Life Extension Magazine.
The leading cause of human suffering is not what you read in the media.
The culprit is biological aging, whereby our body becomes increasingly susceptible to diseases that diminish quality of life, leading to pain, disability, and eventual personal extinction.1
Research that aims to reverse degenerative aging holds immense societal benefits. This includes slashing healthcare costs,2 enhancing productivity, and retaining the cumulative wisdom of millions of humans.3
As scientists identify new aging mechanisms, interventions that target degenerative decline are rapidly being investigated.
Successful clinical outcomes will revolutionize humanity in ways analogous to Gutenberg’s invention of the movable-type printing press in the 1440-1450 period.
Gutenberg’s innovation played a crucial role in the spread of knowledge and the advancement of literacy, leading to significant cultural and economic changes, along with exponential improvements in living standards.4
(Before Gutenberg’s invention, there were virtually no "books" to read and learn from.)
Extending healthy lifespans by enabling older people to grow biologically younger may eliminate age-related ailments, while restoring mobility, cognition, and functional independence.2
With populations rapidly aging across the globe, the need for effective interventions to mitigate degenerative diseases and extend healthy lifespans is urgent.3
This article describes research findings published over the last 14 months…most overlooked by mainstream media.
Young Plasma Increases Median Lifespan
Healthy young plasma contains tens of thousands of extracellular vesicles, exosomes, cytokines, growth factors, regulatory proteins and other molecules that may revitalize function and restore organ integrity in elderly individuals.5
Published studies demonstrate systemic effects of young plasma delivered to older organisms,6-14 including preliminary data on humans.15,16
Rejuvenation Studies Published in 202417-19
I helped fund a university study that assessed the effects of young plasma on the lifespan, epigenetic age, and healthspan of old rats.17
Beginning at age 25.6 months one group of rats was infused every other week with plasma from young rats until their natural death. The same age control group received no treatment. Blood samples were collected every other week. The rats were human equivalent of 60-65 years.
Survival curves showed that none of the young plasma animals died from age 26 to 30 months, whereas the survival curve of the no treatment group began to decline at age 26 months.
The chart on this page shows a 9.1% improvement in median lifespan of the young plasma treated group compared with controls. In addition, the young plasma treated rats appeared and behaved significantly younger than the control group. (See photo below)
This chart shows a significant 9.1% improvement in the median lifespan of the young plasma treated group compared with no treatment controls. However, the most remarkable effect of the young plasma treatment is the clear improvement in healthspan of the old rats that the photos reveal. The treated old rats look biologically younger than controls and the clock data (DNA methylation age) confirm that they are epigenetically younger. Although a 9.1% increase in lifespan seems rather modest, this figure is associated with a marked revitalization of the old rats. If applied to healthy humans aged 65, this might increase median lifespan by about 7 years, thus extending the remaining median life expectancy from age 85 to about 92 years.17
DNA methylation testing measures epigenetic age and is considered the most accurate method of assessing future morbidity/mortality risk.20
In this study, DNA methylation age of young plasma treated rats beneficially fell below the control group. In numerical terms, it remained consistently lower in the young plasma group until natural death. (We all want lower, younger, DNA methylation age measures.)
Analysis of epigenetic age in genes revealed favorable insulin signaling indicators, better immune/metabolic health, and reduced inflammation.
The authors of this published study summarized:
"We conclude that young plasma therapy may constitute a natural noninvasive intervention for epigenetic rejuvenation and health enhancement."17
This 2024 published study had an interesting nuance. The longest-lived animal was in the control (no treatment) group. This indicates that young plasma can delay median age of death but may not confer super longevity. That’s where gene therapy can come to the rescue as I describe later in this editorial.17
Plasma Fraction Cuts Old Rat Age in Half
A study I reported on at scientific conferences in 2020 was formally published in 2024 and demonstrated robust epigenetic age reversal in response to a young plasma fraction rich in exosomes.18
Exosomes are microscopic vesicles secreted by cells. They carry various biological factors, such as RNA and proteins, and are an important part of cell-to-cell communication. Exosomes secreted by stem cells and young cells are being investigated for their potential rejuvenating effects.22
In this study, researchers treated old rats with an exosome-fortified plasma fraction from the blood of young adult pigs.18
What is Epigenetic Age?
Epigenetic age is a biomarker of aging associated with future disease risks and all-cause mortality.21
The epigenetic age is measured by a composite of cellular DNA methylation (DNAm) levels.21
Epigenetic age is considered the most accurate way of assessing whether individuals are aging biologically faster or slower than their chronological age. (You don’t want to "age faster.")
Slower (younger) epigenetic age (what you want) occurs among long-lived individuals.21
Older (worsening) epigenetic age is associated with lower levels of physical functioning and decline in cognitive functioning among long-lived individuals. 21
The treatment’s effect was measured using several tailor-made epigenetic clocks which measure biological age by analyzing changes in the epigenome.
In plasma-treated old rats, the researchers showed reduced epigenetic ages of blood, liver, and heart by huge margins, i.e., to levels "comparable with young rats."
According to the six epigenetic clocks used, the treatment rejuvenated, on average, liver tissue by 74.6%, blood by 64.3%, heart tissue by 46.5%, and the hypo-thalamus (in the brain) by 24.4%.
According to this published study, the young plasma fraction halved the epigenetic (DNAm) age.
To put these findings into clinical perspective, kidney failure is often first detected by reduced glomerular filtration rate and an elevation of serum creatinine. In response to this young plasma fraction, creatinine levels in old rats, which were significantly elevated (as expected) dropped to virtually the same healthy level as young rats (see chart on this page).
If this translates into improved renal function in the tens of millions of Americans with chronic kidney disease, countless numbers of lives will be spared each year.
The same potential exists in those suffering heart or liver failure, i.e., exosome-rich young plasma could restore youthful organ functionality.
What intrigued us about this study was the dosing schedule of the exosome-rich young plasma fraction. It was infused into the rats every other day for eight days and then repeated 95 days later. If this easy-to comply-with administration schedule applies to old humans, it makes compliance practical for most people.
Published Reports Describing Regenerative Potential of Young Plasma
The Stanford Parkinson’s Disease Plasma Study25
A randomized, controlled clinical trial of plasma exchange with albumin replacement for Alzheimer’s disease: Primary results of the AMBAR Study26
The Plasma for Alzheimer Symptom Amelioration (PLASMA) Study27
Preclinical Assessment of Young Blood Plasma for Alzheimer Disease28
Safety, Tolerability, and Feasibility of Young Plasma Infusion in the Plasma for Alzheimer Symptom Amelioration Study: A Randomized Clinical Trial29
Young Blood Plasma Administration to Fight Alzheimer’s Disease?30
Platelet factors attenuate inflamma tion and rescue cognition in ageing31
Plasma-Based Strategies for Therapeutic Modulation of Brain Aging23
Aging and age-related diseases with a focus on therapeutic potentials of young blood/plasma7
Circulating plasma factors involved in rejuvenation9
Plasma from Young Rats Injected into Old Rats Induce Antiaging Effects6
The effect of aging on the bone healing properties of blood plasma33
Young plasma ameliorates aging-related acute brain injury after intra cerebral hemorrhage32
Undulating changes in human plasma proteome profiles across the lifespan34
Young Plasma Rejuvenates Blood Dna Methylation Profile, Extends Mean Lifespan And Improves Physical Appearance In Old Rats17
Young blood plasma reduces Alzheimer’s disease-like brain pathol ogies and ameliorates cognitive impairment in 3×Tg-AD mice35
Numerous Young Plasma Research Projects
Over the past nine years, published studies have demonstrated the rejuvenation effects of young plasma, including the use of young human plasma to regenerate the brains of old rats.8,18,23,24
Projects are being initiated to assess the effects of healthy young plasma on older humans to ascertain the impact on degenerated organs such as hearts and kidneys. Initial emphasis has been on attempting to reverse various forms of neurodegeneration, with varying indicators of potential efficacy.
What physician/scientists have learned in clinical studies is that plasma from unhealthy young people is not the healthy young plasma you want infused into your body.
Plasma from healthy young donors (18-24 years) is thought to provide a balanced spectrum of regenerating factors, including exosomes secreted by the abundant healthy stem cells in their young bodies.
There are several different protocols being studied, including removing senile proteins from old people’s blood and replacing the senescent proteins with quality albumin + immunoglobulins or healthy young plasma.
In the listing on this page there are titles and links to young plasma research published in recent years. You can find direct links to these studies by visiting:
Follistatin Gene Research
Aging is associated with loss of muscle mass (sarcopenia) that contributes to frailty, falls, fractures, and premature mortality.36
Follistatin is a protein-coded gene that plays several roles in the human body. Young people have high follistatin levels that plummet with normal aging.
Older people are traveling to offshore clinics today for follistatin gene injections based on the following discoveries:
Year 2001: Increased follistatin greatly increases muscle mass in mice.37
Year 2009: Follistatin gene delivery in monkeys increases muscle growth and strength.38
Year 2020: Follistatin gene delivery reduces inflammation, metabolic dysfunction and osteoarthritis in mice.39
Year 2022: Follistatin gene delivery shown to extend median lifespan of mice by 32.5%.40
A human project published in 2024 shows plasmid*-based follistatin gene delivery resulted in average:40
- Decrease in intrinsic epigenetic age of 6 years,
- Decrease in extrinsic epigenetic age of 8 years,
- Increase in fat-free mass of 1.69 lbs,
- Decrease in body fat of 0.80%, and
- Zero adverse events.
Over 100 people have had plasmid-based follistatin gene therapy at an offshore clinic in Central America operated by American scientists.
The cost is high (around $25,000) but will likely plummet with mass production of plasmid-based follistatin delivery vehicles. It is estimated that each injection of plasmid-based follistatin lasts three to six months.
Note that in order for the gene that produces follistatin to be transported inside cells, it requires a delivery vehicle, with plasmids being the current vehicle of choice, usually along with other substances (like lipid nano particles) that help the gene enter the nucleus of the cell.41
If the findings about follistatin remain consistent, it will likely become a therapy that people over age 40-50 will utilize several times a year to increase fat-free muscle mass and help reverse their epigenetic age.
Alpha-Klotho Gene Research
Alpha-klotho is a gene that has prominent age-regulating effects.
It is being investigated in a plasmid-delivery vehicle to reverse the effects of age-related cognitive decline and neurodegeneration.42
Specific brain cell populations, including neural progenitors require klotho. Human correlational studies and mouse models show that klotho is protective against multiple neurological and psychological disorders.43-45
Klotho gene over-expression in mice resulted in a 30% increase in median lifespan.46
Klotho-deficient mice have shortened lifespans that are accompanied by an array of disorders normally associated with human aging.47
Alpha-klotho has been shown to decrease during middle age in humans, which is when short-term memory loss is often noticed.48,49
Lower alpha-klotho plasma levels in older adults are associated with frailty and all-cause mortality.50-54
A 60-year-old man was the first to receive plasmid-based klotho delivery and reported marked cognitive improvements in 2024 that lasted for over 90 days (internal research awaiting publication).55
Formal alpha-klotho projects are being planned. If it works in people the way it does in mice, alpha-klotho gene therapy injections (perhaps twice per year) may be combined with follistatin to provide broad-spectrum rejuvenating effects in older humans.
Those who don’t want to wait for formal studies plan to travel to offshore clinics.
Interestingly, healthy young plasma may provide some alpha-klotho, follistatin and other regenerative proteins like GDF11.56
What Are Plasmids?
*Plasmids are circular DNA strands that contain within them the gene that codes for the protein of interest (such as follistatin). By encoding genes like follistatin, klotho or OSK into a plasmid and injecting them into the body, the plasmids lodge inside the cell nucleus where they act as a time-release gene addition approach, producing a protein that is released into circulation. After several months (usually three to six months), the plasmids are mostly degraded, and a new injection is required.
Most Promising Youth Restoration Project
Transcription factors are proteins that help turn specific genes "on" or "off" by binding to DNA.
The regenerative power of transcription factors lies in their ability to reprogram old cells back into stem cells or younger cells capable of regenerating tissues throughout the body.
Said differently, transcription factors may transform cells in our aging bodies into a limitless youthful state. They do this by turning "on" genes that promote youthful vitality and turning "off" the expression of genes involved in degenerative aging.
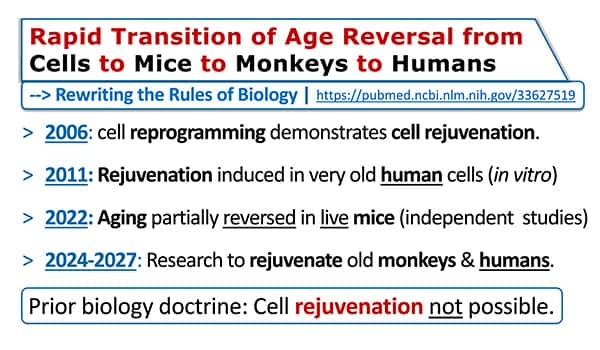
Cyclic Induction of OSK Expression in 2-Year-Old Wild Type Mice19
In March 2022, Salk Institute researchers showed for the first time that partial cell reprogramming using Yamanaka transcription factors reversed systemic aging in old mice.
Salk scientists showed that mice receiving these transcription factors "in many ways resembled younger animals," including having more youthful epigenetic age of skin and kidneys.57
Transcription factor-treated mice had lower inflammation, reduced senescent cell secretions, and looked younger than untreated control mice.
This Salk Institute study found no toxicity but did not assess the lifespans of transcription factor-treated mice.
Move forward to 2024 and a published study showing that transcription factor-treated old mice reversed epigenetic age measures and extended remaining lifespan over 100%!19
The human equivalent age of these old mice was about 77 years, and the transcription factor-treated animals had:
- Improved health relative to control mice,
- Profound age-reversal in heart and liver tissues assessed by DNA methylation clocks,
- Improved Frailty Index scores (another measure of aging), and
- Transcription factor treated mice had remaining lifespans of 18.5 weeks versus 8.86 weeks in the control group.
While these findings further corroborate the Salk Institute transcription factor results published in 2022, there are even more reasons to be optimistic than I’ve revealed so far.
Minimal Transcription Factor Induction = Robust Results
The acronym for the four Yamanaka transcription factors discovered in 2006 is OSKM (Oct3/4, Sox2, Klf4, c-Myc).
A Nobel Prize was awarded to the scientists who showed that delivering OSKM to old cells in a petri dish turned them back into young cells, with greater age reversal occurring in response to longer OSKM induction.58
The initial concern with continuously over-expressing OSKM in live mice was possible side effects. So, the Salk Institute scientists only expressed OSKM intermittently in the 2022 published study.
In the 2024 published study showing age reversal, improved health, and longer remaining lifespans, only OSK transcription factors were used on an intermittent (one week on, one week off) basis. Using only three Yamanaka factors (OSK) is considered safer and likely more effective than OSKM.19
But here is where it gets exciting. Due to a series of typical challenges when studying new technology, only a small fraction of the intended OSK dose was delivered into the cells of the live mice.
Despite this smaller than intended OSK delivery, the results of this study are unprecedented and demonstrate the urgent need to replicate these results using varying doses of OSK in mice while at the same time studying the effects of OSK in old primates.
The unanswered question is whether continuous, or near continuous induction of OSK expression in old monkeys and humans might restore their bodies back to a long-lasting, youthful state.
Effect of OSKM Gene Therapy: Injections Directly into Old Rat Brains
In July 2024, another study we helped fund showed that injecting OSKM transcription factors directly into the hippocampus of old rat brains improved learning and memory skills.59
The charts on this page show the degree of cognitive improvements in old rats using this primitive method of embedding OSKM into the hippocampal region of the brain, but still achieving significant results.59
This method of delivering OSKM improved memory and learning capability. It showed indices of epigenetic age reversal. But it did not restore cognitive capacity back to the young control group.
That’s where enhanced OSKM and OSK delivery methods (such as lipid nano- particles) might yield more robust results by delivering higher precision doses of OSK or OSKM into brain cells.18
We provided funding for this study to prove a concept, i.e., even crudely delivered viral vector OSKM into a small portion of the brain (hippocampus) yielded meaningful results.
Our focus in recent months has been designing systemic delivery studies using large amounts of OSK in old primates with the objective of enabling whole-body rejuvenation.
Are You More Motivated to Stay Alive?
There is published scientific evidence that biological aging is reversible and meaningful extensions of remaining life-spans possible.60
A theoretical basis exists to assert that various cell reprogramming techniques (such as OSK induction) might enable our cells to regain youthful functionality for a sustained period.61,62
This is of little value to those who perish from preventable disorders.
Be it a failure to control blood pressure, ingestion of processed, sugary and over-cooked foods, lack of physical activity, and/or not optimizing blood test markers, most people aren’t doing enough to stay alive.
With the prospect of age-reversal transforming into biological reality, there is more motivation to avoid premature morbidity and mortality than ever before.
There has never been a greater incentive to follow healthy lifestyle patterns to be alive when validated rejuvenation therapies become available.
Your Purchases Fund this Research
We are not the only group studying the restorative potential of young plasma, OSK, and other gene- addition approaches (like follistatin and klotho).
This is good news, as we don’t care who discovers optimal rejuvenation methods, but we want someone to make the breakthroughs fast, as degenerative aging is by far the leading cause of disease and death in modern cultures.
Our research group is spending millions of dollars utilizing differing OSK delivery doses/methods to evaluate safety and assess what rejuvenating effects are occurring in old mice and monkeys.
If these projects find safe ways to restore youthful health, we are targeting OSK human research to initiate in 2026 (or sooner).
Every time you purchase a Life Extension® supplement or blood test, you help fund multiple OSK induction, young plasma, and other research projects aimed at reversing our biological age clocks.
Stay Current with Research Findings
For those who want to stay current with advances occurring on the research front, enroll for free as a member of the Age Reversal Network at:
Newsletter updates are sent several times a year about these emerging sciences and super-longevity clinical projects.
There are thousands of dietary supplement brands on the market. I don’t know of any that dedicates its proceeds to this type of aggressive age-reversal research plus has the expertise to design and evaluate multiple simultaneous scientific studies.
Thank you for supporting these research endeavors with your purchases from Life Extension.
For longer life,
William Faloon, Co-Founder Life Extension Group Age Reversal Group
Meeting the Experts in Person
Each year, scientists, physicians, and interested lay people gather to learn about what can be done to delay and reverse biological aging.
Clinical findings and laboratory research discoveries are presented onstage followed by robust interactions with the entire group.
This year’s 10th annual RAADfest will be in Las Vegas starting Thursday evening, July 10th through Sunday, July 13th, 2025. Special clinic services will be available on the hotel premises throughout the event.
RAADfest is a non-profit event that seeks to unite super-longevity enthusiasts to educate and motivate a revolution against aging and death.
Registration fees, which include two free meals, are a fraction of what commercial conferences charge.
The group meals are designed to keep the entire group together, sharing information and interacting in positive ways to achieve the ultimate super-longevity objectives.
To review the venue registration fees, lig on to www.raadfest.com
References
- Available at: https://www.who.int/news-room/fact-sheets/detail/ageing-and-health. Accessed October 22, 2024.
- Anton SD, Cruz-Almeida Y, Singh A, et al. Innovations in Geroscience to enhance mobility in older adults. Exp Gerontol. 2020 Dec;142:111123.
- Fried LP, Wong JE, Dzau V. A global roadmap to seize the opportunities of healthy longevity. Nat Aging. 2022 Dec;2(12):1080-3.
- Available at: https://www.history.com/news/printing-press-renaissance. Accessed October 23, 2024.
- Di Bella MA. Overview and Update on Extracellular Vesicles: Considerations on Exosomes and Their Application in Modern Medicine. Biology (Basel). 2022 May 24;11(6).
- Tripathi SS, Kumar R, Arya JK, et al. Plasma from Young Rats Injected into Old Rats Induce Antiaging Effects. Rejuvenation Res. 2021 Jun;24(3):206-12.
- Hosseini L, Shahabi P, Fakhari A, et al. Aging and age-related diseases with a focus on therapeutic potentials of young blood/plasma. Naunyn Schmiedebergs Arch Pharmacol. 2024 Jan;397(1):1-13.
- Villeda SA, Plambeck KE, Middeldorp J, et al. Young blood reverses age-related impairments in cognitive function and synaptic plasticity in mice. Nat Med. 2014 Jun;20(6):659-63.
- Kang JS, Yang YR. Circulating plasma factors involved in rejuvenation. Aging (Albany NY). 2020 Nov 16;12(22):23394-408.
- Pandika M. Looking to Young Blood to Treat the Diseases of Aging. ACS Cent Sci. 2019 Sep 25;5(9):1481-4.
- Zhao Y, Qian R, Zhang J, et al. Young blood plasma reduces Alzheimer’s disease-like brain pathologies and ameliorates cognitive impairment in 3xTg-AD mice. Alzheimers Res Ther. 2020 Jun 8;12(1):70.
- Chen X, Luo Y, Zhu Q, et al. Small extracellular vesicles from young plasma reverse age-related functional declines by improving mitochondrial energy metabolism. Nat Aging. 2024 Jun;4(6):814-38.
- Erdogan K, Ceylani T, Teker HT, et al. Young plasma transfer recovers decreased sperm counts and restores epigenetics in aged testis. Exp Gerontol. 2023 Feb;172:112042.
- Liu A, Guo E, Yang J, et al. Young plasma reverses age-dependent alterations in hepatic function through the restoration of autophagy. Aging Cell. 2018 Feb;17(1).
- Kim D, Kiprov DD, Luellen C, et al. Old plasma dilution reduces human biological age: a clinical study. Geroscience. 2022 Dec;44(6):2701-20.
- Liu MN, Lan Q, Wu H, et al. Rejuvenation of young blood on aging organs: Effects, circulating factors, and mechanisms. Heliyon. 2024 Jun 30;10(12):e32652.
- Chiavellini P, Lehmann M, Gallardo MD, et al. Young Plasma Rejuvenates Blood DNA Methylation Profile, Extends Mean Lifespan, and Improves Physical Appearance in Old Rats. J Gerontol A Biol Sci Med Sci. 2024 May 1;79(5).
- Horvath S, Singh K, Raj K, et al. Reversal of biological age in multiple rat organs by young porcine plasma fraction. Geroscience. 2024 Feb;46(1):367-94.
- Macip CC, Hasan R, Hoznek V, et al. Gene Therapy-Mediated Partial Reprogramming Extends Lifespan and Reverses Age-Related Changes in Aged Mice. Cell Reprogram. 2024 Feb;26(1):24-32.
- Reed RG, Carroll JE, Marsland AL, et al. DNA methylation-based measures of biological aging and cognitive decline over 16-years: preliminary longitudinal findings in midlife. Aging (Albany NY). 2022 Nov 11;14(23):9423-44.
- Jain P, Binder AM, Chen B, et al. Analysis of Epigenetic Age Acceleration and Healthy Longevity Among Older US Women. JAMA Netw Open. 2022 Jul 1;5(7):e2223285.
- Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020 Feb 7;367(6478).
- Kheifets V, Braithwaite SP. Plasma-Based Strategies for Therapeutic Modulation of Brain Aging. Neurotherapeutics. 2019 Jul;16(3):675-84.
- Castellano JM, Mosher KI, Abbey RJ, et al. Human umbilical cord plasma proteins revitalize hippocampal function in aged mice. Nature. 2017 Apr 27;544(7651):488-92.
- The Stanford Parkinson’s Disease Plasma (SPDP) Study: Intravenously-Administered Plasma From Young Donors for Treatment of Moderate Parkinson’s Disease. 2016. https://clinicaltrials.gov/study/NCT02968433.
- Boada M, Lopez OL, Olazaran J, et al. A randomized, controlled clinical trial of plasma exchange with albumin replacement for Alzheimer’s disease: Primary results of the AMBAR Study. Alzheimers Dement. 2020 Oct;16(10):1412-25.
- The PLasma for Alzheimer SymptoM Amelioration (PLASMA) Study: Intravenously-Administered Plasma From Young Donors for Treatment of Mild-To-Moderate Alzheimer’s Disease. 2014. https://clinicaltrials.gov/study/NCT02256306.
- Middeldorp J, Lehallier B, Villeda SA, et al. Preclinical Assessment of Young Blood Plasma for Alzheimer Disease. JAMA Neurol. 2016 Nov 1;73(11):1325-33.
- Sha SJ, Deutsch GK, Tian L, et al. Safety, Tolerability, and Feasibility of Young Plasma Infusion in the Plasma for Alzheimer Symptom Amelioration Study: A Randomized Clinical Trial. JAMA Neurol. 2019 Jan 1;76(1):35-40.
- Aicardi G. Young Blood Plasma Administration to Fight Alzheimer’s Disease? Rejuvenation Res. 2018 Apr;21(2):178-81.
- Schroer AB, Ventura PB, Sucharov J, et al. Platelet factors attenuate inflammation and rescue cognition in ageing. Nature. 2023 Aug;620(7976):1071-9.
- Yuan JJ, Zhang Q, Gong CX, et al. Young plasma ameliorates aging-related acute brain injury after intracerebral hemorrhage. Biosci Rep. 2019 May 31;39(5).
- Al-Hamed FS, Rodan R, Ramirez-Garcialuna JL, et al. The effect of aging on the bone healing properties of blood plasma. Injury. 2021 Jul;52(7):1697-708.
- Lehallier B, Gate D, Schaum N, et al. Undulating changes in human plasma proteome profiles across the lifespan. Nat Med. 2019 Dec;25(12):1843-50.
- Zhao Y, Qian R, Zhang J, et al. Young blood plasma reduces Alzheimer’s disease-like brain pathologies and ameliorates cognitive impairment in 3×Tg-AD mice. Alzheimers Res Ther. 2020 Jun 8;12(1):70.
- Hertz K, Santy-Tomlinson J. Fragility Fracture Nursing: Holistic Care and Management of the Orthogeriatric Patient [Internet]. In: Hertz K, Santy-Tomlinson J, editors. Fragility Fracture Nursing: Holistic Care and Management of the Orthogeriatric Patient. Cham (CH)2018.
- Lee SJ, McPherron AC. Regulation of myostatin activity and muscle growth. Proc Natl Acad Sci U S A. 2001 Jul 31;98(16):9306-11.
- Kota J, Handy CR, Haidet AM, et al. Follistatin gene delivery enhances muscle growth and strength in nonhuman primates. Sci Transl Med. 2009 Nov 11;1(6):6ra15.
- Tang R, Harasymowicz NS, Wu CL, et al. Gene therapy for follistatin mitigates systemic metabolic inflammation and post-traumatic arthritis in high-fat diet-induced obesity. Sci Adv. 2020 May;6(19):eaaz7492.
- Jaijyan DK, Selariu A, Cruz-Cosme R, et al. New intranasal and injectable gene therapy for healthy life extension. Proc Natl Acad Sci U S A. 2022 May 17;119(20):e2121499119.
- Walter Patterson RJR. Vol 20242024.
- Zhao Y, Zeng CY, Li XH, et al. Klotho overexpression improves amyloid-beta clearance and cognition in the APP/PS1 mouse model of Alzheimer’s disease. Aging Cell. 2020 Sep 21;19(10):e13239.
- Prather AA, Epel ES, Arenander J, et al. Longevity factor klotho and chronic psychological stress. Transl Psychiatry. 2015 Jun 16;5(6):e585.
- Pavlatou MG, Remaley AT, Gold PW. Klotho: a humeral mediator in CSF and plasma that influences longevity and susceptibility to multiple complex disorders, including depression. Transl Psychiatry. 2016 Aug 30;6(8):e876.
- Dubnov S, Yayon N, Yakov O, et al. Knockout of the longevity gene Klotho perturbs aging- and Alzheimer’s disease-linked brain microRNAs and tRNA fragments. bioRxiv. 2023 Sep 12.
- Cheikhi A, Barchowsky A, Sahu A, et al. Klotho: An Elephant in Aging Research. J Gerontol A Biol Sci Med Sci. 2019 Jun 18;74(7):1031-42.
- Vo HT, Laszczyk AM, King GD. Klotho, the Key to Healthy Brain Aging? Brain Plast. 2018 Aug 10;3(2):183-94.
- Chuang MH, Wang HW, Huang YT, et al. Association between soluble alpha-klotho and mortality risk in middle-aged and older adults. Front Endocrinol (Lausanne). 2023;14:1246590.
- Linghui D, Simin Y, Zilong Z, et al. The relationship between serum klotho and cognitive performance in a nationally representative sample of US adults. Front Aging Neurosci. 2023;15:1053390.
- Guan Z, Ma L, Wu C. Association between Serum Klotho and Physical Frailty in Middle-Aged and Older Adults: Finding From the National Health and Nutrition Examination Survey. J Am Med Dir Assoc. 2023 Aug;24(8):1173-8 e2.
- Shardell M, Semba RD, Rosano C, et al. Plasma Klotho and Cognitive Decline in Older Adults: Findings From the InCHIANTI Study. J Gerontol A Biol Sci Med Sci. 2016 May;71(5):677-82.
- Gao X, Li Y, Sun Z, et al. Could alpha-Klotho Unlock the Key Between Depression and Dementia in the Elderly: from Animal to Human Studies. Mol Neurobiol. 2021 Jun;58(6):2874-85.
- Paroni G, Panza F, De Cosmo S, et al. Klotho at the Edge of Alzheimer’s Disease and Senile Depression. Mol Neurobiol. 2019 Mar;56(3):1908-20.
- Semba RD, Cappola AR, Sun K, et al. Plasma klotho and mortality risk in older community-dwelling adults. J Gerontol A Biol Sci Med Sci. 2011 Jul;66(7):794-800.
- file IrDo. Internal Research: Data on file. 2024.
- Kalampouka I, van Bekhoven A, Elliott BT. Differing Effects of Younger and Older Human Plasma on C2C12 Myocytes in Vitro. Front Physiol. 2018;9:152.
- Ocampo A, Reddy P, Martinez-Redondo P, et al. In Vivo Amelioration of Age-Associated Hallmarks by Partial Reprogramming. Cell. 2016 Dec 15;167(7):1719-33 e12.
- Available at: https://www.ucsf.edu/news/2012/10/104393/shinya-yamanaka-wins-2012-nobel-prize-medicine. Accessed October 22, 2024.
- Available at: https://link.springer.com/article/10.1007/s11357-024-01269-y. Accessed October 22, 2024.
- Life Extension. The Prospect of Human Age Reversal. LifeExtension2022.
- Yang JH, Petty CA, Dixon-McDougall T, et al. Chemically induced reprogramming to reverse cellular aging. Aging (Albany NY) . 2023 Jul 12;15(13):5966-89.
- Lu Y, Brommer B, Tian X, et al. Reprogramming to recover youthful epigenetic information and restore vision. Nature. 2020 Dec;588(7836):124-9.