Life Extension Magazine®

Twenty years ago I was leaving a medical conference when one of our ardent supporters rushed up and handed me a huge textbook.1 She begged I take it home to read.
She was adamant that Life Extension® make a greater effort to enlighten its readers about the underlying cause of most disability and death in persons over age 50.1
The threat described in the textbook occurs when an abnormal blood clot forms inside an artery or vein. The medical term is thrombosis.
Two disorders involving arterial thrombosis are:
- Heart Attack
- Ischemic Stroke
Two disorders involving venous thrombosis are:
- Deep Vein Thrombosis (DVT)
- Pulmonary Embolism
Those stricken with cancer are particularly susceptible to venous thrombosis.2 Chemotherapy patients are up to 6-times more vulnerable.3
One reason we’ve recommended low-dose aspirin since 1983 is its ability to inhibit platelet aggregation, a major factor involved in arterial thrombosis, leading to a heart attack or ischemic stroke.4
Recent studies show that arterial thrombosis occurs more frequently than previously thought.5,6 Minor thrombotic events seldom display outward symptoms and, over time, predispose us to a host of degenerative illnesses including mind-robbing mini-strokes.6,7
Many of the nutrients you take have diverse antiplatelet effects. This is important in protecting against arterial thrombosis, but far less so for venous thrombosis.
The Surgeon General published a report showing that deep vein thrombosis (and subsequent pulmonary embolism) may cause 100,000-180,000 deaths each year in the US.8 To put this in perspective, pancreatic cancer is estimated to kill more than 41,000 Americans in 2016.9 Pancreatic cancer has a decidedly deadly reputation, yet the public is largely unaware that deep vein thrombosis (and subsequent pulmonary embolism) poses a greater overall health risk.

given to me by an enthusiastic
Life Extension® supporter who
wanted the world to know that
thrombosis poses the greatest
threat to healthy longevity.
The Surgeon General was highly critical of mainstream medicine for not recognizing patients at risk for deep vein thrombosis and taking appropriate preventive measures.
Longtime readers of this magazine should be comforted with the knowledge that they are already taking steps to reduce their arterial thrombotic risk.
This issue describes startling new data about deep vein thrombosis, and what can be done to help prevent it.
People often take for granted that blood effortlessly flows through their arteries and veins like water moves through a hose.
The reality is that blood flow is highly dependent upon a complex interplay of different mechanisms, including coagulation factors that regulate the tendency of blood to form a clot.
For example, platelets play an important role in “plugging” holes in our circulatory system, helping to reduce bleeding in conjunction with other clotting factors.
Conversely, when platelets abnormally aggregate (clot) inside a blood vessel in response to arterial plaque and/or endothelial damage, the result is stoppage of blood flow to the affected part of the anatomy. An abnormal blood clot in a cerebral artery can lead to ischemic stroke, whereas a thrombus (clot) that forms in a coronary artery can result in a heart attack.
As humans age, mini-thrombotic events can occur to small arteries in the brain. This includes transient ischemic attacks (TIAs) in the brain that, over time, can cause damage to our cognitive abilities.
Preventing the development of these minor and major thrombotic episodes is critical for healthy longevity. The good news is that we know a lot about what causes pathologic blood clotting inside arteries and veins, and how to prevent it.
Role of Inflammation in Both Arterial and Venous Thrombosis
Inflammation sets in motion a sequence of events that can lead to arterial and venous thrombosis. Normal aging results in increasing levels of vascular inflammation, often without outward symptoms.
Readily obtainable blood markers that can reveal systemic inflammation are homocysteine,10-12 C-reactive protein,13,14and fibrinogen.15-17 Heightened levels of these inflammatory biomarkers are correlated with arterial thrombosis and subsequent risk of cardiovascular disease.18,19
Fish oil,20 vitamin D,21,22 curcumin,23,24 and other plant extracts inhibit many underlying inflammatory factors that increase C-reactive protein. The biologically active form of folic acid (5-MTHF)25,26 along with vitamins B1227 and B627 can slash elevated homocysteine through two distinct detoxification pathways.28
In contrast to the association between risk of arterial thrombosis and C-reactive protein elevation, C-reactive protein is not very useful in predicting future venous thrombosis or pulmonary embolism. Recent evidence does point to the role of proinflammatory cytokines like IL-8 and tumor necrosis factor-alpha in venous thrombosis risk.29
Nattokinase is an enzyme extracted from a Japanese food (natto) prepared from fermented soybeans.30 Venous inflammation tends to raise fibrinogen levels, and fibrinogen is an important factor involved in inflammation as well as venous thrombosis formation.31 Nattokinase has been shown to decrease levels of fibrinogen along with clotting factors VII and VIII, which are involved in the formation of venous thrombosis.32
Conventional Arterial Thrombotic Risk Factors
LDL cholesterol is a common factor involved in development of atherosclerotic plaque in arteries.33
Elevated LDL contributes to deposits of plaque that cause arterial pathways to gradually narrow until normal blood flow is disrupted. When this happens, there is a greater propensity for arterial clot formation (arterial thrombosis).33
Hypertension increases the velocity at which blood is thrust through the arterial system. As blood pressure elevates, platelets become more likely to aggregate and create a thrombotic event.34
Conventional cardiovascular and arterial thrombosis risk factors are diabetes, smoking, abdominal obesity, and hyperlipidemia (elevated LDL and triglycerides).35-38 Some of these same factors are also associated with increased risk of deadly venous thrombotic events.
The encouraging news is that proven methods exist to control underlying causes of thrombosis and the vascular diseases that can develop acutely or chronically.
Epidemic of Deep Vein Thrombosis
Deep venous thrombosis and pulmonary embolism are major causes of disability and death.39
Each year, as many as 900,000 Americans may be affected by venous thromboembolism. Of those diagnosed, up to 30% will die within one month, and the first symptom will be sudden death in about 25% of those who have a pulmonary embolism.40
Venous thrombosis is the formation of a blood clot inside a vein that can obstruct flow in the localized affected part of the venous circulatory system. When a venous blood clot dislodges from its primary location and travels to block circulation in another body part, this is referred to as a venous thromboembolism. When a deep vein thrombosis dislodges and travels to the lungs, this worrisome and potentially life-threatening condition is called a pulmonary embolism.
A variety of factors are implicated in the formation of venous thrombosis. Two major, related risks for the development of deep vein thrombosis are:
- Hemostasis (reduction/stagnation of blood flow)
- Hypercoagulability (propensity of blood to clot inside veins due to lifestyle, cancer or genetics)
The good news is that steps can be taken to reduce the risk of deep vein thrombosis, as well as thrombotic risks throughout the circulatory system. This means that strategies to protect against deep vein thrombosis may also confer protection against stroke and heart attack.
Symptoms of Deep Vein Thrombosis
A blood clot in one of the deep veins can include the following symptoms:83
- Mild to severe pain in the affected arm or leg
- Swelling of an arm or leg
- Redness or color change
- Warmth of the skin
A venous blood clot that has traveled to the lung (called a pulmonary embolism) has symptoms that include:83
- Chest pain
- Shortness of breath
- Fast heartbeat
What Causes Blood Vessel Clots in Arteries?

To sustain life, blood must remain in a fluid state so that it can freely circulate, while simultaneously being able to properly clot at the site of a vascular injury.
Any event that activates platelets can cause them to aggregate to form an occlusive thrombus.
As it relates to aspirin, fish oil and certain plant polyphenols, you’ll often read about their “antiplatelet” properties. What this describes is their ability to interfere with platelet activity, adhesion, and aggregation, thereby reducing arterial thrombotic risk.
Antiplatelet therapies, however, can be sabotaged by dysfunction of our endothelium (inner arterial lining). A healthy endothelium produces substances that stabilize platelets and impede their unwanted adhesion.
When the endothelial lining is lost, platelets are exposed to parts of the arterial wall that cause them to aggregate. Protecting against endothelial dysfunction is thus essential to maintain vascular health as we age. This is where pomegranate and other plant polyphenols play a critical role.
Antiplatelet strategies employed today, utilizing certain medications and nutrients, can greatly mitigate these arterial thrombotic factors.
Nutrients that Help Protect Against Arterial Thrombosis
 |
- Green tea41,42
- Fish oil43-46
- Olive polyphenols47-51
- Quercetin52
- Resveratrol53-56
- Grape seed extract57-61
- French maritime pine bark extract62
- Lycopene63,64
- Pomegranate65
- Garlic66-72
- Flax seed oil73,74
- Ginger66,75-80
- Curcumin81,82
How Arterial and Venous Clots Differ

There are distinctions between the processes that cause arterial and venous thrombosis.
Arterial thrombosis largely involves platelet aggregation forming around clogged/jagged points in the arterial system, or in response to irregular heart beat (atrial fibrillation) or an artificial heart valve.
Deep vein thrombosis typically occurs due to hemostasis (reduction in venous blood flow) and hypercoagulability (tendency of the blood in veins to clot due to genetic, cancer or lifestyle factors).
One major cause of reduction in venous blood flow is chronic venous insufficiency. This frequently occurs from obesity, lack of physical activity/sitting with the legs in a dependent position, and previous deep vein thrombosis, which injures or destroys one or more of the valves that are located in the deep veins of the leg.
In order to efficiently return blood to the heart when a person is sitting or standing, veins contain tiny valves that open and close. Properly functioning valves prevent blood from flowing backward while muscles surrounding the veins compress them, helping pump venous blood back to the heart.8
Veins contain valves while arteries do not. When veins are damaged by prior venous clot, or physical inactivity leads to pooling of blood in the deep veins of the legs, venous blood flow decreases, setting the stage for venous thrombosis.
In contrast with the venous system, platelets in the arterial system are adversely activated as they bump into buildups of plaque along the arterial walls and interact with a dysfunctional endothelium. In this scenario, platelets begin to clump together, causing a cascade that can lead to blood flow being cut off to vital tissue (such as a portion of the heart muscle).
In the venous system, normal blood flow can slow, and if left to stagnate too long, the blood within these veins begins to coagulate (clot). The problem of deep vein thrombosis, however, extends beyond mere stagnating pools of blood in the lower legs.
Formation of Deep Venous Thrombosis
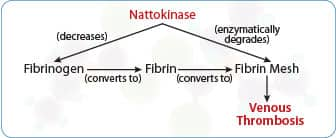 |
Fibrinogen converts to fibrin that creates a fibrin mesh inside a vein. Platelets and red blood cells adhere to fibrin mesh to cause deep vein thrombosis.
Nattokinase reduces fibrinogen and has fibrinolytic effects, which means it can enzymatically break down the fibrin mesh component of blood clots.
Beyond “Antiplatelet” Strategies
Inflammation and some other factors that contribute to arterial thrombosis (such as excess homocysteine) also are linked with an increased risk of deep vein thrombosis.84,85 Several of these dynamics, however, play a larger role in the venous system than in arteries.
What happens with deep vein thrombosis is that fibrinogen, also a proinflammatory regulator, excessively converts to a fibrin mesh that traps red blood cells. In deep vein thrombosis, formation of fibrin is also linked with excess inflammation in veins.86 This helps explain the limited efficacy of antiplatelet drugs (aspirin and Plavix®) in venous thrombosis, i.e., they don’t stop the initiating phase of proinflammatory fibrinogen converting to red blood cell-trapping fibrin.
There are multiple underlying coagulation factors that can initiate a deep venous thrombosis. Some require preventive treatment using anticoagulant drugs (warfarin, Pradaxa®, Eliquis®, Xarelto®). There is a common venous thrombotic mechanism, however, that can be impeded with a low-cost nutrient.
Nattokinase has been shown to decrease fibrinogen levels and help dissolve fibrin clots that obstruct blood flow.32,87
The flow chart on this page shows a simplified version of the complex process of coagulation involved in thrombosis formation. The fibrinogen/fibrin-dissolving effects of nattokinase can help stop this coagulation cascade at several checkpoints.
Cancer-Related Thromboembolism
Cancer is associated with a 4.1-fold increased risk of venous thromboembolism.88
Poor mobility, venous obstruction, and ongoing chemotherapy further increase risk of recurrence.89,90 Venous thromboembolism is associated with advanced and more aggressive cancers.91,92
Cancer patients with venous thromboembolism have worse survival than cancer patients free of this complication.91 For example, after a diagnosis of venous thromboembolism, the mortality rate at 6 months for cancer patients on anticoagulant therapy is 40%.92
Cancer patients today are dying prematurely from venous thromboembolism. This is why Life Extension® long ago recommended aspirin and low-molecular weight heparin as adjuvant cancer therapies. Not only do overly-active platelets contribute to thrombosis, but they facilitate metastasis.93-95
What might surprise you is that venous thromboembolisms can be the first clinical manifestation of cancer somewhere in one’s body. About 10% of patients with unprovoked venous thromboembolism are diagnosed with cancer. Of these, more than 75% are diagnosed within the first year after their thrombotic episode.2
Impressive Human Data!
A recent study highlighted the risk of deep vein thrombosis in response to conditions that predispose to stagnation of venous blood in the lower extremities (legs)—specifically, air travel.96
Many people are not aware that air travel, and associated pooling of venous blood in the legs due to inactivity during flight, is a major risk factor for deep vein thrombosis and potentially life-threatening pulmonary embolism.97,98
Using ultrasound imaging, the presence of venous thrombosis was detected in a startling 5%-7% of passengers of flights lasting 7-8 hours.96 These passengers were asymptomatic, meaning they did not know they had developed a venous blood clot!
When a nondrug approach for prevention of deep vein thrombosis was studied in this group of passengers, no lower leg venous clots were detected and lower leg edema (swelling) was drastically reduced.
Whenever you are faced with long-haul air travel, you should stand up every few hours and take a walk through the plane cabin. Consider obtaining high quality compression stockings to wear whenever you fly to reduce stagnation of blood in lower leg veins.99
As you will read in this month’s issue, ingestion of two nutrients taken two hours before the plane departs and again six hours into the flight drastically reduced detection of venous blood clots and lower leg edema at the end of the flight. These nutrients have a dual effect of inhibiting platelet aggregation and helping to thwart fibrin-induced clots.
The fact that short-term dosing of these two nutrients demonstrated such a profound effect in protecting against deep vein thrombosis implies significant systemic benefits for those who supplement daily.
An intriguing article in this month’s issue describes the robust benefits of these nutrients for reducing deep vein thrombosis risk.
The first article unveils a novel way of enhancing the efficacy of your probiotic by selectively killing off harmful intestinal bacteria.
Surgeon General’s Call to Action
In a report published 8 years ago, the Surgeon General stated:
“DVT [deep vein thrombosis] and PE [pulmonary embolism] are major public health problems in the United States. Much is known about how to reduce their burden, yet this knowledge is not being applied systematically today. Without a concerted effort to stem this public health crisis, the incidence and burden of these diseases will only grow larger as the population ages.”8
Sadly, this medical neglect continues as hurried physicians are not doing enough to prevent thrombotic events that not only cause DVT/pulmonary embolism, but many strokes and heart attacks.
Obtain Nutrient Formulas at Year’s Lowest Prices
This is the time of year when we discount prices on every one of our advanced nutritional formulas.
Longtime supporters take advantage of the once-a-year Super Sale to stock up on their favorite nutrient formulas.
Those who have engaged in healthy lifestyle choices should find comfort knowing that nutrients they may have been using for decades confer considerable protection against thrombosis, which remains the most prevalent underlying cause of disability and death in persons over age 50.
When Anticoagulant Drugs Are Needed
 |
People with artificial heart valves or atrial fibrillation are at high risk for developing a thrombus that breaks loose and travels up a carotid artery, where it can cause an acute ischemia stroke.100,101
There are also inherited conditions in which blood clotting proteins improperly react, either causing blood to overcoagulate or preventing expression of normal clot dissolving factors. Some of these coagulation disorders that result in too much clotting include:
- Factor V Leiden
- Antithrombin III (ATIII) deficiency
- Protein C or protein S deficiency
- Prothrombin (PT) gene mutation
- Antiphospholipid antibody syndrome
Those in a hypercoagulable state are usually prescribed one of the four following anticoagulant drugs:
- Pradaxa®
- Eliquis®
- Xarelto®
- Coumadin® (warfarin)
The major side effect risk of these drugs is unwanted bleeding. These drugs also don’t always prevent a thrombotic event. Those who choose the oldest of these drugs (warfarin) are subjected to severe vitamin K deficiency that rapidly calcifies tissues. This can lead to future degenerative illnesses (such as accelerated atherosclerosis and aortic valve stenosis).
Despite these side effect risks, those at high thrombotic risk should work closely with their physician to use the anticoagulant drug that best meets their individual needs. To review our detailed report on the pros and cons of each of these drugs, log on to:
Our Commitment
No organization is working harder to accelerate human age reversal research than Life Extension.
Your support enables scientists to engage in biomedical research that would have been inconceivable just a few years ago.
To order nutrients you need today at Super Sale prices, call 1-800-544-4440.
For longer life,
William Faloon
References
- Becker Richard C. MD. Textbook of Coronary Thrombosis and Thrombolysis. Boston: Kluwer Academic; 1997.
- Lee AY, Levine MN. Venous thromboembolism and cancer: risks and outcomes. Circulation. 2003;107(23 Suppl 1):I17-21.
- Fennerty A. Venous thromboembolic disease and cancer. Postgrad Med J. 2006;82(972):642-8.
- Weksler BB, Kent JL, Rudolph D, et al. Effects of low dose aspirin on platelet function in patients with recent cerebral ischemia. Stroke. 1985;16(1):5-9.
- ISTH Steering Committee for World Thrombosis Day. Thrombosis: a major contributor to global disease burden. Thromb Res. 2014;134(5):931-8.
- Rosenschein U. Introduction. Intracoronary thrombosis is the largest single cause of morbidity and mortality in the Western World. Semin Interv Cardiol. 2000;5(3):107.
- Available at: http://www.hopkinsmedicine.org/healthlibrary/conditions/nervous_system_disorders/types_of_stroke_85,P00813/. Accessed October 17, 2016.
- Available at: https://www.ncbi.nlm.nih.gov/books/NBK44178/. Accessed October 17, 2016.
- Available at: http://seer.cancer.gov/statfacts/html/pancreas.html. Accessed October 17, 2016.
- Hofmann MA, Lalla E, Lu Y, et al. Hyperhomocysteinemia enhances vascular inflammation and accelerates atherosclerosis in a murine model. J Clin Invest. 2001;107(6):675-83.
- McCully KS. Chemical pathology of homocysteine. IV. Excitotoxicity, oxidative stress, endothelial dysfunction, and inflammation. Ann Clin Lab Sci. 2009;39(3):219-32.
- Sainani GS, Sainani R. Homocysteine and its role in the pathogenesis of atherosclerotic vascular disease. J Assoc Physicians India. 2002;50 Suppl:16-23.
- Jialal I, Devaraj S. Inflammation and atherosclerosis: the value of the high-sensitivity C-reactive protein assay as a risk marker. Am J Clin Pathol. 2001;116 Suppl:S108-15.
- Koenig W, Sund M, Frohlich M, et al. C-Reactive protein, a sensitive marker of inflammation, predicts future risk of coronary heart disease in initially healthy middle-aged men: results from the MONICA (Monitoring Trends and Determinants in Cardiovascular Disease) Augsburg Cohort Study, 1984 to 1992. Circulation. 1999;99(2):237-42.
- Drouet L, Bal dit Sollier C. Is fibrinogen a predictor or a marker of the risk of cardiovascular events? Therapie. 2005;60(2):125-36.
- Paramo JA, Rodriguez JA, Orbe J. Fibrinogen. An old hemostatic protein with a new function: non-invasive marker of subclinical atherosclerosis. Med Clin (Barc). 2005;124(20):790-4.
- Canseco-Avila LM, Jerjes-Sanchez C, Ortiz-Lopez R, et al. Fibrinogen. Cardiovascular risk factor or marker? Arch Cardiol Mex. 2006;76 Suppl 4:S158-72.
- Huang W, Chen QW, Lei H, et al. Predictive value of fibrinogen and high-sensitivity C-reaction protein for cardiovascular events in patients with stable coronary artery disease. Zhonghua Xin Xue Guan Bing Za Zhi. 2006;34(8):718-21.
- Ganguly P, Alam SF. Role of homocysteine in the development of cardiovascular disease. Nutr J. 2015;14:6.
- Mozaffarian D, Lemaitre RN, King IB, et al. Circulating long-chain omega-3 fatty acids and incidence of congestive heart failure in older adults: the cardiovascular health study: a cohort study. Ann Intern Med. 2011;155(3):160-70.
- Suzuki Y, Ichiyama T, Ohsaki A, et al. Anti-inflammatory effect of 1alpha,25-dihydroxyvitamin D(3) in human coronary arterial endothelial cells: Implication for the treatment of Kawasaki disease. J Steroid Biochem Mol Biol. 2009;113(1-2):134-8.
- Liefaard MC, Ligthart S, Vitezova A, et al. Vitamin D and C-Reactive Protein: A Mendelian Randomization Study. PLoS One. 2015;10(7):e0131740.
- Aggarwal BB, Harikumar KB. Potential therapeutic effects of curcumin, the anti-inflammatory agent, against neurodegenerative, cardiovascular, pulmonary, metabolic, autoimmune and neoplastic diseases. Int J Biochem Cell Biol. 2009;41(1):40-59.
- Menon VP, Sudheer AR. Antioxidant and anti-inflammatory properties of curcumin. Adv Exp Med Biol. 2007;595:105-25.
- Venn BJ, Green TJ, Moser R, et al. Comparison of the effect of low-dose supplementation with L-5-methyltetrahydrofolate or folic acid on plasma homocysteine: a randomized placebo-controlled study. Am J Clin Nutr. 2003;77(3):658-62.
- Caruso R, Campolo J, Sedda V, et al. Effect of homocysteine lowering by 5-methyltetrahydrofolate on redox status in hyperhomocysteinemia. J Cardiovasc Pharmacol. 2006;47(4):549-55.
- Schnyder G, Roffi M, Flammer Y, et al. Effect of homocysteine-lowering therapy with folic acid, vitamin B12, and vitamin B6 on clinical outcome after percutaneous coronary intervention: the Swiss Heart study: a randomized controlled trial. Jama. 2002;288(8):973-9.
- Selhub J. Homocysteine metabolism. Annu Rev Nutr. 1999;19:217-46.
- Poredos P, Jezovnik MK. The role of inflammation in venous thromboembolism and the link between arterial and venous thrombosis. Int Angiol. 2007;26(4):306-11.
- Suzuki Y, Kondo K, Ichise H, et al. Dietary supplementation with fermented soybeans suppresses intimal thickening. Nutrition. 2003;19(3):261-4.
- Davalos D, Akassoglou K. Fibrinogen as a key regulator of inflammation in disease. Semin Immunopathol. 2012;34(1):43-62.
- Hsia CH, Shen MC, Lin JS, et al. Nattokinase decreases plasma levels of fibrinogen, factor VII, and factor VIII in human subjects. Nutr Res. 2009;29(3):190-6.
- Badimon L, Vilahur G. LDL-cholesterol versus HDL-cholesterol in the atherosclerotic plaque: inflammatory resolution versus thrombotic chaos. Ann N Y Acad Sci. 2012;1254:18-32.
- Lip GY. Hypertension, platelets, and the endothelium: the “thrombotic paradox” of hypertension (or “Birmingham paradox”) revisited. Hypertension. 2003;41(2):199-200.
- Ageno W, Becattini C, Brighton T, et al. Cardiovascular risk factors and venous thromboembolism: a meta-analysis. Circulation. 2008;117(1):93-102.
- Pomp ER, Rosendaal FR, Doggen CJ. Smoking increases the risk of venous thrombosis and acts synergistically with oral contraceptive use. Am J Hematol. 2008;83(2):97-102.
- Hansson PO, Eriksson H, Welin L, et al. Smoking and abdominal obesity: risk factors for venous thromboembolism among middle-aged men: “the study of men born in 1913”. Arch Intern Med. 1999;159(16):1886-90.
- Vaya A, Mira Y, Ferrando F, et al. Hyperlipidaemia and venous thromboembolism in patients lacking thrombophilic risk factors. Br J Haematol. 2002;118(1):255-9.
- Available at: http://www.worldthrombosisday.org/issue/vte/. Accessed October 18, 2016.
- Available at: http://www.cdc.gov/ncbddd/dvt/data.html. Accessed September 20, 2016.
- Kang WS, Lim IH, Yuk DY, et al. Antithrombotic activities of green tea catechins and (-)-epigallocatechin gallate. Thromb Res. 1999;96(3):229-37.
- Sagesaka-Mitane Y, Miwa M, Okada S. Platelet aggregation inhibitors in hot water extract of green tea. Chem Pharm Bull (Tokyo). 1990;38(3):790-3.
- Mori TA, Beilin LJ, Burke V, et al. Interactions between dietary fat, fish, and fish oils and their effects on platelet function in men at risk of cardiovascular disease. Arterioscler Thromb Vasc Biol. 1997;17(2):279-86.
- Vanschoonbeek K, Feijge MA, Paquay M, et al. Variable hypocoagulant effect of fish oil intake in humans: modulation of fibrinogen level and thrombin generation. Arterioscler Thromb Vasc Biol. 2004;24(9):1734-40.
- Akiba S, Murata T, Kitatani K, et al. Involvement of lipoxygenase pathway in docosapentaenoic acid-induced inhibition of platelet aggregation. Biol Pharm Bull. 2000;23(11):1293-7.
- Ikeda I, Yoshida H, Tomooka M, et al. Effects of long-term feeding of marine oils with different positional distribution of eicosapentaenoic and docosahexaenoic acids on lipid metabolism, eicosanoid production, and platelet aggregation in hypercholesterolemic rats. Lipids. 1998;33(9):897-904.
- Correa JA, Lopez-Villodres JA, Asensi R, et al. Virgin olive oil polyphenol hydroxytyrosol acetate inhibits in vitro platelet aggregation in human whole blood: comparison with hydroxytyrosol and acetylsalicylic acid. Br J Nutr. 2009;101(8):1157-64.
- Junker R, Kratz M, Neufeld M, et al. Effects of diets containing olive oil, sunflower oil, or rapeseed oil on the hemostatic system. Thromb Haemost. 2001;85(2):280-6.
- Leger CL, Carbonneau MA, Michel F, et al. A thromboxane effect of a hydroxytyrosol-rich olive oil wastewater extract in patients with uncomplicated type I diabetes. Eur J Clin Nutr. 2005;59(5):727-30.
- Singh I, Mok M, Christensen AM, et al. The effects of polyphenols in olive leaves on platelet function. Nutr Metab Cardiovasc Dis. 2008;18(2):127-32.
- Zbidi H, Salido S, Altarejos J, et al. Olive tree wood phenolic compounds with human platelet antiaggregant properties. Blood Cells Mol Dis. 2009;42(3):279-85.
- Hubbard GP, Wolffram S, Lovegrove JA, et al. Ingestion of quercetin inhibits platelet aggregation and essential components of the collagen-stimulated platelet activation pathway in humans. J Thromb Haemost. 2004;2(12):2138-45.
- Gresele P, Pignatelli P, Guglielmini G, et al. Resveratrol, at concentrations attainable with moderate wine consumption, stimulates human platelet nitric oxide production. J Nutr. 2008;138(9):1602-8.
- Malinowska J, Olas B. Response of blood platelets to resveratrol during a model of hyperhomocysteinemia. Platelets. 2011;22(4):277-83.
- Olas B, Wachowicz B. Resveratrol, a phenolic antioxidant with effects on blood platelet functions. Platelets. 2005;16(5):251-60.
- Yang Y, Wang X, Zhang L, et al. Inhibitory effects of resveratrol on platelet activation induced by thromboxane a(2) receptor agonist in human platelets. Am J Chin Med. 2011;39(1):145-59.
- Shenoy SF, Keen CL, Kalgaonkar S, et al. Effects of grape seed extract consumption on platelet function in postmenopausal women. Thromb Res. 2007;121(3):431-2.
- Zhang Y, Shi H, Wang W, et al. Antithrombotic effect of grape seed proanthocyanidins extract in a rat model of deep vein thrombosis. J Vasc Surg. 2011;53(3):743-53.
- Sano T, Oda E, Yamashita T, et al. Anti-thrombotic effect of proanthocyanidin, a purified ingredient of grape seed. Thromb Res. 2005;115(1-2):115-21.
- Vitseva O, Varghese S, Chakrabarti S, et al. Grape seed and skin extracts inhibit platelet function and release of reactive oxygen intermediates. J Cardiovasc Pharmacol. 2005;46(4):445-51.
- de Lange DW, Verhoef S, Gorter G, et al. Polyphenolic grape extract inhibits platelet activation through PECAM-1: an explanation for the French paradox. Alcohol Clin Exp Res. 2007;31(8):1308-14.
- Nocun M, Ulicna O, Muchova J, et al. French maritime pine bark extract Pycnogenol reduces thromboxane generation in blood from diabetic male rats. Biomed Pharmacother. 2008;62(3):168-72.
- Hsiao G, Wang Y, Tzu NH, et al. Inhibitory effects of lycopene on in vitro platelet activation and in vivo prevention of thrombus formation. J Lab Clin Med. 2005;146(4):216-26.
- Dutta-Roy AK, Crosbie L, Gordon MJ. Effects of tomato extract on human platelet aggregation in vitro. Platelets. 2001;12(4):218-27.
- Aviram M, Dornfeld L, Rosenblat M, et al. Pomegranate juice consumption reduces oxidative stress, atherogenic modifications to LDL, and platelet aggregation: studies in humans and in atherosclerotic apolipoprotein E-deficient mice. Am J Clin Nutr. 2000;71(5):1062-76.
- Srivas KC. Effects of aqueous extracts of onion, garlic and ginger on platelet aggregation and metabolism of arachidonic acid in the blood vascular system: in vitro study. Prostaglandins Leukot Med. 1984;13(2):227-35.
- Bordia A, Verma SK, Srivastava KC. Effect of garlic (Allium sativum) on blood lipids, blood sugar, fibrinogen and fibrinolytic activity in patients with coronary artery disease. Prostaglandins Leukot Essent Fatty Acids. 1998;58(4):257-63.
- Macan H, Uykimpang R, Alconcel M, et al. Aged garlic extract may be safe for patients on warfarin therapy. J Nutr. 2006;136(3 Suppl):793s-5s.
- Rahman K. Effects of garlic on platelet biochemistry and physiology. Mol Nutr Food Res. 2007;51(11):1335-44.
- Steiner M, Li W. Aged garlic extract, a modulator of cardiovascular risk factors: a dose-finding study on the effects of AGE on platelet functions. J Nutr. 2001;131(3s):980s-4s.
- Rahman K, Billington D. Dietary supplementation with aged garlic extract inhibits ADP-induced platelet aggregation in humans. J Nutr. 2000;130(11):2662-5.
- Ali M, Thomson M. Consumption of a garlic clove a day could be beneficial in preventing thrombosis. Prostaglandins Leukot Essent Fatty Acids. 1995;53(3):211-2.
- Allman MA, Pena MM, Pang D. Supplementation with flaxseed oil versus sunflowerseed oil in healthy young men consuming a low fat diet: effects on platelet composition and function. Eur J Clin Nutr. 1995;49(3):169-78.
- Ristic-Medic D, Ristic G, Tepsic V. Alpha-linolenic acid and cardiovascular diseases. Med Pregl. 2003;56 Suppl 1:19-25.
- Bordia A, Verma SK, Srivastava KC. Effect of ginger (Zingiber officinale Rosc.) and fenugreek (Trigonella foenumgraecum L.) on blood lipids, blood sugar and platelet aggregation in patients with coronary artery disease. Prostaglandins Leukot Essent Fatty Acids. 1997;56(5):379-84.
- Lumb AB. Effect of dried ginger on human platelet function. Thromb Haemost. 1994;71(1):110-1.
- Srivastava KC. Effect of onion and ginger consumption on platelet thromboxane production in humans. Prostaglandins Leukot Essent Fatty Acids. 1989;35(3):183-5.
- Verma SK, Singh J, Khamesra R, et al. Effect of ginger on platelet aggregation in man. Indian J Med Res. 1993;98:240-2.
- Jantan I, Raweh SM, Sirat HM, et al. Inhibitory effect of compounds from Zingiberaceae species on human platelet aggregation. Phytomedicine. 2008;15(4):306-9.
- Nurtjahja-Tjendraputra E, Ammit AJ, Roufogalis BD, et al. Effective antiplatelet and COX-1 enzyme inhibitors from pungent constituents of ginger. Thromb Res. 2003;111(4-5):259-65.
- Vachharajani V, Wang SW, Mishra N, et al. Curcumin modulates leukocyte and platelet adhesion in murine sepsis. Microcirculation. 2010;17(6):407-16.
- Shah BH, Nawaz Z, Pertani SA, et al. Inhibitory effect of curcumin, a food spice from turmeric, on platelet-activating factor- and arachidonic acid-mediated platelet aggregation through inhibition of thromboxane formation and Ca2+ signaling. Biochem Pharmacol. 1999;58(7):1167-72.
- Available at: http://www.webmd.com/dvt/deep-vein-thrombosis-dvt-symptoms-diagnosis. Accessed September 22, 2016.
- Anderson FA, Jr., Spencer FA. Risk factors for venous thromboembolism. Circulation. 2003;107(23 Suppl 1):I9-16.
- Langman LJ, Ray JG, Evrovski J, et al. Hyperhomocyst(e)inemia and the increased risk of venous thromboembolism: more evidence from a case-control study. Arch Intern Med. 2000;160(7):961-4.
- Saha P, Humphries J, Modarai B, et al. Leukocytes and the natural history of deep vein thrombosis: current concepts and future directions. Arterioscler Thromb Vasc Biol. 2011;31(3):506-12.
- Sumi H, Hamada H, Nakanishi K, et al. Enhancement of the fibrinolytic activity in plasma by oral administration of nattokinase. Acta Haematol. 1990;84(3):139-43.
- Khalil J, Bensaid B, Elkacemi H, et al. Venous thromboembolism in cancer patients: an underestimated major health problem. World J Surg Oncol. 2015;13:204.
- Lopez JA, Kearon C, Lee AY. Deep venous thrombosis. Hematology Am Soc Hematol Educ Program. 2004:439-56.
- Levine MN, Gent M, Hirsh J, et al. The thrombogenic effect of anticancer drug therapy in women with stage II breast cancer. N Engl J Med. 1988;318(7):404-7.
- Wun T, White RH. Epidemiology of cancer-related venous thromboembolism. Best Pract Res Clin Haematol. 2009;22(1):9-23.
- Lee AY, Levine MN, Baker RI, et al. Low-molecular-weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med. 2003;349(2):146-53.
- Erpenbeck L, Schon MP. Deadly allies: the fatal interplay between platelets and metastasizing cancer cells. Blood. 2010;115(17):3427-36.
- Labelle M, Begum S, Hynes RO. Platelets guide the formation of early metastatic niches. Proc Natl Acad Sci U S A. 2014;111(30):E3053-61.
- Nieswandt B, Hafner M, Echtenacher B, et al. Lysis of tumor cells by natural killer cells in mice is impeded by platelets. Cancer Res. 1999;59(6):1295-300.
- Cesarone MR, Belcaro G, Nicolaides AN, et al. Prevention of venous thrombosis in long-haul flights with Flite Tabs: the LONFLIT-FLITE randomized, controlled trial. Angiology. 2003;54(5):531-9.
- Geroulakos G, Hossain J, Tran T. Economy-class syndrome presenting as phlegmasia caerulea dolens. Eur J Vasc Endovasc Surg. 2000;20(1):102-4.
- Scurr JH, Machin SJ, Bailey-King S, et al. Frequency and prevention of symptomless deep-vein thrombosis in long-haul flights: a randomised trial. Lancet. 2001;357(9267):1485-9.
- Belcaro G, Cesarone MR, Nicolaides AN, et al. Prevention of venous thrombosis with elastic stockings during long-haul flights: the LONFLIT 5 JAP study. Clin Appl Thromb Hemost. 2003;9(3):197-201.
- Bollag L, Attenhofer Jost CH, Vogt PR, et al. Symptomatic mechanical heart valve thrombosis: high morbidity and mortality despite successful treatment options. Swiss Med Wkly. 2001;131(9-10):109-16.
- Noel P, Gregoire F, Capon A, et al. Atrial fibrillation as a risk factor for deep venous thrombosis and pulmonary emboli in stroke patients. Stroke. 1991;22(6):760-2.

