Life Extension Magazine.
In the June 2016 issue of Life Extension Magazine® we reported on a prostate cancer treatment being used on an outpatient basis that was showing good results with minimal side effects in most patients.1
The name of this approach is focal therapy. It is available in several forms including cryo, laser, ultrasound, and others.
The goal with focal therapy is to enable optimal oncological outcomes while reducing side effects, and to improve recovery times compared with conventional treatment options.
Focal Therapy and Immune Responses
The type of focal therapy discussed in this article uses image-guided techniques to damage part of the tumor via a freeze and thaw process while leaving the rest of the organ or tissue intact.
The objective as you will read is to generate a systemic immune response while leaving most of the affected organ or tissue intact.
By damaging the cancer cells, it causes them to release their antigens for recognition by the immune system.
Most focal therapies seek to completely ablate (destroy) the tumor. The majority of reports on focal therapy do not discuss the value of exposing the patient’s very own tumor cell antigens so that immune responses are activated.
As noted in a 2019 publication by Abdo, et al.:
“Cryosurgery releases hundreds of unique antigens from a population of tumor cells that make up the invading cancer.”2
Checkpoint Inhibitor Drugs
Antigens are present in cancer cells and can trigger a targeted immune response against those very same cancer cells.
The problem is that cancer cells erect “checkpoints” that act as barriers to impede immune attack.
That’s why a class of drugs called “checkpoint inhibitors” like pembrolizumab (Keytruda®) and ipilimumab (Yervoy®) are increasingly used against a variety of malignancies.
But when checkpoint inhibitors are delivered via the intravenous route there are often systemic side effects which may be serious.3
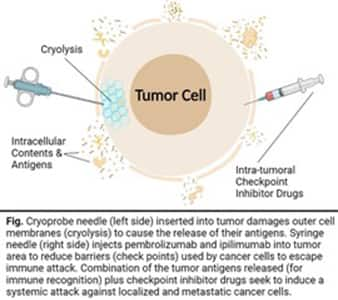
This prompted some prostate cancer treatment centers to trigger damage in part of the tumor with localized cryo-focal therapy and inject checkpoint inhibitor drugs into the tumor area.
The combination of damaging (freezing-thawing) and uniquely injecting checkpoint inhibitors is part of the spectrum of “cryo-immune focal therapy.”4-6
Cryo-immune focal therapy damages the tumor instead of destroying it.
This causes antigens from the tumor to be released into the circulation where they provoke a systemic immune response against both local and distant (metastatic) tumor cells that migrated elsewhere in the body.
An Emerging Science
Life Extension® has been referring prostate cancer patients for cryo-immune focal therapies since at least 2015.
We’ve received much positive feedback about the results from earlier versions of this treatment. We have now learned that this therapy has become more comprehensive, adding additional drugs to augment the overall immune response.
An upcoming clinical trial is open and recruiting new patients with metastatic disease. The trial will assess the effects of cryo-immune focal therapy plus a systemic immune drug called granulocyte-macrophage colony-stimulating factor (GM-CSF).
The trial will also use a low dose of cyclophosphamide to lessen an obstacle to a more robust immune response against tumor cells.
Information about registration and the trial can be found at: https://ramparthealth.com/ or https://www.clinicaltrials.gov/ct2/show/NCT04713371
This article describes recent findings and a clinical trial that is testing this enhanced cryo-immune therapy against common malignancies.
More than 600,000 Americans will perish from cancer this year.7
Modern treatments are curing more cancer patients than ever before.
Unfortunately, the immediate and long-term side effects of conventional treatments like surgeries, radiation, chemo, and other toxic treatments are too often ignored or trivialized.
As just one example, breast cancer patients treated with aggressive chemotherapy (and surgery/radiation) incur serious adverse effects including immune suppression and increased risks of leukemia and coronary artery disease over time.8,9
Although it is experimental, a cancer research group is treating metastatic cancer in an innovative way that could reduce or eliminate most side effects by damaging part of the tumor with cryotherapy and injecting the area with checkpoint inhibitor drugs.
Granulocyte-macrophage colony-stimulating factor or GM-CSF will be used to boost blood cell counts to enhance immune response.
Low-dose cyclophosphamide is also given to suppress a regulatory cell population that curtails the anti-tumor immune response. Cyclophosphamide may have an anti-angiogenesis benefit as well.
The checkpoint inhibitor ipilimumab (Yervoy®) also functions in this manner and potentially adds to the effectiveness of cyclophosphamide.
Basis for Focal Cryo + Checkpoint Inhibitor Therapy
- Cancer cell necrosis inflicted by cryo results in the release of antigens from the cancer cell that act as immune triggers. It is believed that this can induce a tumor-specific immune response.
- Immunotherapy uses the patient's immune system for treatment of the tumor. Not all patients respond to immunotherapy with checkpoint inhibitors or other drugs.
- The combination of cryo + immunotherapy may enhance the effect of both therapies for improved tumor destruction—both locally and systemically.
Although investigational at this point, the administration of checkpoint inhibitor drugs directly into a part of the tumor should generate a broader, both local and systemic, immune response against residual primary malignant cells as well as regional malignant and distant metastasized cells and with a lower risk of side effects compared to the systemic intravenous administration of checkpoint inhibitors.
The use of focal therapy alone against prostate cancer continues to be favorable. The goal now is to study the effects of cryo-immune focal therapy + systemic immune-boosting drugs against not only prostate cancer, but other metastatic malignancies.
Cryoablation + Immune Treatments Against Common Cancers
In December 2019 a literature review by Aarts, et al., found favorable effects for cryoablation therapy combined with systemically administered immune checkpoint inhibitors and other conventional treatments in human studies.5
In most of these trials, the checkpoint inhibitors were given intravenously and not via direct injection into the tumor. The doses used in those studies cited by Aarts were significantly higher.
This 2019 report described human studies demonstrating varying degrees of efficacy using cryoablation + whole-body immune therapies against a variety of cancer types including:
- Breast cancer (2 studies)
- Kidney cancer (2 studies)
- Lung cancer (1 study)
- Melanoma (2 studies)
- Prostate cancer (4 studies)
Researchers are intrigued about first priming the immune system with immune-stimulating drugs and damaging part of the tumor with focal therapy to expose tumor antigens, and injecting checkpoint inhibitor drugs into the area to facilitate an immune response against residual local and metastatic cancer cells.
This is the primary approach that will be used in a multi-modal clinical trial, described next, that plans to treat a variety of solid metastatic malignancies including breast and prostate cancer.
Clinical Trial on Cryo-Immune Focal + Systemic Immune Therapy
A clinical trial is currently recruiting metastatic cancer patients who have not responded to conventional therapy, or who refuse conventional therapy.
The clinical trial will evaluate the effectiveness of cryo-immunotherapy. 10 The current medical center is in Rochester, Michigan, but more sites should soon be open.
The trial design is as follows:
1. Identify an accessible location of tumor mass(es) inside or on the surface of the patient’s body.
2. Administer low-dose cyclophosphamide five days before the focal procedure to suppress a sub-population of lymphocytes (called Tregs) to further enhance T-cell destruction of cancer cells.
3. Use image-guided technology to precisely cryo-damage a portion of the malignant lesion.
4. Precisely inject into the tumor(s) two immune-therapeutic drugs:
a. Keytruda® (pembrolizumab), which is a monoclonal antibody drug that inhibits PD-1 (programmed death -1 receptor) and impedes a cancer cell’s ability to escape the body’s normal immune response. PLUS,
b. Yervoy® (ipilimumab), an anti-CTLA-4 (cytotoxic T-lymphocyte-associated antigen 4) monoclonal antibody drug that works to enhance the immune response by targeting a subset of T-cells called Tregs that inhibit the immune response.
5. The treatment also involves an immune drug called GM-CSF (granulocyte-macrophage colony stimulating factor) that has been used for decades to mobilize the bone marrow release of granulocytes and macrophages to protect against bone marrow suppression caused by toxic chemotherapy drugs. The objective in this cryo-immune clinical trial is to use GM-CSF to potentiate the effects of the immune checkpoint inhibitors Keytruda® and Yervoy®. The GM-CSF is given under the skin (subcutaneous) and is injected for about 30 days after cryo-immune focal therapy is administered.
6. Up to two cancer areas for each patient will be selected and treated during each treatment. The clinical trial will involve up to three treatments using the above approach for each patient.
By using a sequence of cryo-damaging the tumor (to release its antigens), followed by four different immune therapies (Keytruda® + Yervoy® + GM-CSF+ cyclo-phosphamide), a cryo-immune synergy is created, and some researchers believe that a clinically significant systemic anti-cancer immune response may be elicited.
Moreover, the intra-tumoral injection of drugs at a lower dose is likely to cause fewer side effects than higher-dose intravenous systemic therapy.
How to Determine if you Qualify for this Study
There is no cost to those eligible for the study other than possible travel expenses and lodging near the clinical trial study site medical facility. The study center may bill your insurance if it covers the cost of some laboratory tests and imaging, but patients are not expected to pay anything out of pocket.
If you or someone you know has a diagnosed metastatic malignancy involving a solid tumor and all available treatments have failed or the patient chooses against conventional therapy, you can register at the following website to ascertain your eligibility:
Additional information about the trial can be found at https://www.clinicaltrials.gov/ct2/show/NCT04713371
If you are eligible, you will be contacted for full medical records and a trial coordinator(s) will guide you through the trial process.
Summary
I’ve interacted for over 20 years with some of the oncology experts involved with designing this clinical trial.
Neither I, nor Life Extension® have any financial interest in this cryo-immune focal therapy.
I am grateful the parties have worked together to enable this clinical trial to launch this year in the United States.
When confronted with difficult medical issues (not cancer), I’ve been fortunate enough to have alternative medicine doctors recommend simpler solutions that have spared me from surgery and other toxic treatments.
If this combined cryo-immune focal therapy proves efficacious it may revolutionize cancer treatment against many common malignancies.
We look forward to learning the results of this clinical trial that has recently been initiated.
This article may be updated as we learn more about this treatment and the clinical trial. Please go to www.LifeExtension.com/cryo to view any updates to this print article.
For longer life,
William Faloon
Side Effects of Focal Therapies
Before the advent of focal therapies, men diagnosed with prostate cancer were confronted with harsh choices.
The so-called gold standard of treatment entails complete removal of the prostate gland and suspicious lymph nodes (radical prostatectomy). The side effects of this mutilating surgery are often horrendous.
Many men instead choose external beam radiation which has its own litany of potential side effects.
Improvements made over the years include “nerve sparing” radical prostatectomy and insertion of radioactive seeds in the malignant portions of the prostate gland and image-guided radiation therapy.
Currently available focal therapy options include:11,12
- Focal Cryotherapy
- Focal HIFU (high intensity focused ultrasound)
- Focal Laser Ablation (FLA)
- Irreversible Electroporation (IRE)
- Vascular-Targeted Photodynamic Therapy (VTP)
- Focal Cryo-Immune Therapy (investigational)
- Radiofrequency Ablation (RFA)
- HDR-brachytherapy (high-dose rate brachytherapy)
- Focal Brachytherapy (FB) using seeds
- Stereotactic Body Radiation Therapy (SBRT)
The primary advantage demonstrated to date with focal therapy is a significantly improved adverse event profile versus whole-gland treatment.
However, side effects do occur, usually peri-operatively and temporarily that include:13
- Urinary tract infection (in up to 20% of patients) and acute urinary retention (in up to 17% of patients).
- Painful urination and blood in the urine.
- Urinary incontinence following focal therapy in approximately 5% of patients.
The majority of patients recover in a few weeks.
Erectile dysfunction can occur after focal therapy, but usually resolves over time.
Side-effect risks are greatly reduced when treatment is performed by an experienced clinician but are increased if the malignancy is located near the nerve bundle or urethra running through the prostate gland.
References
- Available at: https://lifeextension.com/magazine/2016/6/major-advance-in-screening-and-treating-prostate-cancer. Accessed June 23, 2022.
- Abdo J, Cornell DL, Mittal SK, et al. Immunotherapy Plus Cryotherapy: Potential Augmented Abscopal Effect for Advanced Cancers. Front Oncol. 2018;8:85.
- Available at: https://www.cancer.org/treatment/treatments-and-side-effects/treatment-types/immunotherapy/immune-checkpoint-inhibitors.html. Accessed June 28, 2022.
- Onik G, Bostwick D, Vaughan DJ, et al. Abstract 6540: Regression of metastatic cancer and abscopal effects following in situ vaccination by cryosurgical tumor cell lysis and intratumoral immunotherapy: A case series. Cancer Research. 2020;80(16_Supplement):6540-.
- Aarts BM, Klompenhouwer EG, Rice SL, et al. Cryoablation and immunotherapy: an overview of evidence on its synergy. Insights Imaging. 2019 May 20;10(1):53.
- Yakkala C, Chiang CL, Kandalaft L, et al. Cryoablation and Immunotherapy: An Enthralling Synergy to Confront the Tumors. Front Immunol. 2019;10:2283.
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2022. CA Cancer J Clin. 2022 Jan;72(1):7-33.
- Yang H, Bhoo-Pathy N, Brand JS, et al. Risk of heart disease following treatment for breast cancer – results from a population-based cohort study. eLife. 2022 2022/03/16;11:e71562.
- Available at: https://www.breastcancer.org/research-news/leukemia-risk-higher-than-thought. Accessed June 28, 2022.
- Available at: https://clinicaltrials.gov/ct2/show/NCT04713371. Accessed June 24, 2022.
- Hayes M, Lin-Brande M, Isharwal S. Primary Focal Therapy for Localized Prostate Cancer: A Review of the Literature. Oncology (Williston Park). 2021 May 13;35(5):261-8.
- Atluri S, Mouzannar A, Venkatramani V, et al. Focal therapy for localized prostate cancer - Current status. Indian J Urol. 2022 Jan-Mar;38(1):7-14.
- Rakauskas A, Marra G, Heidegger I, et al. Focal Therapy for Prostate Cancer: Complications and Their Treatment. Front Surg. 2021 2021-July-12;8:696242.