobs 1998). A more recent double-blind trial of 1000 mg calcium carbonate daily for three months found that calcium treatment effectively relieved PMS-related fatigue, appetite changes, and depression (Ghanbari 2009). A literature review of pharmaceutical and integrative treatments for PMS recommended calcium supplementation as first-line treatment for mild-to-moderate PMS symptoms (Douglas 2002).
Women with lower dietary intake and blood levels of vitamin D may have an increased risk of PMS. An analysis was performed in 401 women who were free of PMS at baseline but later developed PMS. It was found that among those whose vitamin D levels were measured before they were diagnosed with PMS, lower vitamin D levels were related to a significantly higher risk of developing premenstrual breast tenderness, fatigue, depression, and constipation or diarrhea (Bertone-Johnson 2014). A case-control study compared 1057 women who developed PMS with 1968 controls; researchers found that women in the highest 20% total vitamin D intake group had a 41% lower risk of PMS compared to women in the lowest 20% total vitamin D intake group. In this study, those who consumed the most calcium had a 30% reduced risk of developing PMS (Bertone-Johnson 2005).
St. John’s wort (Hypericum perforatum)
Hypericum perforatum, more commonly known as St. John’s wort, is frequently used to treat depression in Europe (NCCAM 2014). A randomized controlled trial in women with mild PMS used 900 mg per day of a St. John’s wort extract, standardized to hypericin and hyperforin, or placebo, for two menstrual cycles. St. John’s wort significantly improved a range of physical and behavioral PMS symptoms such as food cravings, swelling, poor coordination, insomnia, confusion, headaches, crying, and fatigue compared with placebo. St. John’s wort also appeared to improve pain and mood symptoms towards the end of the trial (Canning 2010).
Another randomized, controlled trial used a similar dosage of St. John’s wort extract in women with PMS over two menstrual cycles and found that it markedly improved PMS scores compared to placebo. Overall, symptoms were reduced by 40%. The symptoms most strongly impacted were tearfulness, which improved 71%, and depression, which improved 52%. The side effect profile of St. John’s wort in this trial was similar to placebo (Ghazanfarpour 2011).
In an open-label trial, St. John’s wort extract was administered for two menstrual cycles to 19 women with PMS. The treatment resulted in an average reduction in symptom scores of 51% by the end of the trial. Over two-thirds of the participants experienced a 50% reduction in symptom severity. The treatment was well-tolerated (Stevinson 2000).
A case report told of a woman with PMDD who discontinued SSRIs due to intolerable gastrointestinal side effects. St John’s wort extract at a dosage of 900 mg per day was substituted, and her symptoms improved over five months of follow up. The authors proposed that St. John’s wort extract be considered an alternative treatment option for PMDD, particularly in those who experienced undesirable side effects from SSRIs (Huang 2003).
It is important to note that St. John’s wort interacts with some prescription and over-the-counter medications, including OCPs and antidepressants, so women taking such medications should consult with their physicians before taking St. John’s wort (NCCAM 2014).
B Vitamins
Two separate analyses, including data from 9 and 13 randomized controlled trials, found that 100 mg vitamin B6 may be more effective than placebo for the treatment of PMS symptoms. One of these reviews found that, among 541 women from four of the studies, those taking vitamin B6 had 2.3 times greater likelihood of an improvement in their overall PMS symptoms compared to those taking placebo, and 1.7 times greater likelihood of an improvement in depressive symptoms (Nevatte 2013; Whelan 2009; Wyatt 1999). Vitamin B6 has been shown to improve symptoms of bloating, headache, breast pain, depression, and irritability (Gaby 2011).
A large 10-year study found that women with the highest dietary intake of vitamins B1 (thiamin) and B2 (riboflavin) had a lower incidence of PMS. Women whose dietary intake of riboflavin was in the highest one-fifth of the distribution had a 35% lower risk of developing PMS compared to those in the lowest one-fifth (Chocano-Bedoya 2011).
Magnesium
Magnesium may play an important role in the treatment of PMS (Higdon 2013; UMMC 2013a). Some studies have found that women with PMS have lower blood levels of magnesium than women without PMS (Posaci 1994; Rosenstein 1994). Several successful trials have used magnesium to treat symptoms of PMS. A randomized, placebo-controlled trial found that supplementation with 200 mg of magnesium daily significantly reduced weight gain, bloating, and breast tenderness in women with PMS (Walker 1998). Results from two additional randomized, placebo-controlled trials demonstrated that supplementation with 360 mg magnesium daily significantly improved symptoms related to mood, and the incidence of migraines (Facchinetti, Borella 1991; Facchinetti, Sances 1991). Women may also benefit from the combination of magnesium and vitamin B6. Two randomized controlled trials showed that magnesium (200–250 mg) combined with vitamin B6 (40–50 mg) reduced general symptoms of PMS and anxiety more than either supplement alone or placebo (Fathizadeh 2010; De Souza 2000).
Saffron
Saffron, derived from the plant Crocus sativus, has historically been a part of traditional Persian medicine. Saffron has been shown to modulate serotonin neurotransmitter signaling, and has been investigated for treatment of depression and PMDD (Agha-Hosseini 2008). In fact, several clinical trials have found that saffron effectively relieves depression, with efficacy comparable to that of some antidepressant medications (Noorbala 2005; Akhondzadeh 2004; Akhondzadeh 2005).
A randomized, controlled trial enrolled 50 women between the ages of 20 and 45 who had been experiencing PMS symptoms for six months or more. For two menstrual cycles, 25 of the women received 30 mg of saffron extract daily and 25 received placebo. In the saffron group, 76% of women experienced a 50% or greater reduction in overall premenstrual symptom severity, while in the placebo group only 8% of the women did. In the saffron group, 60% of women experienced a 50% or greater reduction in depression symptoms, while in the placebo group only 4% did. In the saffron extract group, there was a significant improvement from the first to the second month in depression score and overall premenstrual symptom scores. Women in the saffron group had significantly greater reductions in depression and overall premenstrual symptom scores compared with those who received a placebo (Agha-Hosseini 2008).
Omega-3 Fatty Acids
Omega-3 fatty acids are found in fish and some plant foods such as flaxseeds and walnuts. Omega-3 fatty acids include eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA), and alpha-linolenic acid (ALA) (UMMC 2013b; Huang 2010). Within the body, omega-3 fatty acids help suppress inflammation and are important structural components of cells (Calder 2015).
A randomized, controlled trial assessed the ability of omega-3 fatty acids to decrease symptoms of PMS. Compared with placebo, 90 days of treatment with 2 g omega-3 fatty acids daily led to significant reductions in depression, anxiety, lack of concentration, bloating, nervousness, headaches, and breast tenderness (Sohrabi 2013). A controlled trial found that krill oil or fish oil were both effective for the treatment of PMS, but krill oil was more effective than fish oil (Sampalis 2003).
Ginkgo Extract
In a randomized controlled trial in 85 women with PMS, Ginkgo biloba extract, 40 mg three times daily, taken from the 16th day of the menstrual cycle to the 5th day of the next cycle, was compared with placebo. Ginkgo extract resulted in a significantly greater average decrease in severity of physical and psychological symptoms than placebo (Ozgoli 2009). A controlled study in 143 women with PMS also administered ginkgo extract from the 16th day of one menstrual cycle to the 5th day of the next, for two cycles. Ginkgo extract was significantly more effective than placebo for breast pain symptoms, and also improved psychological symptoms (Tamborini 1993).
Gamma-Linolenic Acid
Gamma-linolenic acid (GLA) is a fatty acid derived from seeds of plants such as evening primrose, borage, and black currant (EBSCO 2014). One of these, evening primrose oil, has been used to treat symptoms of PMS, but studies have been inconclusive (UMMC 2013c). However, one randomized controlled trial in 120 women with PMS or PMDD compared a GLA-containing formula with placebo. Subjects received one or two capsules per day containing 210 mg each of GLA along with other fatty acids, or placebo. This trial followed these women over six months, evaluating the results at three and six months. Both dosages significantly reduced PMS symptoms, with the two capsule dosage resulting in greater improvement than one capsule (Rocha Filho 2011).
Tryptophan
Tryptophan is an amino acid that can be metabolized into serotonin, and altered serotonin activity is thought to contribute to premenstrual symptoms (Higdon 2014; Rapkin 2012). In a randomized controlled trial, women with PMDD who received 2 g of L-tryptophan three times daily reported 34.5% reductions in mood, tension, and irritability symptoms, compared with 10.4% reductions in women who took placebo (Steinberg 1999).
Ginger
Ginger contains compounds, such as gingerols, that have demonstrated anti-inflammatory properties. Ginger preparations have been used traditionally as a remedy for a variety of ailments, including menstrual pain and cramping (Shimoda 2010). A randomized placebo-controlled trial of ginger in 66 women used 250 mg ginger capsules twice daily, beginning seven days before menstrual bleeding started and continuing until three days after the onset of bleeding. The ginger treatment produced significant improvements in mood as well as physical and behavioral symptoms of PMS. It also improved overall PMS symptom scores. This symptom reduction was significantly greater after one, two, and three months in the ginger group, compared with placebo (Khayat 2014).
Another randomized controlled trial including 50 female subjects aged 18–35 years assessed the effect of 100 mg ginger extract (GINFORT) twice daily for 56 days on symptoms of PMS (Nirvanashetty 2023). Participants had a regular menstrual cycle with reported menstrual discomfort the previous three months. Supplementation with ginger extract significantly reduced the intensity of pain associated with menstrual cramps. After 28 and 56 days, compared with placebo, the ginger extract also mitigated the pain-induced disruption of daily activities during a PMS period. After 56 days, no participants taking the ginger extract reported a need for pain medication. Also, compared with women taking the placebo, significantly fewer participants taking the ginger extract experienced low back pain, fatigue, and nausea.
Chamomile Extract
A randomized trial in 90 women with PMS compared 100 mg of chamomile extract three times daily to 250 mg of the NSAID mefenamic acid three times daily. Women who received chamomile extract had greater improvement in emotional symptoms than women who took mefenamic acid, and mefenamic acid was not significantly superior to chamomile extract in providing relief from physical symptoms (Sharifi 2014).
Disclaimer and Safety Information
This information (and any accompanying material) is not intended to replace the attention or advice of a physician or other qualified health care professional. Anyone who wishes to embark on any dietary, drug, exercise, or other lifestyle change intended to prevent or treat a specific disease or condition should first consult with and seek clearance from a physician or other qualified health care professional. Pregnant women in particular should seek the advice of a physician before using any protocol listed on this website. The protocols described on this website are for adults only, unless otherwise specified. Product labels may contain important safety information and the most recent product information provided by the product manufacturers should be carefully reviewed prior to use to verify the dose, administration, and contraindications. National, state, and local laws may vary regarding the use and application of many of the therapies discussed. The reader assumes the risk of any injuries. The authors and publishers, their affiliates and assigns are not liable for any injury and/or damage to persons arising from this protocol and expressly disclaim responsibility for any adverse effects resulting from the use of the information contained herein.
The protocols raise many issues that are subject to change as new data emerge. None of our suggested protocol regimens can guarantee health benefits. Life Extension has not performed independent verification of the data contained in the referenced materials, and expressly disclaims responsibility for any error in the literature.
ACOG. Premenstrual Syndrome. International Journal of Gynecology and Obstetrics. 2001;73:183-191.
Agha-Hosseini M, Kashani L, Aleyaseen A, Ghoreishi A, Rahmanpour H, Zarrinara AR, Akhondzadeh S. Crocus sativus L. (saffron) in the treatment of premenstrual syndrome: a double-blind, randomised and placebo-controlled trial. BJOG : an international journal of obstetrics and gynaecology. Mar 2008;115(4):515-519.
Ajala OM, Ogunro PS, Elusanmi GF, Ogunyemi OE, Bolarinde AA. Changes in serum leptin during phases of menstrual cycle of fertile women: relationship to age groups and fertility. International journal of endocrinology and metabolism. Winter 2013;11(1):27-33.
Akhondzadeh S, Fallah-Pour H, Afkham K, Jamshidi AH, Khalighi-Cigaroudi F. Comparison of Crocus sativus L. and imipramine in the treatment of mild to moderate depression: a pilot double-blind randomized trial [ISRCTN45683816]. BMC complementary and alternative medicine. Sep 2 2004;4:12.
Akhondzadeh S, Tahmacebi-Pour N, Noorbala AA, Amini H, Fallah-Pour H, Jamshidi AH, Khani M. Crocus sativus L. in the treatment of mild to moderate depression: a double-blind, randomized and placebo-controlled trial. Phytotherapy research : PTR. Feb 2005;19(2):148-151.
Alvero R. Ferri's Clinical Advisor. Premenstrual Syndrome. Available at: www.clinicalkey.com. Copyright © 2014. Accessed 8/11/2014.
Ambrosini A, Di Lorenzo C, Coppola G, et al. Use of Vitex agnus-castus in migrainous women with premenstrual syndrome: an open-label clinical observation. Acta Neurologica Belgica. 2013;113(1):25-29.
AMS. Australasian Menopause Society. Surgical Menopause. Available at: https://www.menopause.org.au/for-women/information-sheets/736-surgical-menopause. Last updated 5/2013. Accessed 12/23/2014.
Andreen L, Nyberg S, Turkmen S, van Wingen G, Fernandez G, Backstrom T. Sex steroid induced negative mood may be explained by the paradoxical effect mediated by GABAA modulators. Psychoneuroendocrinology. Sep 2009;34(8):1121-1132.
Andreen L, Sundstrom-Poromaa I, Bixo M, Nyberg S, Backstrom T. Allopregnanolone concentration and mood--a bimodal association in postmenopausal women treated with oral progesterone. Psychopharmacology. Aug 2006;187(2):209-221.
ARHP. Association of Reproductive Health Professionals. Publications and Resources page. Managing Premenstrual Symptoms. 6/2008. Accessed 2/23/2015.
Atmaca M, Kumru S, Tezcan E. Fluoxetine versus Vitex agnus castus extract in the treatment of premenstrual dysphoric disorder. Human psychopharmacology. Apr 2003;18(3):191-195.
Backstrom T, Haage D, Lofgren M, Johansson IM, Stromberg J, Nyberg S, . . . Bengtsson SK. Paradoxical effects of GABA-A modulators may explain sex steroid induced negative mood symptoms in some persons. Neuroscience. Sep 15 2011;191:46-54.
Backstrom T, Sanders D, Leask R, Davidson D, Warner P, Bancroft J. Mood, sexuality, hormones, and the menstrual cycle. II. Hormone levels and their relationship to the premenstrual syndrome. Psychosomatic medicine. Dec 1983;45(6):503-507.
Benmansour S, Weaver RS, Barton AK, Adeniji OS, Frazer A. Comparison of the effects of estradiol and progesterone on serotonergic function. Biol Psychiatry. Apr 1 2012;71(7):633-641.
Bernardi F, Pluchino N, Begliuomini S, Lenzi E, Palumbo M, Luisi M, Genazzani AR. Disadaptive disorders in women: allopregnanolone, a sensitive steroid. Gynecological endocrinology : the official journal of the International Society of Gynecological Endocrinology. Dec 2004;19(6):344-353.
Bertone-Johnson ER, Hankinson SE, Bendich A, Johnson SR, Willett WC, Manson JE. Calcium and vitamin D intake and risk of incident premenstrual syndrome. Archives of internal medicine. Jun 13 2005;165(11):1246-1252.
Bertone-Johnson ER, Hankinson SE, Forger NG, Powers SI, Willett WC, Johnson SR, Manson JE. Plasma 25-hydroxyvitamin D and risk of premenstrual syndrome in a prospective cohort study. BMC women's health. 2014;14:56.
Bertone-Johnson ER, Hankinson SE, Johnson SR, Manson JE. Cigarette smoking and the development of premenstrual syndrome. American journal of epidemiology. Oct 15 2008;168(8):938-945.
Bertone-Johnson ER, Hankinson SE, Willett WC, Johnson SR, Manson JE. Adiposity and the development of premenstrual syndrome. Journal of women's health (2002). Nov 2010;19(11):1955-1962.
Bhatia SC, Bhatia SK. Diagnosis and treatment of premenstrual dysphoric disorder. American family physician. Oct 1 2002;66(7):1239-1248.
Boruban MC, Altundag K, Kilic GS, Blankstein J. From endometrial hyperplasia to endometrial cancer: insight into the biology and possible medical preventive measures. European journal of cancer prevention : the official journal of the European Cancer Prevention Organisation (ECP). Apr 2008;17(2):133-138.
BreastCancer.org Staff. Is it Menopause? Available at: http://www.breastcancer.org/tips/menopausal/understand/is_it. Last updated 5/9/13. Accessed 8/25/14.
Bryant M, Truesdale KP, Dye L. Modest changes in dietary intake across the menstrual cycle: implications for food intake research. The British journal of nutrition. Nov 2006;96(5):888-894.
Brzyski RG, Knudtson J. Menstrual Cycle; from The Merck Manual Home Edition. Available at: http://www.merckmanuals.com/home/womens_health_issues/biology_of_the_female_reproductive_system/menstrual_cycle.html. Last updated 7/13. Accessed 8/20/14.
Busse JW, Montori VM, Krasnik C, Patelis-Siotis I, Guyatt GH. Psychological intervention for premenstrual syndrome: a meta-analysis of randomized controlled trials. Psychotherapy and psychosomatics. 2009;78(1):6-15.
Calder PC. Marine omega-3 fatty acids and inflammatory processes: Effects, mechanisms and clinical relevance. Biochimica et biophysica acta. Apr 2015;1851(4):469-484.
Canning S, Waterman M, Orsi N, Ayres J, Simpson N, Dye L. The efficacy of Hypericum perforatum (St John's wort) for the treatment of premenstrual syndrome: a randomized, double-blind, placebo-controlled trial. CNS drugs. Mar 2010;24(3):207-225.
Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, . . . Poulton R. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science (New York, N.Y.). Jul 18 2003;301(5631):386-389.
Chocano-Bedoya PO, Manson JE, Hankinson SE, Willett WC, Johnson SR, Chasan-Taber L, . . . Bertone-Johnson ER. Dietary B vitamin intake and incident premenstrual syndrome. The American journal of clinical nutrition. May 2011;93(5):1080-1086.
Cirillo PC, Passos RB, Bevilaqua MC, Lopez JR, Nardi AE. Bipolar disorder and Premenstrual Syndrome or Premenstrual Dysphoric Disorder comorbidity: a systematic review. Revista brasileira de psiquiatria (Sao Paulo, Brazil : 1999). Dec 2012;34(4):467-479.
Clayton AH, Keller AE, Leslie C, Evans W. Exploratory study of premenstrual symptoms and serotonin variability. Archives of women's mental health. Jan 2006;9(1):51-57.
CRUK. Cancer Research UK. About Cancer: Cancer questions and answers. Endometrial hyperplasia. Available at: http://www.cancerresearchuk.org/about-cancer/cancers-in-general/cancer-questions/endometrial-hyperplasia. Accessed 12/23/2014.
Cunningham J, Yonkers KA, O'Brien S, Eriksson E. Update on research and treatment of premenstrual dysphoric disorder. Harvard review of psychiatry. 2009;17(2):120-137.
De Souza MC, Walker AF, Robinson PA, Bolland K. A synergistic effect of a daily supplement for 1 month of 200 mg magnesium plus 50 mg vitamin B6 for the relief of anxiety-related premenstrual symptoms: a randomized, double-blind, crossover study. Journal of women's health & gender-based medicine. Mar 2000;9(2):131-139.
Dennerstein L, Spencer-Gardner C, Gotts G, Brown JB, Smith MA, Burrows GD. Progesterone and the premenstrual syndrome: a double blind crossover trial. British medical journal (Clinical research ed.). Jun 1 1985;290(6482):1617-1621.
Dhingra V, Magnay JL, O'Brien PM, Chapman G, Fryer AA, Ismail KM. Serotonin receptor 1A C(-1019)G polymorphism associated with premenstrual dysphoric disorder. Obstetrics and gynecology. Oct 2007;110(4):788-792.
Douglas S. Premenstrual syndrome. Evidence-based treatment in family practice. Canadian family physician Medecin de famille canadien. Nov 2002;48:1789-1797.
Duckworth K, Freedman JL. NAMI: National Alliance on Mental Health. Treatment & Services page. Cognitive Behavioral Therapy (CBT)? Available at: http://www2.nami.org/Content/NavigationMenu/Inform_Yourself/About_Mental_Illness/About_Treatments_and_Supports/Cognitive_Behavioral_Therapy1.htm. 7/2012. Accessed 2/23/2015.
EBSCO. NYU Langone Medical Center. Gamma-Linolenic Acid. Available at: http://www.med.nyu.edu/content?ChunkIID=21587. Copyright 2014. Accessed 12/23/2014.
Endicott J, Nee J, Harrison W. Daily Record of Severity of Problems (DRSP): reliability and validity. Archives of women's mental health. Jan 2006;9(1):41-49.
Engin E, Treit D. The anxiolytic-like effects of allopregnanolone vary as a function of intracerebral microinfusion site: the amygdala, medial prefrontal cortex, or hippocampus. Behavioural pharmacology. Sep 2007;18(5-6):461-470.
Epperson CN, Steiner M, Hartlage SA, Eriksson E, Schmidt PJ, Jones I, Yonkers KA. Premenstrual dysphoric disorder: evidence for a new category for DSM-5. The American journal of psychiatry. May 2012;169(5):465-475.
Facchinetti F, Borella P, Sances G, Fioroni L, Nappi RE, Genazzani AR. Oral magnesium successfully relieves premenstrual mood changes. Obstetrics and gynecology. Aug 1991;78(2):177-181.
Facchinetti F, Sances G, Borella P, Genazzani AR, Nappi G. Magnesium prophylaxis of menstrual migraine: effects on intracellular magnesium. Headache. May 1991;31(5):298-301.
Fathizadeh N, Ebrahimi E, Valiani M, Tavakoli N, Yar MH. Evaluating the effect of magnesium and magnesium plus vitamin B6 supplement on the severity of premenstrual syndrome. Iranian journal of nursing and midwifery research. Dec 2010;15(Suppl 1):401-405.
FDA. U.S. Food and Drug Administration. Drugs page. FDA Drug Safety Communication: Updated information about the risk of blood clots in women taking birth control pills containing drospirenone. Last updated 2/15/2013. Accessed 2/23/2015.
Files JA, Ko MG, Pruthi S. Bioidentical hormone therapy. Mayo Clinic proceedings. Mayo Clinic. Jul 2011;86(7):673-680, quiz 680.
Finocchi C, Ferrari M. Female reproductive steroids and neuronal excitability. Neurological sciences : official journal of the Italian Neurological Society and of the Italian Society of Clinical Neurophysiology. May 2011;32 Suppl 1:S31-35.
Freeman EW, Halbreich U, Grubb GS, Rapkin AJ, Skouby SO, Smith L, . . . Constantine GD. An overview of four studies of a continuous oral contraceptive (levonorgestrel 90 mcg/ethinyl estradiol 20 mcg) on premenstrual dysphoric disorder and premenstrual syndrome. Contraception. May 2012;85(5):437-445.
Freeman EW, Sondheimer SJ. Premenstrual Dysphoric Disorder: Recognition and Treatment. Primary care companion to the Journal of clinical psychiatry. Feb 2003;5(1):30-39.
Freeman EW. Treatment of depression associated with the menstrual cycle: premenstrual dysphoria, postpartum depression, and the perimenopause. Dialogues in clinical neuroscience. 2002;4(2):177-191.
Fugh-Berman A, Bythrow J. Bioidentical hormones for menopausal hormone therapy: variation on a theme. Journal of general internal medicine. Jul 2007;22(7):1030-1034.
Gaby AR. Nutritional Medicine. Premenstrual Syndrome. Concord, NH: Fritz Perlberg Publishing; 2011.
Ghanbari Z, Haghollahi F, Shariat M, Foroshani AR, Ashrafi M. Effects of calcium supplement therapy in women with premenstrual syndrome. Taiwanese journal of obstetrics & gynecology. Jun 2009;48(2):124-129.
Ghazanfarpour M, Kaviani M, Asadi N, Ghaffarpasand F, Ziyadlou S, Tabatabaee HR, Dehghankhalili M. Hypericum perforatum for the treatment of premenstrual syndrome. International Journal of Gynecology and Obstetrics. 2011;113(1):84-85.
Gingnell M, Comasco E, Oreland L, Fredrikson M, Sundstrom-Poromaa I. Neuroticism-related personality traits are related to symptom severity in patients with premenstrual dysphoric disorder and to the serotonin transporter gene-linked polymorphism 5-HTTPLPR. Archives of women's mental health. Oct 2010;13(5):417-423.
Girdler SS, Klatzkin R. Neurosteroids in the context of stress: implications for depressive disorders. Pharmacology & therapeutics. Oct 2007;116(1):125-139.
Girdler SS, Pedersen CA, Light KC. Thyroid axis function during the menstrual cycle in women with premenstrual syndrome. Psychoneuroendocrinology. 1995;20(4):395-403.
Girdler SS, Straneva PA, Light KC, Pedersen CA, Morrow AL. Allopregnanolone levels and reactivity to mental stress in premenstrual dysphoric disorder. Biol Psychiatry. May 1 2001;49(9):788-797.
Goker A, Ulkumen BA, Aktenk F, Ikiz N. Premenstrual syndrome in Turkish medical students and their quality of life. Journal of obstetrics and gynaecology : the journal of the Institute of Obstetrics and Gynaecology. Aug 20 2014:1-4.
Goncharenko VM, Beniuk VA, Kalenska OV, Demchenko OM, Spivak MY, Bubnov RV. Predictive diagnosis of endometrial hyperplasia and personalized therapeutic strategy in women of fertile age. The EPMA journal. 2013;4(1):24.
Haddad P. The SSRI discontinuation syndrome. Journal of psychopharmacology (Oxford, England). 1998;12(3):305-313.
Hainer V, Kabrnova K, Aldhoon B, Kunesova M, Wagenknecht M. Serotonin and norepinephrine reuptake inhibition and eating behavior. Annals of the New York Academy of Sciences. Nov 2006;1083:252-269.
Hawkins SM, Matzuk MM. The menstrual cycle: basic biology. Annals of the New York Academy of Sciences. 2008;1135:10-18.
Higdon J. Linus Pauling Institute. Micronutrient Information Center. Magnesium. Data on file.
Higdon J. Linus Pauling Institute. Micronutrient Information Center. Vitamin B6. Data on file.
Holtorf K. The bioidentical hormone debate. Postgraduate medicine. 2009;121(1):4-9.
Huang CB, Ebersole JL. A novel bioactivity of omega-3 polyunsaturated fatty acids and their ester derivatives. Molecular oral microbiology. Feb 2010;25(1):75-80.
Huang KL, Tsai SJ. St. John's wort (Hypericum perforatum) as a treatment for premenstrual dysphoric disorder: case report. International journal of psychiatry in medicine. 2003;33(3):295-297.
Hudson T. Chapter 202: Premenstrual Syndrome. pp 1740 - 1747. In: Textbook of Natural Medicine. Pizzorno JE and Murray MT, eds. Copyright 2013 by Churchill Livingstone, an Imprint of Elsevier Inc. 2013.
Ibrahim RM, Soliman SM, Mahmoud HM. Effect of Vitex agnus Custus (VAC) on premenstrual syndromes among nursing students. Journal of American Science. 2012;8(4):144-153.
Jang SH, Kim DI, Choi MS. Effects and treatment methods of acupuncture and herbal medicine for premenstrual syndrome/premenstrual dysphoric disorder: systematic review. BMC complementary and alternative medicine. 2014;14:11.
Jick SS, Hernandez RK. Risk of non-fatal venous thromboembolism in women using oral contraceptives containing drospirenone compared with women using oral contraceptives containing levonorgestrel: case-control study using United States claims data. BMJ (Clinical research ed.). 2011;342:d2151.
Justice AJ, de Wit H. Acute effects of d-amphetamine during the follicular and luteal phases of the menstrual cycle in women. Psychopharmacology. Jul 1999;145(1):67-75.
Khayat S, Kheirkhah M, Behboodi Moghadam Z, Fanaei H, Kasaeian A, Javadimehr M. Effect of treatment with ginger on the severity of premenstrual syndrome symptoms. ISRN obstetrics and gynecology. 2014;2014:792708.
Kim DR, Gyulai L, Freeman EW, Morrison MF, Baldassano C, Dube B. Premenstrual dysphoric disorder and psychiatric co-morbidity. Archives of women's mental health. Feb 2004;7(1):37-47.
Kim SY, Park HJ, Lee H, Lee H. Acupuncture for premenstrual syndrome: a systematic review and meta-analysis of randomised controlled trials. BJOG : an international journal of obstetrics and gynaecology. Jul 2011;118(8):899-915.
Klatzkin RR, Lindgren ME, Forneris CA, Girdler SS. Histories of major depression and premenstrual dysphoric disorder: Evidence for phenotypic differences. Biological psychology. May 2010;84(2):235-247.
Klatzkin RR, Morrow AL, Light KC, Pedersen CA, Girdler SS. Histories of depression, allopregnanolone responses to stress, and premenstrual symptoms in women. Biological psychology. Jan 2006;71(1):2-11.
Kleinstauber M, Witthoft M, Hiller W. Cognitive-behavioral and pharmacological interventions for premenstrual syndrome or premenstrual dysphoric disorder: a meta-analysis. Journal of clinical psychology in medical settings. Sep 2012;19(3):308-319.
Kuhl H. [Influence of the ovarian cycle on the central nervous system]. Therapeutische Umschau. Revue therapeutique. Apr 2002;59(4):175-181.
Kumar P, Sharma A. Gonadotropin-releasing hormone analogs: Understanding advantages and limitations. Journal of human reproductive sciences. Jul 2014;7(3):170-174.
Liou LS. Prolactin: from MedlinePlus. Available at: http://www.nlm.nih.gov/medlineplus/ency/article/003718.htm. Last updated 9/17/12. Accessed 8/22/14.
Lombardi I, Luisi S, Quirici B, Monteleone P, Bernardi F, Liut M, . . . Genazzani AR. Adrenal response to adrenocorticotropic hormone stimulation in patients with premenstrual syndrome. Gynecological endocrinology : the official journal of the International Society of Gynecological Endocrinology. Feb 2004;18(2):79-87.
Lopez LM, Kaptein AA, Helmerhorst FM. Oral contraceptives containing drospirenone for premenstrual syndrome. The Cochrane database of systematic reviews. 2012;2:CD006586.
Ma L, Lin S, Chen R, Wang X. Treatment of moderate to severe premenstrual syndrome with Vitex agnus castus (BNO 1095) in Chinese women. Gynecol Endocrinol. 2010;26(8):612-6.
Ma L, Lin S, chen R, Zhang Y, et al. Evaluating therapeutic effect in symptoms of moderate-to-severe premenstrual syndrome with Vitex agnus castus (BNO 1095) in Chinese women. Aust N Z J Obstet Gynaecol. 2010;50(2):189-93.
Magnay JL, El-Shourbagy M, Fryer AA, O'Brien S, Ismail KM. Analysis of the serotonin transporter promoter rs25531 polymorphism in premenstrual dysphoric disorder. American journal of obstetrics and gynecology. Aug 2010;203(2):181.e181-185.
Magnay JL, Ismail KM, Chapman G, Cioni L, Jones PW, O'Brien S. Serotonin transporter, tryptophan hydroxylase, and monoamine oxidase A gene polymorphisms in premenstrual dysphoric disorder. American journal of obstetrics and gynecology. Nov 2006;195(5):1254-1259.
Magon N. Gonadotropin releasing hormone agonists: Expanding vistas. Indian journal of endocrinology and metabolism. Oct 2011;15(4):261-267.
Marjoribanks J, Brown J, O'Brien PM, Wyatt K. Selective serotonin reuptake inhibitors for premenstrual syndrome. The Cochrane database of systematic reviews. 2013;6:Cd001396.
Marsden T. Bioidentical Hormone Replacement: Guiding Principles for Practice. Natural Medicine Journal. 2010;2(3).
Masho SW, Adera T, South-Paul J. Obesity as a risk factor for premenstrual syndrome. Journal of psychosomatic obstetrics and gynaecology. Mar 2005;26(1):33-39.
Mayo Clinic. Premenstrual syndrome. Available at: http://www.mayoclinic.org/diseases-conditions/premenstrual-syndrome/basics/treatment/con-20020003. Last updated 1/18/12. Accessed 8/31/14.
MGH. Massachusetts General Hospital Center for Women's Health. Specialty Areas page. PMS & PMDD. Available at: http://womensmentalhealth.org/specialty-clinics/pms-and-pmdd/. Copyright 2013. Accessed 12/23/2014.
Mira M, McNeil D, Fraser IS, Vizzard J, Abraham S. Mefenamic acid in the treatment of premenstrual syndrome. Obstetrics and gynecology. Sep 1986;68(3):395-398.
Miyaoka Y, Akimoto Y, Ueda K, Ujiie Y, Kametani M, Uchiide Y, Kamo T. Fulfillment of the premenstrual dysphoric disorder criteria confirmed using a self-rating questionnaire among Japanese women with depressive disorders. BioPsychoSocial medicine. 2011;5:5.
Momoeda M, Sasaki H, Tagashire E, et al. Efficacy and safety of Vitex agnus-castus extract for treatment of premenstrual syndrome in Japanese patients: a prospective, open-label study. Adv Ther. 2014;31(3):362-73.
Monteleone P, Luisi S, Tonetti A, Bernardi F, Genazzani AD, Luisi M, . . . Genazzani AR. Allopregnanolone concentrations and premenstrual syndrome. European journal of endocrinology / European Federation of Endocrine Societies. Mar 2000;142(3):269-273.
Monti JM. Serotonin control of sleep-wake behavior. Sleep medicine reviews. Aug 2011;15(4):269-281.
Mortola JF, Girton L, Yen SS. Depressive episodes in premenstrual syndrome. American journal of obstetrics and gynecology. Dec 1989;161(6 Pt 1):1682-1687.
Nagata C, Hirokawa K, Shimizu N, Shimizu H. Soy, fat and other dietary factors in relation to premenstrual symptoms in Japanese women. BJOG : an international journal of obstetrics and gynaecology. Jun 2004;111(6):594-599.
NCCAM. National Center for Complementary and Alternative Medicine. St. John’s Wort and Depression. Available at: http://nccam.nih.gov/health/stjohnswort/sjw-and-depression.htm. Last updated 1/30/2014. Accessed 2/23/2015.
Nevatte T, O'Brien PM, Backstrom T, Brown C, Dennerstein L, Endicott J, . . . Yonkers K. ISPMD consensus on the management of premenstrual disorders. Archives of women's mental health. Aug 2013;16(4):279-291.
NIH. Information About Sleep. In: NIH Curriculum Supplement Series [Internet]. National Institutes of Health (US): Biological Sciences Curriculum Study. Bethesda (MD): 2007. Available at: http://www.ncbi.nlm.nih.gov/books/NBK20359/. Accessed 12/23/2014.
Nillni YI, Toufexis DJ, Rohan KJ. Anxiety sensitivity, the menstrual cycle, and panic disorder: a putative neuroendocrine and psychological interaction. Clinical psychology review. Nov 2011;31(7):1183-1191.
Nirvanashetty S, Panda S. K., Michel S. J. High Potency Ginger Extract Reduces Menstrual Discomfort in Healthy Participants with Recurrent Dysmenorrhea Linked to Hypercontractility of the Uterus: a Randomized, Double-Blind, Placebo-Controlled Trial. Open Acc J Comp & Alt Med. 2023;5(1). DOI: 10.32474/OAJCAM.2023.05.000203
NLM. U.S. National Library of Medicne. PubMed Health webpage. Mefenamic Acid (By mouth). Available at: http://www.ncbi.nlm.nih.gov/pubmedhealth/PMHT0011062/?report=details. Copyright 2012. Accessed 2/2/2015.
Noorbala AA, Akhondzadeh S, Tahmacebi-Pour N, Jamshidi AH. Hydro-alcoholic extract of Crocus sativus L. versus fluoxetine in the treatment of mild to moderate depression: a double-blind, randomized pilot trial. Journal of ethnopharmacology. Feb 28 2005;97(2):281-284.
O'Brien PM, Backstrom T, Brown C, Dennerstein L, Endicott J, Epperson CN, . . . Yonkers K. Towards a consensus on diagnostic criteria, measurement and trial design of the premenstrual disorders: the ISPMD Montreal consensus. Archives of women's mental health. Feb 2011;14(1):13-21.
OpenStax College. Wikimedia Commons. File: Figure 28 02 07. In: Anatomy & Physiology, Connexions Website. Available at: http://commons.wikimedia.org/wiki/File:Figure_28_02_07.jpg. 5/2/2013. Accessed 2/5/2015.
Ounis-Skali N, Mitchell GF, Solomon CG, Solomon SD, Seely EW. Changes in central arterial pressure waveforms during the normal menstrual cycle. Journal of investigative medicine : the official publication of the American Federation for Clinical Research. Sep 2006;54(6):321-326.
OWH. Office On Women's Health. Premenstrual syndrome (PMS) fact sheet. Available at: http://www.womenshealth.gov/publications/our-publications/fact-sheet/premenstrual-syndrome.html#f. Last updated 7/16/2012. Accessed 10/27/2014.
Ozaki A, Bartholomew JR. Venous Thromboembolism (Deep Venous Thrombosis & Pulmonary Embolism). Available at: http://www.clevelandclinicmeded.com/medicalpubs/diseasemanagement/cardiology/venous-thromboembolism/#s0045. Published 12/12. Accessed 9/2/14.
Ozgoli G, Selselei EA, Mojab F, Majd HA. A randomized, placebo-controlled trial of Ginkgo biloba L. in treatment of premenstrual syndrome. Journal of alternative and complementary medicine (New York, N.Y.). Aug 2009;15(8):845-851.
Parkin L, Sharples K, Hernandez RK, Jick SS. Risk of venous thromboembolism in users of oral contraceptives containing drospirenone or levonorgestrel: nested case-control study based on UK General Practice Research Database. BMJ (Clinical research ed.). 2011;342:d2139.
Parsey RV, Hastings RS, Oquendo MA, Huang YY, Simpson N, Arcement J, . . . Mann JJ. Lower serotonin transporter binding potential in the human brain during major depressive episodes. The American journal of psychiatry. Jan 2006;163(1):52-58.
Pearlstein T, Steiner M. Premenstrual dysphoric disorder: burden of illness and treatment update. Journal of psychiatry & neuroscience : JPN. Jul 2008;33(4):291-301.
PEPI Trial Writing Group. Effects of hormone replacement therapy on endometrial histology in postmenopausal women. The Postmenopausal Estrogen/Progestin Interventions (PEPI) Trial. The Writing Group for the PEPI Trial. JAMA : the journal of the American Medical Association. Feb 7 1996;275(5):370-375.
Perkonigg A, Yonkers KA, Pfister H, Lieb R, Wittchen HU. Risk factors for premenstrual dysphoric disorder in a community sample of young women: the role of traumatic events and posttraumatic stress disorder. The Journal of clinical psychiatry. Oct 2004;65(10):1314-1322.
Pilver CE, Desai R, Kasl S, Levy BR. Lifetime discrimination associated with greater likelihood of premenstrual dysphoric disorder. J Womens Health (Larchmt). Jun 2011;20(6):923-931.
Pilver CE, Levy BR, Libby DJ, Desai RA. Posttraumatic stress disorder and trauma characteristics are correlates of premenstrual dysphoric disorder. Archives of women's mental health. Oct 2011;14(5):383-393.
Pinkerton J. Premenstrual Syndrome; from The Merck Manual Professional Edition. Available at: http://www.merckmanuals.com/professional/gynecology_and_obstetrics/menstrual_abnormalities/premenstrual_syndrome_pms.html?qt=premenstrual%20syndrome&alt=sh#v1062695. Last updated 10/13. Accessed 10/15/14.
Posaci C, Erten O, Uren A, Acar B. Plasma copper, zinc and magnesium levels in patients with premenstrual tension syndrome. Acta obstetricia et gynecologica Scandinavica. Jul 1994;73(6):452-455.
Rani A, Sharma A. The genus Vitex: A review. Pharmacognosy reviews. Jul 2013;7(14):188-198.
Rapkin AJ, Akopians AL. First Consult. Premenstrual syndrome. Available at: www.clinicalkey.com. Last updated 1/7/2014. Accessed 8/11/2014.
Rapkin AJ, Akopians AL. Pathophysiology of premenstrual syndrome and premenstrual dysphoric disorder. Menopause Int. Jun 2012;18(2):52-59.
Rapkin AJ, Lewis EI. Treatment of premenstrual dysphoric disorder. Women's health (London, England). Nov 2013;9(6):537-556.
Rapkin AJ, Morgan M, Goldman L, Brann DW, Simone D, Mahesh VB. Progesterone metabolite allopregnanolone in women with premenstrual syndrome. Obstetrics and gynecology. Nov 1997;90(5):709-714.
Rapkin AJ, Winer SA. Premenstrual syndrome and premenstrual dysphoric disorder: quality of life and burden of illness. Expert review of pharmacoeconomics & outcomes research. Apr 2009;9(2):157-170.
Rasgon N, Serra M, Biggio G, Pisu MG, Fairbanks L, Tanavoli S, Rapkin A. Neuroactive steroid-serotonergic interaction: responses to an intravenous L-tryptophan challenge in women with premenstrual syndrome. European journal of endocrinology / European Federation of Endocrine Societies. Jul 2001;145(1):25-33.
Reddy DS. Neurosteroids: endogenous role in the human brain and therapeutic potentials. Progress in brain research. 2010;186:113-137.
Reed SC, Levin FR, Evans SM. Changes in mood, cognitive performance and appetite in the late luteal and follicular phases of the menstrual cycle in women with and without PMDD (premenstrual dysphoric disorder). Hormones and behavior. Jun 2008;54(1):185-193.
Rees M. ePocrates online. Premenstrual syndrome/dysphoric disorder. Available at: https://online.epocrates.com/u/2911419/Premenstrual+syndrome+/dysphoric+disorder. Last updated 4/3/2014. Accessed 8/11/2014.
Renoir T. Selective serotonin reuptake inhibitor antidepressant treatment discontinuation syndrome: a review of the clinical evidence and the possible mechanisms involved. Frontiers in pharmacology. 2013;4:45.
Rocha Filho EA, Lima JC, Pinho Neto JS, Montarroyos U. Essential fatty acids for premenstrual syndrome and their effect on prolactin and total cholesterol levels: a randomized, double blind, placebo-controlled study. Reproductive health. 2011;8:2.
Rodriguez AL, Wojcik BM, Wrobleski SK, Myers DD, Jr., Wakefield TW, Diaz JA. Statins, inflammation and deep vein thrombosis: a systematic review. Journal of thrombosis and thrombolysis. May 2012;33(4):371-382.
Rosenstein DL, Elin RJ, Hosseini JM, Grover G, Rubinow DR. Magnesium measures across the menstrual cycle in premenstrual syndrome. Biological psychiatry. Apr 15 1994;35(8):557-561.
Rossignol AM, Bonnlander H. Caffeine-containing beverages, total fluid consumption, and premenstrual syndrome. American journal of public health. Sep 1990;80(9):1106-1110.
Rossignol AM, Bonnlander H. Prevalence and severity of the premenstrual syndrome. Effects of foods and beverages that are sweet or high in sugar content. The Journal of reproductive medicine. Feb 1991;36(2):131-136.
Roth-Deri I, Green-Sadan T, Yadid G. Beta-endorphin and drug-induced reward and reinforcement. Progress in neurobiology. Sep 2008;86(1):1-21.
Rubinow DR, Hoban MC, Grover GN, Galloway DS, Roy-Byrne P, Andersen R, Merriam GR. Changes in plasma hormones across the menstrual cycle in patients with menstrually related mood disorder and in control subjects. American journal of obstetrics and gynecology. Jan 1988;158(1):5-11.
Samadi Z, Taghian F, Valiani M. The effects of 8 weeks of regular aerobic exercise on the symptoms of premenstrual syndrome in non-athlete girls. Iranian journal of nursing and midwifery research. Jan 2013;18(1):14-19.
Sampalis F, Bunea R, Pelland MF, Kowalski O, Duguet N, Dupuis S. Evaluation of the effects of Neptune Krill Oil on the management of premenstrual syndrome and dysmenorrhea. Alternative medicine review : a journal of clinical therapeutic. May 2003;8(2):171-179.
Schellenberg R, Zimmermann C, Drewe J, Hoexter G, Zahner C. Dose-dependent efficacy of the Vitex agnus castus extract Ze 440 in patients suffering from premenstrual syndrome. Phytomedicine : international journal of phytotherapy and phytopharmacology. Nov 15 2012;19(14):1325-1331.
Schellenberg R. Treatment for the premenstrual syndrome with agnus castus fruit extract: prospective, randomised, placebo controlled study. BMJ (Clinical research ed.). Jan 20 2001;322(7279):134-137.
Schmidt PJ, Grover GN, Roy-Byrne PP, Rubinow DR. Thyroid function in women with premenstrual syndrome. The Journal of clinical endocrinology and metabolism. Mar 1993;76(3):671-674.
Segebladh B, Bannbers E, Moby L, Nyberg S, Bixo M, Backstrom T, Sundstrom Poromaa I. Allopregnanolone serum concentrations and diurnal cortisol secretion in women with premenstrual dysphoric disorder. Archives of women's mental health. Apr 2013;16(2):131-137.
Sharifi F, Simbar M, Mojab F, Majd HA. Comparison of the effects of Matricaria chamomila (Chamomile) extract and mefenamic acid on the intensity of premenstrual syndrome. Complementary therapies in clinical practice. Feb 2014;20(1):81-88.
Shimoda H, Shan SJ, Tanaka J, Seki A, Seo JW, Kasajima N, . . . Murakami N. Anti-inflammatory properties of red ginger (Zingiber officinale var. Rubra) extract and suppression of nitric oxide production by its constituents. Journal of medicinal food. Feb 2010;13(1):156-162.
Smith RN, Studd JW, Zamblera D, Holland EF. A randomised comparison over 8 months of 100 micrograms and 200 micrograms twice weekly doses of transdermal oestradiol in the treatment of severe premenstrual syndrome. British journal of obstetrics and gynaecology. Jun 1995;102(6):475-484.
Smith SM, Vale WW. The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress. Dialogues in clinical neuroscience. 2006;8(4):383-395.
Sohrabi N, Kashanian M, Ghafoori SS, Malakouti SK. Evaluation of the effect of omega-3 fatty acids in the treatment of premenstrual syndrome: "a pilot trial". Complementary therapies in medicine. Jun 2013;21(3):141-146.
Sprouse-Blum AS, Smith G, Sugai D, Parsa FD. Understanding endorphins and their importance in pain management. Hawaii medical journal. Mar 2010;69(3):70-71.
Stegeman BH, de Bastos M, Rosendaal FR, van Hylckama Vlieg A, Helmerhorst FM, Stijnen T, Dekkers OM. Different combined oral contraceptives and the risk of venous thrombosis: systematic review and network meta-analysis. BMJ (Clinical research ed.). 2013;347:f5298.
Steinberg S, Annable L, Young SN, Liyanage N. A placebo-controlled clinical trial of L-tryptophan in premenstrual dysphoria. Biol Psychiatry. Feb 1 1999;45(3):313-320.
Steiner M, Dunn E, Born L. Hormones and mood: from menarche to menopause and beyond. Journal of affective disorders. Mar 2003;74(1):67-83.
Steiner M, Pearlstein T, Cohen LS, Endicott J, Kornstein SG, Roberts C, . . . Yonkers K. Expert guidelines for the treatment of severe PMS, PMDD, and comorbidities: the role of SSRIs. Journal of women's health (2002). Jan-Feb 2006;15(1):57-69.
Stevinson C, Ernst E. A pilot study of Hypericum perforatum for the treatment of premenstrual syndrome. BJOG : an international journal of obstetrics and gynaecology. Jul 2000;107(7):870-876.
Studd JW. A guide to the treatment of depression in women by estrogens. Climacteric : the journal of the International Menopause Society. Dec 2011;14(6):637-642.
Sulak PJ, Scow RD, Preece C, Riggs MW, Kuehl TJ. Hormone withdrawal symptoms in oral contraceptive users. Obstetrics and gynecology. Feb 2000;95(2):261-266.
Tamborini A, Taurelle R. [Value of standardized Ginkgo biloba extract (EGb 761) in the management of congestive symptoms of premenstrual syndrome]. Revue francaise de gynecologie et d'obstetrique. Jul-Sep 1993;88(7-9):447-457.
Tchaikovski SN, Rosing J. Mechanisms of estrogen-induced venous thromboembolism. Thrombosis research. Jul 2010;126(1):5-11.
Thys-Jacobs S, Ceccarelli S, Bierman A, Weisman H, Cohen MA, Alvir J. Calcium supplementation in premenstrual syndrome: a randomized crossover trial. Journal of general internal medicine. May-Jun 1989;4(3):183-189.
Thys-Jacobs S, McMahon D, Bilezikian JP. Cyclical changes in calcium metabolism across the menstrual cycle in women with premenstrual dysphoric disorder. The Journal of clinical endocrinology and metabolism. Aug 2007;92(8):2952-2959.
Thys-Jacobs S, Starkey P, Bernstein D, Tian J. Calcium carbonate and the premenstrual syndrome: effects on premenstrual and menstrual symptoms. Premenstrual Syndrome Study Group. American journal of obstetrics and gynecology. Aug 1998;179(2):444-452.
Thys-Jacobs S. Micronutrients and the premenstrual syndrome: the case for calcium. Journal of the American College of Nutrition. Apr 2000;19(2):220-227.
Tricotel A, Raguideau F, Collin C, Zureik M. Estimate of venous thromboembolism and related-deaths attributable to the use of combined oral contraceptives in france. PloS one. 2014;9(4):e93792.
UMMC. University of Maryland Medical Center. Magnesium. Available at: http://umm.edu/health/medical/altmed/supplement/magnesium. Last updated 5/7/2013a. Accessed 12/23/2014.
UMMC. University of Maryland Medical Center. Omega-3 fatty acids. Available at: http://umm.edu.health/medical/altmed/supplement/omega3-fatty-acids. Last updated 6/24/2013b. Accessed 9/7/2014.
UMMC. University of Maryland Medical Center. Omega-6 fatty acids. Available at: http://umm.edu/health/medical/altmed/supplement/omega6-fatty-acids. Last updated 6/20/2013c. Accessed 9/8/2014.
van Die MD, Burger HG, Teede HJ, Bone KM. Vitex agnus-castus extracts for female reproductive disorders: a systematic review of clinical trials. Planta medica. May 2013;79(7):562-575.
Walker AF, De Souza MC, Vickers MF, Abeyasekera S, Collins ML, Trinca LA. Magnesium supplementation alleviates premenstrual symptoms of fluid retention. Journal of women's health / the official publication of the Society for the Advancement of Women's Health Research. Nov 1998;7(9):1157-1165.
Watson NR, Studd JW, Savvas M, Garnett T, Baber RJ. Treatment of severe premenstrual syndrome with oestradiol patches and cyclical oral norethisterone. Lancet. Sep 23 1989;2(8665):730-732.
Whelan AM, Jurgens TM, Naylor H. Herbs, vitamins and minerals in the treatment of premenstrual syndrome: a systematic review. The Canadian journal of clinical pharmacology = Journal canadien de pharmacologie clinique. Fall 2009;16(3):e407-429.
Willacy H, Kenny T. Patient. Health Information page. Premenstrual Syndrome. Available at: http://www.patient.co.uk/health/premenstrual-syndrome. Last updated 4/20/2012. Accessed 10/27/2014.
Wittchen HU, Perkonigg A, Pfister H. Trauma and PTSD - an overlooked pathogenic pathway for premenstrual dysphoric disorder? Archives of women's mental health. Nov 2003;6(4):293-297.
Wu CQ, Grandi SM, Filion KB, Abenhaim HA, Joseph L, Eisenberg MJ. Drospirenone-containing oral contraceptive pills and the risk of venous and arterial thrombosis: a systematic review. BJOG : an international journal of obstetrics and gynaecology. Jun 2013;120(7):801-810.
Wyatt KM, Dimmock PW, Jones PW, Shaughn O'Brien PM. Efficacy of vitamin B-6 in the treatment of premenstrual syndrome: systematic review. BMJ (Clinical research ed.). May 22 1999;318(7195):1375-1381.
Yen JY, Chang SJ, Ko CH, Yen CF, Chen CS, Yeh YC, Chen CC. The high-sweet-fat food craving among women with premenstrual dysphoric disorder: emotional response, implicit attitude and rewards sensitivity. Psychoneuroendocrinology. Sep 2010;35(8):1203-1212.
Yen JY, Tu HP, Chen CS, Yen CF, Long CY, Ko CH. The effect of serotonin 1A receptor polymorphism on the cognitive function of premenstrual dysphoric disorder. European archives of psychiatry and clinical neuroscience. Oct 26 2013.
Yonkers KA, O'Brien PM, Eriksson E. Premenstrual syndrome. Lancet. Apr 5 2008;371(9619):1200-1210.
Zamani M, Neghab N, Torabian S. Therapeutic effect of Vitex Agnus Castus in Patients with Premenstrual Syndrome. Acta Medica Iranica. 2012;50(2):101-106.
