Life Extension Magazine®
Diabetes is a disease in which the body does not produce and/or properly use insulin—in other words, the body is insulin resistant.1 The treatment of type I and some cases of type II diabetes with subcutaneous insulin injections is sometimes associated with lack of compliance due to the pain of multiple daily injections.2 Hence, there is a big demand for insulin that can be administered without painful shots. Development of such an insulin delivery system could open the way to a multibillion-dollar market, while making diabetics more treatment-compliant.
The search for a non-injectable form of insulin continues as the diabetic population all over the world continues to explode.3-5 An apparent advance arrived with the development of a preparation that could simply be inhaled.6
While the FDA had deemed this novel insulin preparation safe and effective, many questions regarding its long-term health effects remained unresolved.7-9 After an article was published on the potential cancer-causing effects of inhaled insulin using a medication called Exubera®, the Pfizer company withdrew the drug, taking a $2.5 billion loss.10-12 Pfizer later reported the development of lung cancer in six patients who had used inhaled insulin. Pfizer’s timely withdrawal potentially saved hundreds of diabetics using inhaled insulin from developing cancer.13
Unfortunately, on June 27, 2014, the FDA approved another inhaled insulin drug.7 It is obvious that the FDA did not thoroughly look at the ill effects of inhaled insulin.
What Causes Diabetes?

Insulin is a hormone secreted by endocrine cells (specifically beta cells located in the islets of Langerhans) of the pancreas and is essential for human life. It works by interacting with the insulin receptors on cell membranes to facilitate the entry of glucose and other nutrients into cells for energy production.14,15 Insulin facilitates various cellular metabolic functions. In addition to removing excess sugar from the blood, insulin also promotes cell division.16,17
Type I diabetes is characterized by a lack of insulin in the blood due to a deficiency of its production in the pancreas.18 In type II diabetes, the pancreas does produce insulin, but the body’s cells (estimated to be around 37 trillion in adults)19 are resistant to insulin’s action—it is as if the doors that allow glucose to move from the blood into the cells are shut. The result is high levels of unused insulin and glucose in the blood, the hallmarks of early-stage type II diabetes.20 In the later stages of type II diabetes, the pancreas fails to secrete enough insulin,21 and the patient becomes reliant on either drugs that artificially stimulate pancreatic insulin secretion, or on exogenously administered insulin with or without oral antidiabetic therapeutic agents.
Presently, type I diabetes is treated with daily insulin injections, whereas type II diabetes is treated with oral antidiabetic therapeutic agents, either alone or in combination with insulin shots.22,23 Other modalities to curtail, control, and cure diabetes are under intense research. It is the intent of researchers to develop a simple therapy to treat both of these types of diabetes. The pharmaceutical industry is waiting in the wings for a blockbuster moneymaking drug. It will come, but it will not be inhaled insulin. It will come in a combination that enhances glucose uptake at the cellular level, along with therapeutic agents that act the same as insulin when taken orally.
The Problem With Insulin Therapy Today
The disadvantage of repeated insulin injections is the pain, which makes it more difficult to properly manage type I diabetes.24 To replace injections, repeated attempts have been made to deliver the insulin through alternative routes.
Based on today’s diabetes epidemic—often due to obesity associated with a lack of exercise—there is a large and growing demand for insulin medications. However, the inconvenience and disruption of lifestyle associated with multiple daily insulin injections leads many patients to abandon their doctor-recommended treatment plans.2 As a result, many patients fail to effectively manage their condition, causing systemic disease associated with complications and early death.
To eliminate pain and improve patient compliance, and thus treatment outcome, research is focusing on alternatives to repeated subcutaneous insulin injections. Some of the areas of investigation include aerosolized insulin for inhalation, oral insulin, insulin-producing stem cell implantation, insulin delivery pumps, and more.25 There is even possible development of microneedles to deliver insulin subcutaneously, along with various transdermal and transmucosal delivery of insulin without needles.
What you need to know
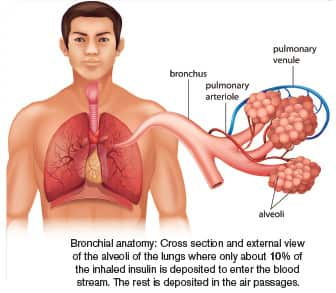 |
Inhaled Insulin’s Health Risks
- As the number of diabetes cases continues to increase, the search for a non-injectable form of insulin increases. In June 2014, the FDA approved an inhaled insulin drug, despite the fact that an earlier inhaled insulin drug was linked to lung cancer and withdrawn from the market.
- A potential problem with inhaled insulin is that it is only effective when the dose is three to 10 times higher than that in an injection because only about 10% is absorbed by the bloodstream.
- Another area of concern is that the possible effect on tissue the inhaled insulin comes in contact with upon delivery. Insulin induces cell division wherever it is deposited. This may lead to aberrant cell growth, triggering cancer.
- Further concerns regarding inhaled insulin include respiratory tract irritation, hypoglycemia, exacerbation of asthma symptoms, and adverse effects in those with pre-existing respiratory diseases.
- It may be years before the benefits versus risks of inhaled insulin are known.
Risk Of Using Inhaled Insulin
The problem with inhaled forms of insulin is that it is effective only when the administered dose is more than three to 10 times the amount given by subcutaneous injection. That’s because little more than 10% of the inhaled insulin reaches the alveoli in the lungs where it is absorbed into the bloodstream.6,26
Another area of potential concern regarding inhaled insulin is the possible effect on the tissues that it comes in contact with on its way to the alveoli, including the linings of the mouth, throat, tongue, cheeks, gums, tonsils, trachea, bronchial tree, vocal cords, larynx, nasal air sinuses, and olfactory mucosa (which has a direct connection to the brain).27 Even the modified dry form of insulin is of concern. The powdered insulin will stick to the above-mentioned breathing passages on the way to the lungs. It is a known fact that insulin induces cell division wherever it is deposited.28
Furthermore, since insulin is a growth factor, there is also the potential concern that inhaled insulin could support aberrant cell growth, and potentially even change precancerous lesions into cancer. Cancer cells and precancerous cells have numerous insulin receptors that bind to the inhaled insulin. The fundamental problem with the insulin-inhalation delivery method is that the powder particulates stick to the naso-oro-pharyngeal-laryngeal-tracheobronchial tree. By sticking to these structures before it reaches the alveoli, inhaled insulin can facilitate the malignant transformation of cells.29
Researchers have noted that those with elevated blood sugar due to type II diabetes and other conditions are more prone to develop certain types of cancers than the healthy population.30 Numerous cancers have more than the normal amount of insulin receptors to facilitate the entry of large amounts of glucose into the tumor cells, thus promoting their growth, multiplication, and spread.31,32
Inhaled insulin may potentially increase the risk of lung cancer. Studies of human epithelial cells suggest that insulin-receptor activation is in itself insufficient for malignant transformation. However, once malignant transformation has been induced by other agents, the insulin receptor pathway to promote malignant progression of these cells can be activated.32 Since inhaled insulin comes in contact with so many tissues, it is crucial that future research examines its impact on normal, precancerous, and cancerous cells of the upper respiratory and digestive systems.
Potential Health Risks Of Inhaled Insulin Therapy
The following are documented and possible health risks of insulin inhalation therapy:
- Increased risk of respiratory tract irritation,33 which causes coughing, shortness of breath, sore throat, and dry mouth.34,35
- Development of hypoglycemia, with adverse outcomes in those who exercise immediately after inhalation and those who smoke. These effects may occur due to the rapid absorption of inhaled insulin from the alveoli.36,37
- Exacerbation of existing conditions in asthmatics that would require more inhaled insulin to control blood sugar.7,38 Furthermore, inhaled insulin could lead to smooth muscle contraction of the airway, which could precipitate or exacerbate chronic obstructive pulmonary disease (COPD) or episodes of asthma.39
- Adverse effects in people with pre-existing respiratory diseases such as chronic bronchitis, tuberculosis, tumors, and other chronic lung afflictions.8
- Increased insulin antibodies. In one study, inhaled insulin increased the level of insulin antibodies in the body from baseline levels of 6 to 35%.40 This could delay and retard the action of soluble insulin in the blood, since the removal of an insulin immune complex could make less insulin available to lower blood sugar.
- Unwanted tissue growth in normal and precancerous cells, which may lead to genetic defects and ultimately cause cancer. Long-term effects of supraphysiologic doses of insulin in the human lung or on neoplastic lung tissue have begun to unfold.13 Many of the insulin particles are deposited on the oro-pharyngeal-laryngeal-tracheobronchial tree, and nose lining. This might increase the incidence of tumors of the oral cavity, tongue, larynx, pharynx, trachea, bronchial tree, lungs, tonsils, nasal mucosa, nasal air sinuses, nasal polyps, vocal cords, esophagus, and any other structure where the insulin particulates are deposited during inhalation and nasal spray delivery methods.
- A potential increased tumor incidence in the tissues of the respiratory tract, although no evidence of this has been presented to date due to the withdrawal of inhalation insulin by Pfizer. With large-scale use of inhalation insulin, this may become apparent with increased tumors of the nasal sinuses, nasopharyngeal cavity, laryngeal, and respiratory tracheobronchial passages as well as the esophagus. The true health risks could take a long time to reveal themselves, as occurred with other drugs such as Vioxx® (an anti-inflammatory), Avandia® (an oral antidiabetic drug), and the diet drug combination, fenfluramine/phentermine (fen-phen).41-43
If you visit www.pubmed.gov and search for “insulin and cancer,” you will find almost 30,000 citings; if you search for “insulin causes cancer,” you will find nearly 17,000 citings. This is one indication of the intense research underway on the relationship of insulin to cancer. Many cancer and precancerous cells have two to four times more insulin receptors (IR) and more insulin-like growth factor 1 (IGF-1) receptors,32,44,45 which thrive on the high blood sugar and high insulin with which they come in direct contact.
Should You Use Inhaled Insulin?
The FDA’s recently approved inhaled insulin is a different formulation than Pfizer’s Exubera®, which was removed from the market,7,46,47 but that does not make it the method of choice for insulin delivery in type I and some type II diabetics. That’s because the powder still has to pass through the same air passages that Exubera® did, and almost 80 to 90% of it is going to be lost when it sticks to the respiratory passages before it ever reaches the lungs and is then delivered to all cells in the body.
I recommend that inhaled insulin should not be used by diabetics who smoke or patients with underlying lung diseases such as asthma or chronic obstructive pulmonary disease (COPD), chronic bronchitis, lung infections (including tuberculosis), and patients suspected of lung carcinomas and sarcomas. Until the drug’s full health risks versus benefits are known, I further discourage its use in patients with precancerous lesions (such as polyps, dysplasia, and leukoplakia), those with changes caused by tobacco use, those with chronic exposure to dust and other hydrocarbons, and patients with chronic infections.
It may take years before we know the benefits versus risks of inhaled insulin. The safety of using this type of product in pregnant women, adolescents, and children has not been established. I hope that the FDA and drug companies involved in licensing and developing an inhaled method of insulin delivery will fully investigate these health risks and concerns on a post-approval surveillance basis.
The Future Of Insulin Delivery

The future of insulin delivery with the fewest side effects and a less painful delivery method may come from:
- The development of slow-release injectable insulin that lasts days or weeks with a single shot,
- The implantation of insulin-producing stem cells,
- An insulin pump or painless microneedles that deliver insulin under the skin,
- A method to activate and induce primordial stem cells in the pancreas’ insulin-producing islet cells, or
- A transmucosal delivery patch as described in US patent publication 2009/0304776 Al.48
The market for new antidiabetic therapeutic agents is a multibillion-dollar market. I am sure the drug companies and research scientists are in a race to develop a method to control the blood sugar to treat diabetes, which has become an endemic disease in the current century.49
Summary
As the diabetes epidemic continues to grow, drug manufacturers are eager to develop new methods of insulin delivery. The FDA recently approved an inhaled insulin drug, despite the fact that Pfizer withdrew its inhaled insulin product due to its potential to cause cancer. Inhaled insulin affects all tissue it comes in contact with upon delivery, and since insulin induces cell division, this can lead to aberrant cell growth. Hypoglycemia, exacerbation of asthma symptoms, and adverse effects in those with pre-existing respiratory diseases are also areas of concern regarding inhaled insulin.
If you have any questions on the scientific content of this article, please call a Life Extension® Wellness Specialist at 1-866-864-3027.
Dr. T.R. Shantha has relentlessly battled and suffered at the hands of the entrenched medical establishment. He is currently pursuing novel approaches
to better treat today’s diabetes epidemic. His exposés of the risks of the first FDA-approved inhaled insulin drug possibly saved countless lives.
For more information, call, write, or email Dr. Shanta. 1946 Carrington Court Stone Mountain, GA 30087. Phone/Fax: 678-580-5446, Cell: 678-640-7705
Email: shantha35@aol.com; www.wedgetherapeutics.com
References
- Available at: http://www.ncbi.nlm.nih.gov/pubmedhealth/pmh0015919. Accessed October 2, 2014.
- Mohan V, Shah SN, Joshi SR, et al. Current status of management, control, complications and psychosocial aspects of patients with diabetes in India: Results from the DiabCare India 2011 Study. Indian J Endocrinol Metab. 2014 May;18(3):370-8.
- Heinemann L, Traut T, Heise T. Time-action profile of inhaled insulin. Diabet Med. 1997 Jan;14(1):63-72.
- Tabish SA. Is diabetes becoming the biggest epidemic of the twenty-first century? Int J Health Sci (Qassim). 2007 Jul;1(2):V-VI.
- Verma R, Khanna P, Mehta B. National programme on prevention and control of diabetes in India: Need to focus. Australas Med J. 2012;5(6):310-5.
- Heinemann L, Scheuch G, Heise T. Inhaled insulin: take a deep breath, but how? J Diabetes Sci Technol. 2008 Mar;2(2):297-9.
- Available at: http://www.fda.gov/newsevents/newsroom/pressannouncements/ucm403122.htm. Accessed October 17, 2014.
- Available at: http://www.news.mannkindcorp.com/phoenix.zhtml?c=147953&p=irol-newsArticle&id=1943390. Accessed October 17, 2014.
- Available at: http://www.nytimes.com/2014/06/28/business/afrezza-a-new-inhaled-insulin-is-approved-by-fda.html?_r=0. Accessed October 17, 2014.
- Shantha TR. Unknown health risks of inhaled insulin. Life Extension. 2007 Sept;13(9):79-82.
- Shantha TR. Inhaled insulin increases lung cancer risk confirmed. Life Extension. 2008 Sept;14(9):20.
- Available at: http://www.nj.com/business/index.ssf/2008/04/pfizer warns patients. Accessed October 9, 2014.
- Available at: http://www.fda.gov/safety/medwatch/safetyinformation/safety-relateddruglabelingchanges/ucm122978.htm. Accessed October 9, 2014.
- Lee J, Pilch PF. The insulin receptor: structure, function, and signaling. Am J Physiol. 1994 Feb;266(2 Pt 1):C319-34.
- Bonadonna RC, Saccomani MP, Cobelli C, DeFronzo RA. Effect of insulin on system A amino acid transport in human skeletal muscle. J Clin Invest. 1993 Feb;91(2):514-21.
- Ish-Shalom D, Christoffersen CT, Vorwerk P, Sacerdoti-Sierra N, Shymko RM, Naor D, et al. Mitogenic properties of insulin and insulin analogues mediated by the insulin receptor. Diabetologia. 1997 Jul;40 Suppl 2:S25-31.
- Messina JL. Inhibition and stimulation of c-myc gene transcription by insulin in rat hepatoma cells. Insulin alters the intragenic pausing of c-myc transcription. J Biol Chem. 1991 Sep 25;266(27):17995-8001.
- Available at: http://www.diabetes.org/diabetes-basics/type-1. Accessed October 17, 2014.
- Bianconi E, Piovesan A, Facchin F, et al. An estimation of the number of cells in the human body. Ann Hum Biol. 2013 Nov-Dec;40(6):463-71.
- Vaidyula VR, Rao AK, Mozzoli M, Homko C, Cheung P, Boden G. Effects of hyperglycemia and hyperinsulinemia on circulating tissue factor procoagulant activity and platelet CD40 ligand. Diabetes. 2006 Jan;55(1):202-8.
- Available at: https://diabetes.org/tools-support/diabetes-prevention/high-blood-sugar. Accessed October 17, 2014.
- Available at: http://www.diabetes.org/living-with-diabetes/treatment-and-care/medication. Accessed October 17, 2014.
- Mastrandrea LD. Inhaled insulin: overview of a novel route of insulin administration. Vasc Health Risk Manag. 2010 Mar 3;6:47-58.
- Iwanaga M, Kamoi K. Patient perceptions of injection pain and anxiety: a comparison of NovoFine 32-gauge tip 6mm and Micro Fine Plus 31-gauge 5mm needles. Diabetes Technol Ther. 2009 Feb;11(2):81-6.
- Owens DR, Zinman B, Bolli G. Alternative routes of insulin delivery. Diabet Med. 2003 Nov;20(11):886-98.
- Agu RU, Ugwoke MI, Armand M, Kinget R, Verbeke N. The lung as a route for systemic delivery of therapeutic proteins and peptides. Respir Res. 2001;2(4):198-209.
- Graff CL, Pollack GM. Nasal drug administration: potential for targeted central nervous system delivery. J Pharm Sci. 2005 Jun;94(6):1187-95.
- Draznin B. Mechanism of the mitogenic influence of hyperinsulinemia. Diabetol Metab Syndr. 2011 Jun 13;3(1):10.
- von Kriegstein E, von Kriegstein K. Inhaled insulin for diabetes mellitus. N Engl J Med. 2007 May 17;356(20):2106.
- Stattin P, Bjor O, Ferrari P, et al. Prospective study of hyperglycemia and cancer risk. Diabetes Care. 2007 Mar;30(3):561-7.
- Shen MR, Hsu YM, Hsu KF, et al. Insulin-like growth factor 1 is a potent stimulator of cervical cancer cell invasiveness and proliferation that is modulated by alphavbeta3 integrin signaling. Carcinogenesis. 2006 May;27(5):962-71.
- Zhao YL, Piao CQ, Wu LJ, Suzuki M, Hei TK. Differentially expressed genes in asbestos-induced tumorigenic human bronchial epithelial cells: implication for mechanism. Carcinogenesis. 2000 Nov;21(11):2005-10.
- Sélam JL. Inhaled insulin: promises and concerns. J Diabetes Sci Technol. 2008 Mar;2(2):311-5.
- Siekmeier R , Scheuch G. Inhaled insulin--does it become reality? J Physiol Pharmacol. 2008 Dec;59 Suppl 6:81-113.
- Available at: http://www.rxlist.com/exubera-side-effects-drug-center.htm. Accessed October 17, 2014.
- Kohler D. Aerosols for systemic treatment. Lung. 1990;168. Suppl:677-84.
- Himmelmann A, Jendle J, Mellen A, et al. The impact of smoking on inhaled insulin. Diabetes Care. 2003 Mar;26(3):677-82.
- Henry RR, Mudaliar SR, Howland WC, III, et al. Inhaled insulin using the AERx Insulin Diabetes Management System in healthy and asthmatic subjects. Diabetes Care. 2003 Mar;26(3):764-9.
- Schaafsma D, Gosens R, Ris JM, et al. Insulin induces airway smooth muscle contraction. Br J Pharmacol. 2007 Jan;150(2):136-42.
- Hermansen K, Ronnemaa T, Petersen AH, Bellaire S, Adamson U. Intensive therapy with inhaled insulin via the AERx insulin diabetes management system: a 12-week proof-of-concept trial in patients with type II diabetes. Diabetes Care. 2004 Jan;27(1):162-7.
- Baron JA, Sandler RS, Bresalier RS, et al. Cardiovascular events associated with rofecoxib: final analysis of the APPROVe trial. Lancet. 2008 Nov 15;372(9651):1756-64.
- Breunig IM, Shaya FT, McPherson ML, Snitker S. Development of heart failure in medicaid patients with type II diabetes treated with pioglitazone, rosiglitazone, or metformin. J Manag Care Pharm. 2014 Sep;20(9):895-903.
- Cheung BM, Cheung TT, Samaranayake NR. Safety of antiobesity drugs. Ther Adv Drug Saf. 2013 Aug;4(4):171-81.
- Belfiore A, Malaguarnera R. Insulin receptor and cancer. Endocr Relat Cancer. 2011 Jul 4;18(4):R125-47.
- Aleem E, Nehrbass D, Klimek F, Mayer D, Bannasch P. Upregulation of the insulin receptor and type I insulin-like growth factor receptor are early events in hepatocarcinogenesis. Toxicol Pathol. 2011 Apr;39(3):524-43.
- Yaturu S. Insulin therapies: Current and future trends at dawn. World J Diabetes. 2013 Feb 15;4(1):1-7.
- Hollander PA. Evolution of a pulmonary insulin delivery system (Exubera) for patients with diabetes. MedGenMed. 2007 Mar 5;9(1):45.
- Available at: http://www.google.com/patents/us20090304776. Accessed October 17, 2014.
- Lam DW, LeRoith D. The worldwide diabetes epidemic. Curr Opin Endocrinol Diabetes Obes. 2012 Apr;19(2):93-6.

