Life Extension Magazine®

We are zeroing in on a prime culprit behind Alzheimer’s, stroke, and age-related cognitive impairment.
The term for this reversible disorder is “hypoperfusion.” It means an inadequate supply of blood to a body part.
Hypoperfusion of the brain occurs in response to reduced blood flow. The result of hypoperfusion is a series of harmful changes that severely diminish neurological function.
We have long known about structural changes that adversely impact the aging brain. Preceding this structural deterioration, however, is a decline in microvascular blood flow.
What researchers are increasingly recognizing is that most aging humans suffer from obstructions to cerebral blood flow that result in chronic hypoperfusion.1 This sets in motion a cascade of neuronal injuries that can manifest as memory loss,2 depression,3-6 and cognitive dysfunction.7-9 The long-term impact of hypoperfusion is a higher risk of stroke,10,11 vascular dementia,12,13 and Alzheimer’s disease.14-16
Life Extension® members will find comfort that their healthy lifestyle choices have been proven to help protect against hypoperfusion. We must never underestimate, however, the fragile nature of our aging circulatory systems.
This article represents a compilation of new findings that will profoundly change how neurodegenerative disease is viewed. It provides a rational basis to prevent and reverse the circulatory deficits that cripple and destroy our aging brains.
Don’t Let Your Brain Shrink!

Normal aging is associated with diminished blood flow to the brain. This pathology is known as hypoperfusion and causes cell injury and death.17
Hypertension (high blood pressure) accelerates brain atrophy in humans.18 It does this by damaging the cerebral circulatory system to the point that it cannot adequately transport blood.19,20
Blood vessels damaged by hypertension (and other factors) lose their ability to nourish cells, which can result in chronic hypoperfusion and loss of brain function.20
The combination of hypertension and hypoperfusion is associated with smaller brain volume.18
Once the cerebral vasculature is damaged, lowering blood pressure will not reverse brain shrinkage, and shrinkage may continue despite successful blood pressure control.20 The reason is that deformed and dysfunctional cerebral arteries may require higher blood pressure to avoid hypoperfusion.19 In other words, in some people with cerebrovascular damage, higher blood pressure may be needed to “squeeze” blood into their brain. This “squeezing” process results in additional blood vessel damage and increased stroke risk.19
While hypertension is a significant cause of arterial damage and hypoperfusion, aging humans have to do more than lower their blood pressure to reverse hypoperfusion.
Role Of Hypoperfusion In Alzheimer’s Disease

Hypoperfusion is no longer a controversial aspect of Alzheimer’s disease.15,21
Disrupted blood flow (hypoperfusion) is evident when Alzheimer’s manifests in its initial stage as mild cognitive impairment all the way to full-blown dementia.7,14-16,21
Hypoperfusion is also evident in cognitively healthy persons at high-risk for developing Alzheimer’s due to family history or genetic factors.21
Through the advent of advanced imaging technologies, it is now known that Alzheimer’s disease is associated with both global and regional cerebral hypoperfusion.21,22 Scientists have discovered that perfusion deficits in regions of the brain observed in Alzheimer’s disease patients are also present in people at increased risk for Alzheimer’s.21
While there is still debate as to whether decreased blood flow in Alzheimer’s is a cause or consequence of the disease, hypoperfusion is definitively associated with both structural and functional changes in the Alzheimer’s brain.21
Aging humans now have documented opportunities to aggressively explore treatments to prevent, or at least slow the progression of diseases like Alzheimer’s and stroke by guarding against hypoperfusion,also known as cerebrovascular insufficiency.
Hypoperfusion Associated With Reduced Memory Function
Metabolic syndrome is a cluster of cardiovascular risk factors that is also associated with cognitive decline and dementia.2
Common characteristics of metabolic syndrome include elevated glucose,23 high triglycerides,23 insulin resistance,24 abdominal obesity,23,24 low testosterone (in men),25,26 and hypertension.24
A study of late middle-aged adults showed that mean cerebral blood flow was 15% lower in those with metabolic syndrome compared to age-matched controls. The metabolic syndrome group also had lower immediate memory function. In this study,abdominal obesity and elevated triglycerides were most strongly associated with lower cerebral blood flow (hypoperfusion).2
Our “Tiny” Capillaries
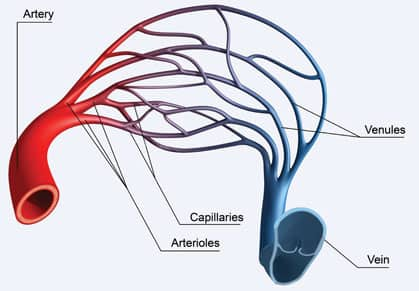 |
With each heartbeat, blood is thrust into arteries that branch into smaller arterioles that branch further into capillaries where they deliver oxygen and nutrients to cells.27 Even medically-educated individuals forget just how tiny capillaries that oxygenate our neurons really are.
A typical red blood cell is 6-10 micrometers, but capillary diameter is only 8-10 micrometers on average.27,28 Capillaries are so narrow that red blood cells often have to bend their shape to squeeze through them.29
Platelets are usually 2-4 micrometers,30 but anything that causes abnormal platelet clumping (thrombosis) creates a mass that cannot fit through thread-like capillaries.31 This helps explain how precarious our aging cerebral vascular system is and how readily hypoperfusion develops via disrupted capillary beds.
Not only are capillaries tiny, but they are also extremely delicate. Instead of the tough layers that make up arteries, capillaries consist only of a single layer of endothelial cells lying on a basement membrane.29,32 Hypertension destroys fragile capillaries leaving in its wake hypoperfused regions of the brain, often described as cerebral perfusion deficits .33
Capillaries surround neurons and diffuse oxygen and nutrients into them.33 Any interruption to capillary blood flow has the potential to injure or kill neurons.33 This is why hypoperfusion must be prevented or reversed if we are to preserve our cognitive integrity.
Abnormal platelet aggregation increases as humans age, which explains why thrombosis is an increasing threat with aging.34,35 Any blood particle larger than 5-10 micrometers can clog capillaries, and if enough capillaries become occluded in the brain, an ischemic stroke can occur.33
Risk factors in the blood that cause brain vasculature to become blocked include excess homocysteine,36 fibrinogen,37 C-reactive protein,38 and triglycerides.39 Homocysteine creates more havoc at the capillary level than it does in large blood vessels.40 Fibrinogen promotes occlusive thrombosis.41 Inflammation42 damages the delicate endothelium, and triglycerides clog capillary beds.43
Maintaining capillary integrity is essential to prevent hypoperfusion and the neurodegeneration that invariably accompanies it. Most Life Extension members already take steps to guard their overall health, which confers tremendous benefits in sustaining capillary blood flow, thus protecting against hypoperfusion.
Hypoperfusion Associated With Weakened Heart Function
A group of 211 men aged 68 went through a battery of tests to assess cognitive and cardiac function. These same men were tested 14 years later.44 Those with weakened hearts as measured on an echocardiogram and abnormal EKG patterns at baseline scored lower on verbal and speed-performance neurological tests. The doctors who conducted this study concluded that heart deficiencies in the study subjects were “associated with lower cognitive test results and may predict cognitive decline and silent cerebral perfusion abnormalities 14 years later.”44
Another study found reduced cerebral perfusion in elderly men with abnormal EKGs and nighttime blood pressure dipping. The doctors who conducted this study concluded:
“Silent myocardial ischemia may contribute to cerebrovascular disease in non-demented elderly men. Cerebral perfusion seems to be most vulnerable to myocardial ischemia in elderly with nocturnal blood pressure dipping.”45
These and other studies show that circulatory interruptions caused by even relatively mild cardiac disturbances deprive the brain of blood flow and result in cognitive impairments. So taking supplements like coenzyme Q10,46 lipoic acid,47 carnitine48,49 and PQQ50-53 not only help boost cardiac output to the brain, but also protect the brain and enhance mitochondrial energy production within brain cells (neurons).
Visualizing The Aging Brain

Advanced neuroimaging methods are enabling doctors to observe structural, functional, and biochemical changes in the brain, thus allowing earlier diagnosis of neurodegenerative diseases.54
A review of studies using enhanced neuroimaging techniques showed significant individual differences in the rate of cerebral aging (such as a decay of brain volume and reduction of blood flow) that accompanies loss of cognitive function.54
One neuroimaging study looked at degeneration in regions of the brain (frontal and temporal lobes) and their relationship with hypoperfusion. The researchers found worsening of frontal-temporal degeneration in response to lower cerebral blood flow. More severe hypoperfusion related to greater functional deficit.55
Preventing Progression To Senility
Mild cognitive impairment is considered an early stage of dementia. A group of researchers conducted a 3-year test and found the conversion rate from mild cognitive impairment to dementia was 11.65% each year.56
They found that cognitive decline and hypoperfusion were related to diabetes, carotid stenosis, and changes in the white matter area of the brain. The researchers conducting this study concluded:
“… our findings could imply that controlling blood glucose, removing carotid stenosis, and improving cerebral perfusion could be effective measures to delay cognitive decline in patients with mild cognitive impairment and prevent conversion from mild cognitive impairment to dementia.”56
Another study looked at structural alterations (such as amyloid beta deposition) and vascular organization in brains of aged monkeys and human Alzheimer’s brain tissue. The findings suggest that amyloid plaque brain formation relates to multiple underlying pathologies that occur in partnership with vascular or metabolic deficit.57 This data provides a mechanistic explanation for why senile plaques (as seen in Alzheimer’s) are present preferentially near the cerebral vasculature, and the importance of guarding against hypoperfusion.
Reversing Brain Damage In Former NFL Players
Brain injuries are common in professional football players and severe cases sometimes make headline news stories.59-62
A clinical trial was conducted on 30 retired NFL players who demonstrated brain damage and cognitive impairment. They underwent baseline testing of cognitive function and brain perfusion as measured by SPECT imaging.63
Participants were encouraged to lose weight (if appropriate) and take the following supplements for six months:
 |
Fish oil 64-66 |
1,720 mg EPA |
Vinpocetine 67-72 |
15 mg |
Ginkgo extract 73-78 |
120 mg |
Alpha Lipoic Acid 79-82 |
300 mg |
Acetyl L-Carnitine 80,83-85 |
1,000 mg |
Huperzine A 86-88 |
150 mcg |
N-acetyl-cysteine 89-93 |
600 mg |
High-potency multivitamin 94,95 |
|
The rationale behind using these nutrients was that they were individually shown to enhance blood flow, protect against free radicals, enhance brain cell membrane structure, boost acetylcholine, enhance neuronal metabolic activity, and reduce chronic inflammatory markers.
After six months, the tests were repeated. There were statistically significant increases in scores of attention, memory, reasoning, information processing speed, and accuracy in these retired NFL players. The SPECT scan showed increased perfusion in areas throughout much of the brain. The researchers who conducted this trial concluded:
“This study demonstrates that cognitive and cerebral blood flow improvements are possible in this group with multiple interventions.”63
Neurological trauma during football events accelerates brain aging. Life Extension members should be gratified to know that they have been taking most, if not all of the nutrients shown in this study to reverse brain damage in retired NFL players. This brain damage clearly linked hypoperfusion with cognitive impairment.
Tying This All Together
A review published in 2011 titled “Cerebral microvascular pathology and neurodegeneration” provided a meticulous description as to how cerebro- vascular dysfunction precedes and accompanies cognitive impairment and senility.58 What made this report stand out was that it utilized a novel micro-pathology technique to permit viewing the cerebral vasculature in a 3-diminsional setting.
This 2011 review detailed how perilous our cerebral blood supply becomes with aging, describing tortuous arterioles that barely transport blood, obliterated capillary beds that no longer nourish neurons, and thickened veins that impede blood flow. It went on to describe how hypoperfusion occurs early in Alzheimer’s and other degenerative brain disorders.58
Of interest was the demonstration of a decline in cerebral angiogenesis that precludes natural repair of vascular deficits—and the dangers of particles in the blood (such as circulating clots) that destroy capillary beds, all of which contribute to the hypoperfusion and other vascular deficits that underlie neurodegenerative disease.58 This review is available in full text for members to read at www.lifeextension.com/neuro.
An enormous volume of accumulated research reveals why virtually all aging humans suffer cognitive impairment, and why there are so many cases of crippling stroke and dementia.58
Aggressive intervention is clearly needed to protect our memories and very identities against the microvascular pathologies that have been accepted far too long as a hallmark of “normal” aging.
The encouraging news is that nutrients, hormones, and certain drugs that Life Extension members already take are proving more than ever to protect against cerebral circulatory deficits that occur in the aging brain.
Vinpocetine Reverses Cerebral Hypoperfusion
European doctors prescribe a periwinkle-originated drug called vinpocetine to patients suffering from cognitive problems ranging from short-term memory loss to Alzheimer’s dementia.
Vinpocetine exerts several anti-aging mechanisms, but its most profound effect may be its ability to interfere with processes associated with chronic cerebral hypoperfusion.103 The diverse mechanisms of vinpocetine explain its beneficial effects on clinical signs and symptoms of cerebrovascular insufficiency.
Life Extension has long been familiar with vinpocetine and has recommended it since the 1980s. The FDA tried to shut down our organization and incarcerate me for doing this. The FDA’s rationale was that vinpocetine was not an approved medicinal in the United States, even though it was safely and effectively being prescribed in Europe.
Fortunately, vinpocetine is now sold as a dietary supplement at a fraction of the price it would cost as an FDA-approved prescription drug. Life Extension members have obtained optimal daily doses of vinpocetine for the past three decades in a popular brain boosting formula they take.
Hypoperfusion Facilitates Alzheimer’s Disease
For years, neuroscientists have attributed Alzheimer’s disease to structural malformations observed in the brains of Alzheimer’s patients.96 Terms used to describe these Alzheimer’s alterations include beta-amyloid plaque and neurofibrillary tangles.
Newer findings, however, link hypoperfusion to the formation and progression of these Alzheimer’s malformations. One recent human study found cerebral blood flow to be 20% lower in Alzheimer’s patients compared to a similar aged group with normal cognitive function.97This correlates with other research showing that cerebral blood flow is decreased in Alzheimer’s patients.14-16
Mild cognitive impairment is the transitional clinical stage between loss of cognition in normal aging and severe dementia. Both Alzheimer’s disease and mild cognitive impairment have been linked to abnormalities in brain perfusion.98
A study evaluated brain perfusion in patients with mild Alzheimer’s dementia and patients with mild cognitive impairment, and compared them to cognitively healthy elderly controls. The researchers found lower cerebral perfusion throughout many regions of the brain in patients with mild cognitive impairment and Alzheimer’s and suggested that evaluating cerebral perfusion might better diagnose those with serious neurological impairment.99
In an intriguing study that shatters conventional wisdom, researchers identified elderly people that had significant amounts of beta-amyloid plaque and neurofibrillary tangles, but were not demented. The researchers compared these non-demented individuals to Alzheimer’s patients. The difference was the amount of amyloid plaque found in the vasculature was almost 2-fold higher in the Alzheimer’s patients. This led the scientists to conclude that in addition to Alzheimer’s structural abnormalities, “vascular integrity must play an important role in cognitive failure.”100
Another study performed mental tests and brain perfusion tests (SPECT scans) on normal elderly individuals, those with mild cognitive impairment, and those with Alzheimer’s patients. Over a two-year period, there was a worsening of the mental test scores in the two cognitively dysfunctional groups. In the mild cognitive impairment and Alzheimer’s groups, cerebral perfusion fell in the left postsubicular area of the brain.101 The postsubicular region is necessary for the recognition of familiar environments, and is required for the formation of new object–place associations that support recognition memory.102
This study showed that Alzheimer’s patients had extensive cerebral perfusion reductions. Worsening of mental test scores was related to decreased perfusion in multiple regions of the brain (bilateral middle, posterior cingulate, left frontal, temporal and parietal areas, and postsubicular area).101
This corroborates other studies that correlate cerebral hypoperfusion with diagnosis of Alzheimer’s disease.
Exercise Reverses Brain Decay
Several human studies show that aerobic exercise increases the size of the cognitive centers of the brain and improves memory.104,105
One study showed that 1-2 years of aerobic exercise increased hippocampal volume by 2%, which was accompanied by improved memory function.104 Considering hippocampal volume often shrinks with aging, this improvement in size should be viewed as substantial.
A review of several studies showed better physical fitness to be associated with improved cognitive functioning. This review showed that beneficial mechanisms behind the effect of exercise on cognitive health were “increases in brain perfusion and the ability of cerebral blood vessels to respond to demand.”105
Green Tea Inhibits Hypoperfusion Damage

Cerebral hypoperfusion results in oxidative stress that leads to neurodegenerative disease.
Health conscious people today take antioxidant supplements to protect against free radicals and the oxidative damage they inflict.
A study was done on rats where experimental cerebral hypoperfusion was induced and the effects of green tea extract evaluated.106 The scientists wanted to see if two different doses of green tea polyphenols over a 4-8 week time period could prevent cognitive deficits and the oxidative brain cell damage that occurs in response to hypoperfusion.
High-dose green tea extract was found to scavenge oxygen free radicals, enhance antioxidant potential, decrease lipid peroxide production, and reduce oxidative DNA damage. The high-dose group had better spatial learning and memory than saline-treated rats. These beneficial effects, however, were not found in the lower-dose group.106
The human equivalent amount of green tea extract in the high-dose group would be about 4,800 mg/day. The low dose human equivalent amount would be 1,200 mg of green tea extract daily.
The first supplement I take upon wakening is a 725 mg green tea extract capsule. There’s no particular reason for this, but since I don’t drink coffee or tea regularly, it seems to make sense to swallow a tea extract capsule when my day starts. To emulate this rat study, I would have to swallow six of these green tea extract capsules.
I do not believe, however, that I or most of our members need to take anywhere near this high dose of green tea. That’s because we take so many other antioxidants like gamma tocopherol,107 astaxanthin,108,109 benfotiamine,110 PQQ,53,111 lipoic acid,112,113 and carnosine114,115 that are proven to guard against oxidative stress in the brain.
So I will continue my one green tea extract capsule each morning and rely on the many other antioxidants I take to suppress the free radicals that are inevitably generated in my 59-year-old brain.
New Way To Protect Against Brain Aging
Proven methods exist to help reverse hypoperfusion and better oxygenate our brain.63 That alone, however, will not fully restore youthful cerebral functions. Additional pathologic mechanisms underlie age-associated mental impairment.54 These damaging factors should all be corrected if we are to achieve meaningful improvement in our thinking ability.
It is refreshing to know that studies are documenting the brain benefits of fish oil,116 carnitine,80 lipoic acid,80,84 vinpocetine,70,103,117-119 and other nutrients Life Extension members have long used.
What’s needed now is something to fill “missing gaps” that enable degenerative aging processes to destroy our precious neurons.
A solution has been found in an extract from an Oriental orchid called Gastrodia elata, which is used in China to treat neurological disorders,120 just as vinpocetine is prescribed in Europe for conditions relating to hypoperfusion.121
Gastrodia acts as a “brain shield,” calming neurons and protecting them from oxidant,122,123 inflammatory,120,124-127 and excitatory damage122,128-137 associated with hypoperfusion and stroke.122,136-142 As a result, Gastrodia helps prevent cognitive decline and memory loss.123,125,143-147
As you’ll read, Gastrodia has even been shown to protect against cognitive impairment inflicted during heart bypass surgery.
Surgery-Induced Hypoperfusion

Each year, hundreds of thousands of Americans undergo heart surgery that requires that they be placed on a heart-lung machine.148 A tragic side effect to this procedure is that it can cause capillary blockage in the brain that leads to hypoperfusion and severe cognitive deficits.149
Scientists have recently uncovered a unique reason why this occurs. During heart surgery, blood bleeding from surgical wounds is suctioned up into the cardio-pulmonary circuit of the heart-lung machine and then reintroduced into the patient.58
This suctioned blood is laden with lipids (fats), especially from the sternal bone marrow in the chest that has to be cut through to gain access to the heart. These lipid globules slip by the normal filters of the heart-lung machine and travel to the brain where they become lodged in capillaries as microemboli.58
While some of these microemboli pass through the brain in a few hours or days, some remain impacted for weeks or longer.58 These microemboli block capillary blood flow, causing hypoperfusion and eventual death to affected brain cells.
A novel method of protecting the brain against this type of hypoperfusion is to run suctioned blood through a special device called a “cell saver” that cleanses blood of lipids as it separates out red cells. This technique has been documented in experimental models to improve surgical outcomes.58
Some surgical patients undergo accelerated cognitive declines that can continue 3-5 years after heart surgeries and can lead to dementia.58,150 It is thus well worth implementing multiple strategies to protect against the hypoperfusion that results when lipid globules rapidly release into the bloodstream.
Gastrodia Extract Proven Under Toughest Conditions
A study of 200 cardiac surgery patients was done where prior to surgery, half the group was administered Gastrodia extract intravenously and the other half a placebo.151 Five different areas of cognitive function were measured before surgery began.
After the surgery and just prior to being discharged from the hospital, 42% of the placebo patients had a deficit in at least one area of cognitive measurement, which is about the standard number expected. In the group given Gastrodia extract, however, only 9% showed any evidence of cognitive impairment.151
A three-month follow-up evaluation showed that 31% of the placebo arm still had at least one cognitive deficit, as opposed to only 6% of patients given Gastrodia.151This follow-up reveals how long cognitive deficits persist in patients undergoing heart surgery and the statistically and clinically significant protection conferred by Gastrodia extract.
The kind of brain injury suffered during cardiac surgery is analogous to accelerated aging, though much worse in some ways. That’s because the sudden release of lipid globules is not a natural event that your body has a defense against. The most common natural type of emboli comes from blood clots that break lose inside blood vessels. Your body has enzymes that may dissolve these tiny blood clots, but not necessarily the lipid globules released during certain surgeries. While surgery-induced capillary impaction occurs acutely, its effects may persist indefinitely as chronically hypoperfused areas of the brain slowly die.58
The ability of Gastrodia to protect humans undergoing this massive attack of lipid (fat) globules signifies a tremendous ability of this orchid extract to protect against “normal” pathologies in the aging brain. These include inflammation, excitotoxicity, oxidation, hypoperfusion, and structural changes in neurons.120,122-137 The science, in fact, shows that Gastrodia provides a virtual “shield” against the most common causes of brain aging.
Gastrodia extract has been added to the most popular formula Life Extension members take to protect and enhance their neurological functions. It’s also available as a stand-alone supplement.
Our “Fragile” Aging Brains

The most important organ in our body is also the most fragile.
Stroke is a leading cause of death in the United States.152 Alzheimer’s incidence is spiraling upwards.153 Both are related to hypoperfusion, as is the mental slowdown that aging people encounter.
We will soon be publishing an article on a disease that virtually none of you knew existed. This disease (leukoaraiosis) involves deleterious changes in the brain’s vital white matter where transmission of nerve impulses enables one part of the brain to communicate with other parts of the brain.154 Enhanced imaging technologies are enabling doctors to identify this cognitive-robbing disorder in huge numbers of aging individuals.154,155 It shouldn’t surprise you to learn that an underlying culprit behind this white matter disorder is hypoperfusion.154 This means all the good steps you are taking to protect against known brain disorders may also shield you against this new one.
We’re also going to discuss the science behind keeping one’s overall neurological function in the most youthful condition possible, such as exercising your brain by reading articles like this that inundate you with new information.
An achievable New Year’s resolution is to take assertive steps to improve your cognitive function while slashing your risk of neurodegenerative disease. This article has provided practical steps that can be initiated immediately, including adding Gastrodia to one’s daily supplement program.
Time Of Year To Stock Up On Life-Saving Supplements
Once a year, we discount all of our cutting-edge formulas so that our members can stock up at extra-low prices.
We hope you’ll take advantage of this year’s Super Sale to obtain premium-grade supplements to protect your health today, while helping to support biomedical research aimed at achieving unprecedented life span extensions.
In 2012, Life Extension spent a record $14.6 million on some of the world’s most ambitious projects to halt aging and eliminate premature death. In this issue, we describe recent grants made to pioneering young scientists. These aggressive research programs are only made possible through the generous support of our many members.
I cannot tell you how much your support through product purchases is needed and appreciated to battle inept bureaucrats who would prefer our non-profit research foundation cease to exist.
Until February 3, 2014, members take advantage of Super Sale discounts to stock up on cutting-edge formulas designed to circumvent aging processes (including loss of neurological function) that used to be considered inevitable consequences of living too long!
For longer life,
William Faloon
References
- Available at: http://stroke.ahajournals.org/content/24/1/94.full.pdf. Accessed October 7, 2013.
- Birdsill AC, Carlsson CM, Willette AA, et al. Low cerebral blood flow is associated with lower memory function in metabolic syndrome. Obesity (Silver Spring). 2013 Jul;21(7):1313-20.
- Alosco ML, Spitznagel MB, Cohen R, et al. Reduced cerebral perfusion predicts greater depressive symptoms and cognitive dysfunction at a 1-year follow-up in patients with heart failure. Int J Geriatr Psychiatry. 2013 Sep 10.
- Bonne O, Krausz Y, Gorfine M, et al. Cerebral hypoperfusion in medication resistant, depressed patients assessed by Tc99m HMPAO SPECT. J Affect Disord. 1996 Dec 16;41(3):163-71.
- Grasso MG, Pantano P, Ricci M, et al. Mesial temporal cortex hypoperfusion is associated with depression in subcortical stroke. Stroke. 1994 May;25(5):980-5.
- Onoda K, Kuroda Y, Yamamoto Y, et al. Post-stroke apathy and hypoperfusion in basal ganglia: SPECT study. Cerebrovasc Dis. 2011 31(1):6-11.
- Farkas E, de Wilde MC, Kiliaan AJ, Luiten PG. Chronic cerebral hypoperfusion-related neuropathologic changes and compromised cognitive status: window of treatment. Drugs Today (Barc). 2002 May;38(5):365-76.
- Vicente E, Degerone D, Bohn L, et al. Astroglial and cognitive effects of chronic cerebral hypoperfusion in the rat. Brain Res. 2009 Jan 28;1251:204-12.
- Bennett SA, Tenniswood M, Chen JH, et al. Chronic cerebral hypoperfusion elicits neuronal apoptosis and behavioral impairment. Neuroreport. 1998 Jan 5;9(1):161-6.
- Hillis AE, Wityk RJ, Barker PB, et al. Subcortical aphasia and neglect in acute stroke: the role of cortical hypoperfusion. Brain. 2002 May;125(Pt 5):1094-104.
- Pullicino PM, McClure LA, Wadley VG, et al. Blood pressure and stroke in heart failure in the REasons for Geographic And Racial Differences in Stroke (REGARDS) study. Stroke. 2009 Dec;40(12):3706-10.
- Román GC. Brain hypoperfusion: a critical factor in vascular dementia. Neurol Res. 2004 Jul;26(5):454-8.
- Schuff N, Matsumoto S, Kmiecik J, et al. Cerebral blood flow in ischemic vascular dementia and Alzheimer’s disease, measured by arterial spin-labeling magnetic resonance imaging. Alzheimers Dement. 2009 Nov;5(6):454-62.
- de la Torre JC. Critical threshold cerebral hypoperfusion causes Alzheimer’s disease? Acta Neuropathol. 1999 Jul;98(1):1-8.
- Kim HA, Miller AA, Drummond GR, et al. Vascular cognitive impairment and Alzheimer’s disease: role of cerebral hypoperfusion and oxidative stress. Naunyn Schmiedebergs Arch Pharmacol. 2012 Oct;385(10):953-9.
- Nishimura T, Hashikawa K, Fukuyama H, et al. Decreased cerebral blood flow and prognosis of Alzheimer’s disease: a multicenter HMPAO-SPECT study. Ann Nucl Med. 2007 Jan;21(1):15-23.
- Liu Y, Zhu X, Feinberg D, et al. Arterial spin labeling MRI study of age and gender effects on brain perfusion hemodynamics. Magn Reson Med. 2012 Sep;68(3):912-22.
- Alosco ML, Brickman AM, Spitznagel MB, et al. Independent and interactive effects of blood pressure and cardiac function on brain volume and white matter hyperintensities in heart failure. J Am Soc Hypertens. 2013 Sep-Oct;7(5):336-43.
- Iadecola C, Davisson RL. Hypertension and cerebrovascular dysfunction. Cell Metab. 2008 Jun;7(6):476-84.
- Jennings JR, Mendelson DN, Muldoon MF, et al. Regional grey matter shrinks in hypertensive individuals despite successful lowering of blood pressure. J Hum Hypertens. 2012 May;26(5):295-305.
- Austin BP, Nair VA, Meier TB, et al. Effects of hypoperfusion in Alzheimer’s disease. J Alzheimers Dis. 2011;26 Suppl 3:123-33.
- Chen W, Song X, Beyea S, D’Arcy R, Zhang Y, Rockwood K. Advances in perfusion magnetic resonance imaging in Alzheimer’s disease. Alzheimers Dement . 2011 Mar;7(2):185-96.
- Pintó X, Corbella E, Valdivielso, Mostaza J. Prevalence of metabolic syndrome in hypertriglyceridaemic patients: higher than it may appear. Curr Med Res Opin. 2013 Oct 16.
- Tsujimura A, Miyagawa Y, Takezawa K, et al. Is low testosterone concentration a risk factor for metabolic syndrome in healthy middle-aged men? Urology. 2013 Oct;82(4):814-9.
- Rabijewski M, Papierska L, Kozakowski J, Zgliczynski W. The relationship between androgens concentrations (testosterone and dehydroepiandrosterone sulfate) and metabolic syndrome in non-obese elderly men. Endokrynol Pol. 2007 Nov-Dec;58(6):496-504.
- Deedwania PC, Gupta R. Management issues in the metabolic syndrome. J Assoc Physicians India. 2006 Oct;54:797-810.
- Available at: http://www.augustatech.edu/anatomy/chapter%2020.htm. Accessed October 22, 2013.
- Available at: http://education.mrsec.wisc.edu/36.htm. Accessed October 22, 2013.
- Available at: http://faculty.stcc.edu/aandp/ap/ap2pages/units18to20/vessels/capillar.htm. Accessed October 22, 2013.
- Available at: http://www.mdconsult.com/books/page.do?eid=4-u1.0-b978-1-4377-0974-2..00030-0&isbn=978-1-4377-0974-2&type=bookpage&from=content&uniqid=428111871-2. Accessed October 22, 2013.
- Rosolowsky M, Weiss HR. Effect of blood coagulation and platelet aggregation on perfusable capillaries and arterioles in ischemic and nonischemic myocardium. Microvasc Res. 1987 Jul;34(1):69-83.
- Available at: http://www.columbia.edu/~kj3/chapter6.html. Accessed October 22, 2013.
- Available at: http://www.jappl.org/content/100/1/328.full. Accessed October 22, 2013.
- Lin HC, Liu HW, Lin SF. Study on the effect of various conditions and age to blood platelet aggregation test. Gaoxiong Yi Xue Ke Xue Za Zhi. 1990 Dec;6(12):636-42.
- Available at: http://www.mdguidelines.com/arterial-embolism-and-thrombosis. Accessed October 22, 2013.
- Terwecoren A, Steen E, Benoit D, Boon P, Hemelsoet D. Ischemic stroke and hyperhomocysteinemia: truth or myth? Acta Neurol Belg. 2009 Sep;109(3):181-8.
- Tang WB, Li MX, Li GQ, Cai JD, Wei S, Wan YB. Changes of mean platelet volume, fibrinogen content and blood rheology in peripheral blood of youth patients with cerebral infarction. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2012 Apr;20(2):390-3.
- Eikelboom JW, Hankey GJ, Baker RI, et al. C-reactive protein in ischemic stroke and its etiologic subtypes. J Stroke Cerebrovasc Dis. 2003 Mar-Apr;12(2):74-81.
- Freiberg JJ, Tybjaerg-Hansen A, Jensen JS, Nordestgaard BG. Nonfasting triglycerides and risk of ischemic stroke--secondary publication. Ugeskr Laeger. 2009 Jun 22;171(26):2188-91.
- Feng C, Bai X, Xu Y, Hua T, Huang J, Liu XY. Hyperhomocysteinemia associates with small vessel disease more closely than large vessel disease. Int J Med Sci. 2013 10(4):408-12.
- Machlus KR, Cardenas JC, Church FC, Wolberg AS. Causal relationship betweenhyperfibrinogenemia, thrombosis, and resistance to thrombolysis in mice. Blood. 2011 May 5;117(18):4953-63.
- Apetrei E, Ciobanu-Jurcut R, Rugina M, Gavrila A, Uscatescu V. C-reactive protein, prothrombotic imbalance and endothelial dysfunction in acute coronary syndromes without ST elevation. Rom J Intern Med. 2004 42(1):95-102.
- Available at: http://atvb.ahajournals.org/content/22/2/211.full. Accessed October 23. 2013.
- Furuäng L, Wollmer P, Siennicki-Lantz A, Elmståhl S. Cardiac ventricular dimensions predict cognitive decline and cerebral blood flow abnormalities in aging men. BMC Geriatr. 2013 May 15;13:45.
- Furuäng L, Siennicki-Lantz A, Elmståhl S. Reduced cerebral perfusion in elderly men with silent myocardial ischaemia and nocturnal blood pressure dipping. Atherosclerosis. 2011 Jan;214(1):231-6.
- Matthews RT, Yang L, Browne S, Baik M, Beal MF. Coenzyme Q10 administration increases brain mitochondrial concentrations and exerts neuroprotective effects. Proc Natl Acad Sci U S A. 1998 Jul 21;95(15):8892-7.
- Hagen TM, Ingersoll RT, Lykkesfeldt J, Liu J, Wehr CM, Vinarsky V, Bartholomew JC, Ames AB. (R)-alpha-lipoic acid-supplemented old rats have improved mitochondrial function, decreased oxidative damage, and increased metabolic rate. FASEB J. 1999 Feb;13(2):411-8.
- Pesce V, Fracasso F, Cassano P, Lezza AM, Cantatore P, Gadaleta MN. Acetyl-L-carnitine supplementation to old rats partially reverts the age-related mitochondrial decay of soleus muscle by activating peroxisome proliferator-activated receptor gamma coactivator-1alpha-dependent mitochondrial biogenesis. Rejuvenation Res. 2010 Apr-Jun;13(2-3):148-51.
- Gomez LA, Heath SH, Hagen TM. Acetyl-L-carnitine supplementation reverses the age-related decline in carnitine palmitoyltransferase 1 (CPT1) activity in interfibrillar mitochondria without changing the L-carnitine content in the rat heart. Mech Ageing Dev. 2012 Feb-Mar;133(2-3):99-106.
- Zhu BQ, Zhou HZ, Teerlink JR, Karliner JS. Pyrroloquinoline quinone (PQQ) decreases myocardial infarct size and improves cardiac function in rat models of ischemia and ischemia/reperfusion. Cardiovasc Drugs Ther. 2004 Nov;18(6):421-31.
- Chowanadisai W, Bauerly KA, Tchaparian E, Wong A, Cortopassi GA, Rucker RB. Pyrroloquinoline quinone stimulates mitochondrial biogenesis through cAMP response element-binding protein phosphorylation and increased PGC-1a expression. J Biol Chem. 2010 Jan 1;285(1):142-52.
- Tao R, Karliner JS, Simonis U, et al. Pyrroloquinoline quinone preserves mitochondrial function and prevents oxidative injury in adult rat cardiac myocytes. Biochem Biophys Res Commun. 2007 Nov 16;363(2):257-62.
- Zhang Y, Feustel PJ, Kimelberg HK. Neuroprotection by pyrroloquinoline quinone (PQQ) in reversible middle cerebral artery occlusion in the adult rat. Brain Res. 2006 Jun 13;1094(1):200-6.
- Schuster L, Essig M, Schröder J. Normal aging and imaging correlations. Radiologe. 2011 Apr;51(4):266-72.
- Borroni B, Agosti C, Premi E, et al. The FTLD-modified Clinical Dementia Rating scale is a reliable tool for defining disease severity in frontotemporal lobar degeneration: evidence from a brain SPECT study. Eur J Neurol. 2010 May;17(5):703-7.
- Li L, Wang Y, Yan J, et al. Chongqing Aging Study Group. Clinical predictors of cognitive decline in patients with mild cognitive impairment: The Chongqing aging study. J Neurol. 2012 Jul;259(7):1303-11.
- Cai Y, Xiong K, Zhang XM, et al. β-Secretase-1 elevation in aged monkey and Alzheimer’s disease human cerebral cortex occurs around the vasculature in partnership with multisystem axon terminal pathogenesis and β-amyloid accumulation. Eur J Neurosci. 2010 Oct;32(7):1223-38.
- Brown WR, Thore CR. Review: cerebral microvascular pathology in ageing and neurodegeneration. Neuropathol Appl Neurobiol. 2011 Feb;37(1):56-74.
- Casson IR, Viano DC, Powell JW, Pellman EJ. Twelve years of national football league concussion data. Sports Health. 2010 Nov;2(6):471-83.
- Available at: http://www.deseretnews.com/article/383760/concussions-force-merril-hoge-to-retire.html?pg=all. Accessed October 22, 2013.
- Available at: http://articles.philly.com/1994-01-24/sports/25822971_1_troy-aikman-cowboys-coach-jimmy-johnson-cowboys-trainer-kevin-o-neill. Accessed October 22, 2013.
- Available at: http://articles.latimes.com/2000/jun/09/sports/sp-39252. Accessed October 22,2013.
- Amen DG, Wu JC, Taylor D, Willeumier K. Reversing brain damage in former NFL players: implications for traumatic brain injury and substance abuse rehabilitation. J Psychoactive Drugs. 2011 Jan-Mar;43(1):1-5.
- Bazan NG, Musto AE, Knott EJ. Endogenous signaling by omega-3 docosahexaenoic acid-derived mediators sustains homeostatic synaptic and circuitry integrity. Mol Neurobiol. 2011 Oct;44(2):216-22.
- Palacios-Pelaez R, Lukiw WJ, Bazan NG. Omega-3 essential fatty acids modulate initiation and progression of neurodegenerative disease. Mol Neurobiol. 2010 Jun;41(2-3):367-74.
- Pu H, Guo Y, Zhang W, et al. Omega-3 polyunsaturated fatty acid supplementation improves neurologic recovery and attenuates white matter injury after experimental traumatic brain injury. J Cereb Blood Flow Metab. 2013 Sep;33(9):1474-84.
- Szilágyi G, Nagy Z, Balkay L, et al. Effects of vinpocetine on the redistribution of cerebral blood flow and glucose metabolism in chronic ischemic stroke patients: a PET study. J Neurol Sci. 2005 Mar 15;229-230:275-84.
- Hadjiev D. Asymptomatic ischemic cerebrovascular disorders and neuroprotection with vinpocetine. Ideggyogy Sz. 2003 May 20;56(5-6):166-72.
- Vishnevskiĭ AA, Korotkevich IG, Zhaparalieva ChO. Membrane and functional effects of vinpocetine and tocopherol in rats with experimental cerebral ischemia. Biomed Khim. 2009 Sep-Oct;55(5):635-42.
- Valikovics A. Investigation of the effect of vinpocetine on cerebral blood flow and cognitive functions. Ideggyogy Sz. 2007 Jul 30;60(7-8):301-10.
- Gaal L, Molnar P. Effect of vinpocetine on noradrenergic neurons in rat locus coeruleus. Eur J Pharmacol. 1990 Oct 23;187(3):537-9.
- Santos MS, Duarte AI, Moreira PI, Oliveira CR. Synaptosomal response to oxidative stress: effect of vinpocetine. Free Radic Res. 2000 Jan;32(1):57-66.
- Chung HS, Harris A, Kristinsson JK, Ciulla TA, Kagemann C, Ritch R. Ginkgo biloba extract increases ocular blood flow velocity. J Ocul Pharmacol Ther. 1999 Jun;15(3):233-40.
- Bridi R, Crossetti FP, Steffen VM, et al. The antioxidant activity of standardized extract of Ginkgo biloba (EGb 761) in rats. Phytother Res 2001 15(5):449-51.
- Stoll S, Scheuer K, Pohl O, Müller WE. Ginkgo biloba extract (EGb 761) independently improves changes in passive avoidance learning and brain membrane fluidity in the aging mouse. Pharmacopsychiatry. 1996 Jul;29(4):144-9.
- Ihl R. Effects of Ginkgo biloba extract EGb 761(®) in dementia with neuropsychiatric features: review of recently completed randomised, controlled trials. Int J Psychiatry Clin Pract. 2013 Nov;17 Suppl 1:8-14.
- DeKosky ST, Williamson JD, Fitzpatrick AL, Kronmal RA, Ives DG, Saxton JA, et al; Ginkgo Evaluation of Memory (GEM) Study Investigators. Ginkgo biloba for prevention of dementia: a randomized controlled trial. JAMA. 2008 Nov 19;300(19):2253-62
- Ahlemeyer B, Krieglstein J. Neuroprotective effects of Ginkgo biloba extract. Cell Mol Life Sci. 2003 Sep;60(9):1779-92.
- Maczurek A, Hager K, Kenklies M, et al. Lipoic acid as an anti-inflammatory and neuroprotective treatment for Alzheimer’s disease. Adv Drug Deliv Rev. 2008 Oct-Nov;60(13-14):1463-70.
- Liu J, Head E, Gharib AM, et al. Memory loss in old rats is associated with brain mitochondrial decay and RNA/DNA oxidation: partial reversal by feeding acetyl-L-carnitine and/or R-alpha -lipoic acid. Proc Natl Acad Sci U S A. 2002 Feb 19;99(4):2356-61.
- Pershadsingh HA. Alpha-lipoic acid: physiologic mechanisms and indications for the treatment of metabolic syndrome. Expert Opin Investig Drugs. 2007 Mar;16(3):291-302.
- Astiz M, de Alaniz MJ, Marra CA. The oxidative damage and inflammation caused by pesticides are reverted by lipoic acid in rat brain. Neurochem Int. 2012 Dec;61(7):1231-41.
- Poon HF, Calabrese V, Calvani M, Butterfield DA. Proteomics analyses of specific protein oxidation and protein expression in aged rat brain and its modulation by L-acetylcarnitine: insights into the mechanisms of action of this proposed therapeutic agent for CNS disorders associated with oxidative stress. Antioxid Redox Signal. 2006 Mar-Apr;8(3-4):381-94.
- Long J, Gao F, Tong L, Cotman CW, Ames BN, Liu J. Mitochondrial decay in the brains of old rats: ameliorating effect of alpha-lipoic acid and acetyl-L-carnitine. Neurochem Res. 2009 Apr;34(4):755-63.
- Wilson AD, Hart A, Wiberg M, Terenghi G. Acetyl-l-carnitine increases nerve regeneration and target organ reinnervation - a morphological study. J Plast Reconstr Aesthet Surg. 2010 Jul;63(7):1186-95.
- Shang YZ, Ye JW, Tang XC. Improving effects of huperzine A on abnormal lipid peroxidation and superoxide dismutase in aged rats. Zhongguo Yao Li Xue Bao. 1999 Sep;20(9):824-8.
- Zhang HY, Yan H, Tang XC. Non-cholinergic effects of huperzine A: beyond inhibition of acetylcholinesterase. Cell Mol Neurobiol. 2008 Feb;28(2):173-83.
- Wang J, Zhang HY, Tang XC. Huperzine a improves chronic inflammation and cognitive decline in rats with cerebral hypoperfusion. J Neurosci Res. 2010 Mar;88(4):807-15.
- Hoffer ME, Balaban C, Slade MD, Tsao JW, Hoffer B. Amelioration of acute sequelae of blast induced mild traumatic brain injury by N-acetyl cysteine: a double-blind, placebo controlled study. PLoS One. 2013;8(1):e54163.
- Holmay MJ, Terpstra M, Coles LD, et al. N-acetylcysteine boosts brain and blood glutathione in Gaucher and Parkinson diseases. Clin Neuropharmacol . 2013 Jul-Aug;36(4):103-6.
- Pawlas N, Małecki A. Neuroprotective effect of N-acetylcysteine in neurons exposed to arachidonic acid during simulated ischemia in vitro. Pharmacol Rep. 2009 Jul-Aug;61(4):743-50.
- Wang X, Svedin P, Nie C, et al. N-acetylcysteine reduces lipopolysaccharide-sensitized hypoxic-ischemic brain injury. Ann Neurol. 2007 Mar;61(3):263-71.
- Khan M, Sekhon B, Jatana M, et al. Administration of N-acetylcysteine after focal cerebral ischemia protects brain and reduces inflammation in a rat model of experimental stroke. J Neurosci Res. 2004 May 15;76(4):519-27.
- Kamphuis PJ, Scheltens P. Can nutrients prevent or delay onset of Alzheimer’s disease? J Alzheimers Dis. 2010;20(3):765-75.
- Kidd PM. Alzheimer’s disease, amnestic mild cognitive impairment, and age-associated memory impairment: current understanding and progress toward integrative prevention. Altern Med Rev. 2008 Jun;13(2):85-115.
- Ramani A, Jensen JH, Helpern JA. Quantitative MR imaging in Alzheimer disease. Radiology. 2006 Oct;241(1):26-44.
- Roher AE, Debbins JP, Malek-Ahmadi M, et al. Cerebral blood flow in Alzheimer’s disease. Vasc Health Risk Manag. 2012 8:599-611.
- Johnson NA, Jahng GH, Weiner MW, et al. Pattern of cerebral hypoperfusion in Alzheimer disease and mild cognitive impairment measured with arterial spin-labeling MR imaging: initial experience. Radiology. 2005 Mar;234(3):851-9.
- Alexopoulos P, Sorg C, Förschler A, et al. Perfusion abnormalities in mild cognitive impairment and mild dementia in Alzheimer’s disease measured by pulsed arterial spin labeling MRI. Eur Arch Psychiatry Clin Neurosci. 2012 Feb;262(1):69-77.
- Maarouf CL, Daugs ID, Kokjohn TA, et al. Alzheimer’s disease and non-demented high pathology control nonagenarians: comparing and contrasting the biochemistry of cognitively successful aging. PLoS One. 2011 6(11):e27291.
- Alegret M, Cuberas-Borrós G, Vinyes-Junqué G, et al. A two-year follow-up of cognitive deficits and brain perfusion in mild cognitive impairment and mild Alzheimer’s disease. J Alzheimers Dis. 2012 30(1):109-20.
- Available at: http://www.jneurosci.org/content/33/16/6928.short. AccessedOctober 7, 2013.
- Bagoly E, Fehér G, Szapáry L. The role of vinpocetine in the treatment of cerebrovascular diseases based in human studies. Orv Hetil. 2007 Jul 22;148(29):1353-8.
- Erickson KI, Voss MW, Prakash RS, et al. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci U S A. 2011 Feb 15;108(7):3017-22.
- Davenport MH, Hogan DB, Eskes GA, Longman RS, Poulin MJ. Cerebrovascular reserve: the link between fitness and cognitive function? Exerc Sport Sci Rev. 2012 Jul;40(3):153-8.
- Xu Y, Zhang JJ, Xiong L, Zhang L, Sun D, Liu H. Green tea polyphenols inhibit cognitive impairment induced by chronic cerebral hypoperfusion via modulating oxidative stress. J Nutr Biochem. 2010 Aug;21(8):741-8.
- Williamson KS, Gabbita SP, Mou S et al. The nitration product 5-nitro-gamma-tocopherol is increased in the Alzheimer brain. Nitric Oxide. 2002 Mar;6(2):221-7.
- Hussein G, Sankawa U, Goto H, Matsumoto K, Watanabe H. Astaxanthin, a carotenoid with potential in human health and nutrition. J Nat Prod. 2006 Mar;69(3):443-9.
- Mattei R, Polotow TG, Vardaris CV, et al. Astaxanthin limits fish oil-related oxidative insult in the anterior forebrain of Wistar rats: putative anxiolytic effects? Pharmacol Biochem Behav. 2011 Sep;99(3):349-55.
- Wu S, Ren J. Benfotiamine alleviates diabetes-induced cerebral oxidative damage independent of advanced glycation end-product, tissue factor and TNF-alpha. Neurosci Lett. 2006 Feb 13;394(2):158-62.
- Ohwada K, Takeda H, Yamazaki M, et al. Pyrroloquinoline quinone (PQQ) prevents cognitive deficit caused by oxidative stress in rats. J Clin Biochem Nutr. 2008 Jan;42:29-34.
- Lovell MA, Xie C, Xiong S, Markesbery WR. Protection against amyloid beta peptide and iron/hydrogen peroxide toxicity by alpha lipoic acid. J Alzheimers Dis. 2003 Jun;5(3):229-39.
- Shirpoor A, Minassian S, Salami S, Khadem-Ansari MH, Yeghiazaryan M. Alpha--lipoic acid decreases DNA damage and oxidative stress induced by alcohol in the developing hippocampus and cerebellum of rat. Cell Physiol Biochem. 2008;22(5-6):769-76.
- Hipkiss AR, Preston JE, Himswoth DT, Worthington VC, Abbot NJ. Protective effects of carnosine against malondialdehyde-induced toxicity towards cultured rat brain endothelial cells. Neurosci Lett. 1997 Dec 5;238(3):135-8.
- Rajanikant GK, Zemke D, Senut MC, et al. Carnosine is neuroprotective against permanent focal cerebral ischemia in mice. Stroke. 2007 Nov;38(11):3023-31.
- Morris MC, Evans DA, Bienias JL, et al. Consumption of fish and n-3 fatty acids and risk of incident Alzheimer disease. Arch Neurol. 2003 Jul;60(7):940-6.
- Patyar S, Prakash A, Modi M, Medhi B. Role of vinpocetine in cerebrovascular diseases. Pharmacol Rep. 2011 63(3):618-28.
- Bönöczk P, Panczel G, Nagy Z. Vinpocetine increases cerebral blood flow and oxygenation in stroke patients: a near infrared spectroscopy and transcranial Doppler study. Eur J Ultrasound. 2002 Jun;15(1-2):85-91.
- Solanki P, Prasad D, Muthuraju S, Sharma AK, Singh SB, Ilavzhagan G. Preventive effect of piracetam and vinpocetine on hypoxia-reoxygenation induced injury in primary hippocampal culture. Food Chem Toxicol. 2011 Apr;49(4):917-22.
- Kim BW, Koppula S, Kim JW, et al. Modulation of LPS-stimulated neuroinflammation in BV-2 microglia by Gastrodia elata: 4-hydroxybenzyl alcohol is the bioactive candidate. J Ethnopharmacol. 2012 Jan 31;139(2):549-57.
- Available at: http://ntp.niehs.nih.gov/ntp/htdocs/chem_background/exsumpdf/vinpocetine091613_508.pdf. Accessed October 23, 2013.
- Kim HJ, Hwang IK, Won MH. Vanillin, 4-hydroxybenzyl aldehyde and 4-hydroxybenzyl alcohol prevent hippocampal CA1 cell death following global ischemia. Brain Res. 2007 Nov 21;1181:130-41.
- Zhao X, Zou Y, Xu H, et al. Gastrodin protect primary cultured rat hippocampal neurons against amyloid-beta peptide-induced neurotoxicity via ERK1/2-Nrf2 pathway. Brain Res. 2012 Oct 30;1482:13-21.
- Ahn EK, Jeon HJ, Lim EJ, Jung HJ, Park EH. Anti-inflammatory and anti-angiogenic activities of Gastrodia elata Blume. J Ethnopharmacol. 2007 Apr 4;110(3):476-82.
- Dai JN, Zong Y, Zhong LM, et al. Gastrodin inhibits expression of inducible NO synthase, cyclooxygenase-2 and proinflammatory cytokines in cultured LPS-stimulated microglia via MAPK pathways. PLoS One. 2011 6(7):e21891.
- Du X, Mao R, Liu Y, Li Y, Shan Y. Gastrodine represses expression of IL-1 beta, IL-6 induced by hyperglycemia in gitter cells. Zhongguo Zhong Yao Za Zhi. 2009 Jun;34(12):1535-9.
- Hwang SM, Lee YJ, Kang DG, Lee HS. Anti-inflammatory effect of Gastrodia elata rhizome in human umbilical vein endothelial cells. Am J Chin Med. 2009 37(2):395-406.
- An SJ, Park SK, Hwang IK, et al. Gastrodin decreases immunoreactivities of gamma-aminobutyric acid shunt enzymes in the hippocampus of seizure-sensitive gerbils. J Neurosci Res. 2003 Feb 15;71(4):534-43.
- Bie X, Chen Y, Han J, Dai H, Wan H, Zhao T. Effects of gastrodin on amino acids after cerebral ischemia-reperfusion injury in rat striatum. Asia Pac J Clin Nutr. 2007 16 Suppl 1:305-8.
- Fu L, Mao YH, Gao Y, Liu L, Wang ZP, Li LC. Expression of NR1 mRNA of NMDA receptor by gastrodine on hypoxia injury in cultured rat cerebral cortical neurons. Zhongguo Zhong Yao Za Zhi. 2008 May;33(9):1049-52.
- Ha JH, Lee DU, Lee JT, et al. 4-Hydroxybenzaldehyde from Gastrodia elata B1. is active in the antioxidation and GABAergic neuromodulation of the rat brain. J Ethnopharmacol. 2000 Nov;73(1-2):329-33.
- Ha JH, Shin SM, Lee SK, et al. In vitro effects of hydroxybenzaldehydes from Gastrodia elata and their analogues on GABAergic neurotransmission, and a structure-activity correlation. Planta Med. 2001 Dec;67(9):877-80.
- Jung JW, Yoon BH, Oh HR, et al. Anxiolytic-like effects of Gastrodia elata and its phenolic constituents in mice. Biol Pharm Bull. 2006 Feb;29(2):261-5.
- Shin EJ, Bach JH, Nguyen TT, et al. Gastrodia elata Bl attenuates cocaine-induced conditioned place preference and convulsion, but not behavioral sensitization in mice: Importance of GABA(A) receptors. Curr Neuropharmacol. 2011 Mar;9(1):26-9.
- Shuchang H, Qiao N, Piye N, Mingwei H, Xiaoshu S, Feng S, Sheng W, Opler M. Protective effects of gastrodia elata on aluminium-chloride-induced learning impairments and alterations of amino acid neurotransmitter release in adult rats. Restor Neurol Neurosci. 2008 26(6):467-73.
- Xu X, Lu Y, Bie X. Protective effects of gastrodin on hypoxia-induced toxicity in primary cultures of rat cortical neurons. Planta Med. 2007 Jun;73(7):650-4.
- Zeng X, Zhang Y, Zhang S, Zheng X. A microdialysis study of effects of gastrodin on neurochemical changes in the ischemic/reperfused rat cerebral hippocampus. Biol Pharm Bull. 2007 Apr;30(4):801-4.
- Descamps E, Petrault-Laprais M, Maurois P, et al. Experimental stroke protection induced by 4-hydroxybenzyl alcohol is cancelled by bacitracin. Neurosci Res. 2009 Jun;64(2):137-42.
- Kam KY, Yu SJ, Jeong N, et al. p-Hydroxybenzyl alcohol prevents brain injury and behavioral impairment by activating Nrf2, PDI, and neurotrophic factor genes in a rat model of brain ischemia. Mol Cells. 2011 Mar;31(3):209-15.
- Yu SS, Zhao J, Zheng WP, Zhao Y. Neuroprotective effect of 4-hydroxybenzyl alcohol against transient focal cerebral ischemia via anti-apoptosis in rats. Brain Res. 2010 Jan 13;1308:167-75.
- Zeng X, Zhang S, Zhang L, Zhang K, Zheng X. A study of the neuroprotective effect of the phenolic glucoside gastrodin during cerebral ischemia in vivo and in vitro. Planta Med. 2006 Dec;72(15):1359-65.
- Zhang CY, Du GY, Wang W, et al. Effects of tianma gouteng fang on transmitter amino acids in the hippocampus extracellular liquids in freely moving rats subjected to brain ischemia. Zhongguo Zhong Yao Za Zhi. 2004 Nov;29(11):1061-5.
- An H, Kim IS, Koppula S, et al. Protective effects of Gastrodia elata Blume on MPP+-induced cytotoxicity in human dopaminergic SH-SY5Y cells. J Ethnopharmacol. 2010 Jul 20;130(2):290-8.
- Kim IS, Choi DK, Jung HJ. Neuroprotective effects of vanillyl alcohol in Gastrodia elata Blume through suppression of oxidative stress and anti-apoptotic activity in toxin-induced dopaminergic MN9D cells. Molecules. 2011 16(7):5349-61.
- Liu ZH, Hu HT, Feng GF, Zhao ZY, Mao NY. Protective effects of gastrodin on the cellular model of Alzheimer’s disease induced by Abeta25-35. Sichuan Da Xue Xue Bao Yi Xue Ban. 2005 Jul;36(4):537-40.
- Mishra M, Huang J, Lee YY, et al. Gastrodia elata modulates amyloid precursor protein cleavage and cognitive functions in mice. Biosci Trends. 2011 5(3):129-38.
- Ramachandran U, Manavalan A, Sundaramurthi H, et al. Tianma modulates proteins with various neuro-regenerative modalities in differentiated human neuronal SH-SY5Y cells. Neurochem Int. 2012 Jun;60(8):827-36.
- Epstein AJ, Polsky D, Yang F, Yang L, Groeneveld PW. Coronary revascularization trends in the United States, 2001-2008. JAMA. 2011 May 4;305(17):1769-76.
- Available at: http://digitalcommons.liberty.edu/cgi/viewcontent.cgi?article=1259&context=honors. Accessed October 7, 2013.
- Newman MF, Kirchner JL, Phillips-Bute B, et al. Longitudinal assessment of neurocognitive function after coronary-artery bypass surgery. N Engl J Med. 2001 Feb 8;344(6):395-402.
- Zhang Z, Ma P, Xu Y, Zhan M, Zhang Y, Yao S, Zhang S. Preventive effect of gastrodin on cognitive decline after cardiac surgery with cardiopulmonary bypass: a double-blind, randomized controlled study. J Huazhong Univ Sci Technolog Med Sci. 2011 Feb;31(1):120-7.
- Available at: http://stroke.ahajournals.org/content/42/8/2351.full.pdf. Accessed October 7, 2013.
- Hebert LE, Weuve J, Scherr PA, Evans DA. Alzheimer disease in the United States (2010-2050) estimated using the 2010 census. Neurology. 2013 May 7;80(19):1778-83.
- Pantoni L, Garcia JH. Pathogenesis of leukoaraiosis: a review. Stroke. 1997 Mar;28(3):652-9.
- O’Sullivan M. Leukoaraiosis. Pract Neurol. 2008 Feb;8(1):26-38.

