Life Extension Magazine®

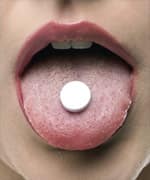
Ask a lay person what to take if you develop cold symptoms, and they will often suggest zinc lozenges.
This recommendation is based on the many media reports surrounding the use of zinc lozenges in preventing or shortening the duration of the common cold.
For too many people, however, zinc lozenges have not worked to alleviate their cold symptoms.
To get to the bottom of this, Life Extension® reached out to George Eby, the scientist who discovered that zinc can eradicate cold symptoms. George Eby has spent decades perfecting the most effective and palatable form and dose of zinc lozenge.
In this report, George Eby relates his serendipitous discovery, and the many avenues of investigative research required to identify how to make zinc effective against cold viruses when administered in lozenge form.
In 1984, an article I wrote about zinc lozenges and the common cold was published in a cancer journal called Antimicrobial Agents and Chemotherapy.1
In 2014, a positive article on zinc and common colds was published in the Journal of the American Medical Association.2 Why did it take 30 years for zinc to be accepted as a viable treatment for common colds?
In this brief article, I won’t delve into a nitty-gritty discussion of each and every clinical trial. Rather, I will cover subjects that I think are of general interest. I refer the academic reader to the most accurate review of the subject ever written, my 2010 article titled “Zinc Lozenges as Cure for the Common Cold—A Review and Hypothesis,”3 and my 1994 book on the subject, Handbook for Curing the Common Cold.4 My review article and handbook show that efficacy depends totally on the amount of ionic zinc (iZn) available in the lozenges when used nine times per day (every two wakeful hours). How it works remains to be determined, although a number of possibilities exist.
Positively charged ionic zinc (iZn), but not bound (neutrally charged) zinc, is a strongly astringent, anti-rhinoviral that:
- Increases interferon-gamma (IFN-g) 10-fold,
- Inhibits intercellular adhesion molecule-1 (ICAM-1), and
- Inhibits the release of the vasoactive compound (histamine) from mast cell granules.3, 4
Interestingly, one 18.75 mg zinc acetate lozenge in the morning and one in the evening controls most allergy symptoms, especially throat congestion. Also, iZn protects cell membranes from a variety of assaults, ranging from viruses to venoms.5 My family has used topically applied supersaturated solutions of zinc gluconate to immediately terminate the sting of yellow jackets, bees, wasps, scorpions, and Portuguese man-of-wars. It quickly heals brown recluse spider bites. I have a friend who has used it topically to effectively treat rattlesnake bites in horses and dogs. It clearly has general anti venom properties.6
But back to the common cold: The main indicator that a zinc lozenge is releasing ionic zinc (iZn) is when a drying and astringent sensation occurs in the mouth. Zinc acetate releases 100% of its zinc as iZn and it can be made into a pleasant-tasting, but astringent and drying, lozenge. It is imperative that strongly astringent and drying zinc acetate lozenges be used if maximum efficacy and pleasant taste are desired.
Ionization is the process that occurs when an atom or a molecule acquires a positive or negative charge by gaining or losing electrons.7 You’ll read why this is important when attacking cold viruses later in this article.
A Serendipitous Discovery
Let me start my remarks with the comment that I was not smart enough to “figure out” how to cure the common cold. Rather, it was discovered totally by accident. When my daughter, Karen, was 3-years old in 1979, she was diagnosed with acute lymphocytic leukemia (T-cell). Upon her diagnosis, I immediately realized that zinc was critical for her survival since she had abnormally low zinc in her blood and zinc was vital to the T-cell lymphocytes,8 and perhaps vital to obtain a rapid remission and recovery.
From the first day of diagnosis, I regularly administered zinc and other supplemental nutrients to her. She was totally free of leukemic blast cells two-weeks after diagnosis and being given chemotherapy, radiation, and zinc which was unheard of at that time. Eventually, I published my hypothesis about zinc being vital for a rapid recovery from leukemia.9,10
Since leukemic children have impaired immune systems due to both the disease and the chemotherapy, a common cold could lead to serious trouble. My daughter developed a horrible cold, and her doctor told us that it might last for months, maybe even until she was taken off of chemotherapy in three years! One afternoon, several months after her diagnosis, she was extremely tired and wanted to take a nap, but it was also time for her to take her 50 mg zinc gluconate tablet. She told me that her throat hurt too much to swallow the pill, so I told her to crush it with her teeth, hold the crushed particles in her mouth, and take a nap. Several hours later, she got up from her nap and was playing and smiling and looked and acted totally well. I told her to go back to bed, and she told me, “No, Daddy, zinc cured my cold.”
There you have it, the cure for the common cold was discovered by a 3-year-old girl with leukemia. How did her leukemia turn out? She ran an 11-minute mile a few months later. She said she could run faster if her legs were longer! She never relapsed. Did physicians pay any attention to my article? No.
Testing The Theory

I tried the same zinc lozenge for common cold experiment with other members of my family, friends, and co-workers at the Texas Department of Health in Austin, Texas. It was always amazingly effective, and the Commissioner of the Department of Health, Dr. Robert Bernstein, MD, suggested to me that I should recruit a local physician and a scientist or two from the University of Texas in Austin to conduct a double-blind clinical trial. Dr. William W. Halcomb, an Austin osteopathic physician, and Ronald R. Davis, PhD, of the Clayton Foundation Biochemical Institute, University of Texas at Austin, decided they would like to participate.
Eventually, we ran the clinical trial in Dr. Halcomb’s office with the help of local TV and radio stations that recruited patients for an “amazing research project.” We ran two clinical trials, one with the 23 mg zinc gluconate tablets (used as throat lozenges), which were only one-half as potent as what our previous experience indicated was needed, and the other trial with 37 mg zinc orotate lozenges used with 10 mmol zinc (gluconate) nasal spray. Upon analysis, we found that the 23 mg zinc gluconate lozenges used every two hours (nine lozenges per day) shortened common colds by about seven days, with strong statistical significance (P=0.001).1 On the other hand, the zinc orotate lozenge and zinc nasal spray study showed zero results. We were hoping that the zinc orotate lozenges would work since they were more palatable, but zinc orotate releases zero iZn, explaining lack of efficacy. Years later, we submitted the results for publication to Alternative Therapies in Health and Medicine.11
Before the zinc gluconate article was accepted and published, I sent a courtesy copy to Jack Merit Gwaltney Jr., MD, the Director of the Department of Internal Medicine, University of Virginia School of Medicine, Charlottesville, Virginia. He ran a common cold research unit there, and was considered to be the leading common cold researcher in the United States. A few days later, he called me on the phone and was very flattering in his comments about my research. We talked for about a half an hour, and he always came back to say how much he liked the article. However, he was a bit concerned that our placebo matching wasn’t as good as he would have liked since our placebos tasted a bit better than the zinc gluconate lozenges. He suggested that it might be best for me to withdraw my article from consideration for publication, and he would run a new study with a better placebo. I thought about his kind offer, but rejected it since it would postpone publication for at least a year. When I told him no, he became angry, and told me that I would definitely need his help. I have always wondered if my rejecting his kind offer had anything to do with the 30-year delay in obtaining widespread acceptance of my discovery. Perhaps it’s not widespread yet, but zinc for common colds did get favorable mention in the April 2014 issue of the ultra-conservative Journal of the American Medical Association.2
What You Need To Know
 |
Zinc Lozenges And The Common Cold
- In 1979, George Eby gave his daughter, who had leukemia and was suffering from a bad cold, a crushed 50 mg zinc gluconate tablet. Several hours later, her cold was completely gone.
- Clinical trials revealed that 23 mg zinc gluconate lozenges used every two waking hours (nine a day) shortened the common cold by about seven days.
- Dr. Ananda Prasad, considered the father of zinc biochemistry, demonstrated zinc acetate shortened cold duration by three days in three separate trials.
- Taken at the first sign of a cold, typically a scratchy throat, zinc lozenges taken every two hours abort a cold by the end of the first day, earning them a special place in your supplement regimen.
The Media Frenzy
Upon publication of our article in 1984,1 we received substantial press coverage and interest by scientists with professional involvement in common cold research. One scientist was Dr. Rinaldo Pellegrini, the Medical-Scientific Director of RBS Pharma-Milan in Milan, Italy. He took the time to visit us in Austin and to listen to our warnings about metallic chelators and the vital solution chemistry of zinc. He agreed to replicate the solution chemistry of our lozenges. No attempt was made to replicate the solution chemistry of our zinc gluconate lozenges by anyone except Dr. Pellegrini, who made the flavor-masked zinc gluconate in 1 gram fructose-based compressed lozenges using a binder with properties similar to high molecular weight hydroxypropyl methylcellulose. Those lozenges were successfully demonstrated by the British Medical Research Council Common Cold Unit in Salisbury, England (MRC), the world’s leading authority in common cold research. The lozenges were quite astringent. Results of that study were similar to our original results, since the products being tested were quite similar in release of iZn.3,4,12 The lozenges never went to market since the company was sold and the new owners were not interested in zinc lozenges for colds.
Commercial Products That Failed

The Quigley Corporation was making and selling zinc gluconate lozenges. There were several studies done on these lozenges, with two showing success, 13,14 while two failed.15,16 Another company, Bristol Myers Squibb, came up with a very pleasant-tasting hard candy zinc gluconate lozenge that was flavor-masked with lemon and citric acid. Their lozenges failed in a clinical trial done at the Department of Internal Medicine, University of Virginia School of Medicine, Charlottesville, Virginia.17 Interestingly, of the many zinc lozenges on the market in the United States, several contain citric acid and release no iZn, thus lacking any efficacy. Warner Lambert Company also wanted to market a zinc lozenge; unfortunately, it too failed in clinical trials.16 Their hard candy zinc acetate lozenges contained 5 or 11.5 mg of zinc. They were flavor-masked with partially hydrogenated cottonseed oil and/or palm kernel oil and soy lecithin. They thought it released iZn, but I am certain that they did not, mainly since there was no hint of oral astringency.
Over the years, there were a number of other scientists and companies that wanted in on producing a zinc lozenge for the common cold. These companies and scientists published both positive and negative reports, the efficacy of which I found to be closely related to the availability of iZn.3,4 Perhaps the most interesting of these was the 1987 study by Robert M. Douglas of the Australian National University, in Canberra, Australia, as it increased the duration of colds by 4.4 days.18 They used “effervescent” 10 mg zinc acetate lozenges. I wrote them to find out what caused the effervescence, and they indicated that they used tartaric acid and sodium bicarbonate.3 Zinc acetate dissociates in the presence of these added ingredients and forms several tightly bound reaction products including zinc carbonate, which is insoluble and nonionizable, plus negatively charged zinc tartrate. These zinc lozenges appear to have released sufficient negatively charged zinc that neutralized native iZn from mast cell granules (allergy symptom-inducing) of the infected nasal epithelium, resulting in significantly worsened cold symptoms. 3 This is why care should be taken in one’s selection of zinc lozenges.
Developing An Effective Lozenge

Around 1990, I developed pleasant-tasting zinc acetate lozenges, which released all of their zinc as iZn, and submitted them to three independent clinical trials. The first clinical trial was conducted in 1998 by Edward Petrus and his colleagues.19 Using 9 mg zinc acetate compressed tablet lozenges, they found significant reductions in mean duration. What was really interesting about this study is that in people with allergy symptoms, the results were twice as good as in those without allergy symptoms. The second and third clinical trials were conducted by Ananda S. Prasad, MD, PhD, and colleagues, of Wayne State University in Detroit, Michigan. In 2000, he was appointed Distinguished Professor of Medicine, Division of Hematology-Oncology. He has long been considered the father of zinc biochemistry in humans, having discovered the essentiality of zinc in the human diet in 1963.20 His first study in 2000 used 12.8 mg of zinc acetate, and these compressed tablet lozenges shortened colds by 3.6 days and reduced severity of colds with strong statistical significance (P < 0.002).21 I still have some of those zinc acetate lozenges, and they still taste the same and are still astringent. Dr. Prasad’s second study was conducted in 2008 and the lozenges contained 13.3 mg of zinc acetate. Mean duration of colds was reduced by about three days (4.0 vs. 7.1 days; P < .0001).22 Theses lozenges were also perceived to be pleasant tasting, but astringent. The zinc acetate lozenges being introduced in this publication are the only zinc lozenges to have ever been shown effective in three independent clinical trials.
Why It Took So Long
Back to the question, why has it taken 30 years for acceptance? First, I am not certain that a single positive article published in JAMA means “acceptance.” It just means we have broken the ice a bit with establishment medicine. The question really has two basic problems.
The first problem is the lack of understanding as to what solution equilibrium chemistry is all about. This is the science that allows the calculation of the availability of ionic zinc at physiologic pH 7.4. This science has found that zinc acetate releases 100% of its zinc as iZn. Zinc gluconate releases 72% as iZn. Zinc gluconate-glycine releases 57% (or less) iZn, while zinc gluconate-citrate releases zero iZn.3,23,24 This particular science is far outside the education of biologists, bio-chemists, physicians, and others who have responsibility in common cold research. Unless researchers use zinc acetate with a tablet base of nothing but directly compressible dextrose and some flavor oils (peppermint) plated onto silica gel, they are not likely to find efficacy if they also want a pleasant-tasting zinc lozenge. Of course, there needs to be enough zinc acetate to do the job, and I have found that 18.75 mg of zinc (from zinc acetate) is enough. You can see the effect of iZn on average duration of colds in the chart on page 76. How do I know about such an esoteric subject? I needed to know, and I was open to suggestion. One day, about 1993, I was invited by Guy Berthon, PhD, to prepare a paper for publication in his new book about metal-ligand interactions in biological fluids. I was delighted and I submitted to him my best work.25 He wrote me back citing a number of errors related to solution chemistry, but instead of rejecting my work, he fixed my article to meet his professional judgment! I was thrilled! He took me under his wing and became my mentor in solution chemistry. I suspect that without Dr. Berthon’s very kind assistance, I would have given up on this line of research long ago. We all owe him deeply. At that time, Dr. Berthon was Director of Research for Unit 305 (Equipe Bioréaciifs: Spéciation et Biodisponibilité), at the Institut National de la Santé et de la Recherche Médicale (INSERM), in Toulouse, France, a part of the Centre National de la Recherche Scientifique (CNRS), Paris, France. That would be roughly the equivalent of a major office at our National Institutes of Health.
The second problem is the total disbelief by almost all common cold researchers that the best way to treat a cold is by using zinc lozenges. Why treat the mouth and not the nose? There have been some nasal zinc treatments used to treat colds and allergies, but intranasal iZn has been known to cause extreme nasal pain and anosmia (loss of the sense of smell) since before 1938, according to E.W. Schultz and L.P. Gebhardt.26 In my web article about zinc and anosmia, I reported that scientists today continue to document this unfortunate effect.27 Does intranasal iZn help with colds? I didn’t find any evidence that it did in my 2006 report.9 However, others did find efficacy, but at what cost?28,29 There used to be a large number of zinc nasal treatment products for colds, but the threat of lawsuits over anosmia has reduced them considerably.
Another Mechanism As To Why Zinc Works

So, administration of zinc is fraught with problems and may or may not work, and I still haven’t explained why zinc lozenges can work. It has to do with medicine’s most exotic science. That is the field of “biologically closed electric circuits.” This is a field pioneered by Björn Nordenström in his amazing 1983 book titled Biologically Closed Electric Circuits.30 Dr. Nordenström showed that electrons moved in circuits within the body outside the nervous system and that by artfully manipulating them, serious illness could be cured.
How did I find out about his research? I was watching a news program on TV in 1988 when they showed a segment about his work. That news report and others like it were later placed on YouTube.31,32 Dr. Nordenström was Head of Diagnostic Radiology at the Karolinska Institute, Stockholm, Sweden. He was a member of the Nobel Assembly from 1967 through 1986, and served as President of the Assembly in 1985. He had extremely good credentials, but his book was so unorthodox that he lost his job and he went to China, where his advice on biologically closed electric circuits (BCEC) and cancer treatment was vastly more respected.
When I saw his amazing TV segment, I immediately went to the University of Texas medical library, checked out his book, and read the whole thing. I was left with the definite feeling that there was something electrical going on in the mouth and nose; perhaps one could call it a mouth-nose-BCEC (mouth-nose-biologically closed electric circuit). Since the voltages that he found were around 0.1 volts, I bought a high quality volt-ohm meter capable of detecting voltages as low as 0.01 volts and began my research. It only took a few seconds to find that there was a 0.1 volt flow of electrons from the nose to the mouth, and that the nose was positively charged relative to the mouth. The voltage fluctuated slightly with the respiratory rhythm.
According to Nordenström and classical electrical physics, there would be an opposing flow of metal ions (but not neutrally charged bound zinc) like iZn from the mouth to the nose. Wow! That was exactly the explanation I needed as to why zinc lozenges worked! The mouth-nose-BCEC moved iZn into the infected tissues of the nose from the mouth and throat!
Previously, I mentioned Edward Petrus and colleagues19 showed that people with allergies were twice as responsive to his lozenges as people who didn’t have allergies. I tested dozens of people with my volt-ohm meter and found that the voltage was always around 0.1 volt, but the electrical resistance between the mouth and nose varied widely between people.
People with allergies had extremely low resistances (1 to 20 kiloohm range), which would result in much more electrons flowing from the nose to the mouth and much more iZn flowing from the mouth to the nose. I suspect that this is why Petrus found those amazing differences. The dozen physicians that I tested had extremely high resistances (100 to 500 kiloohm range) and they reported that they never caught colds. My daughter, Karen, tested each of her middle school classmates and found the same voltage for a research project. (She got an A+ on her report!) Over the years, the mouth-nose-BCEC has become the best known of the BCECs. I published my mouth-nose-BCEC findings in my review,3 and later in Expert Reviews of Respiratory Medicine. 33
There you have it, two major reasons why scientists and physicians have not accepted zinc lozenges for common colds, and how a lozenge sucked in the mouth and delivered to the throat tissues is able to alleviate nasal cold symptoms.
What The “Experts” Still Don’t Understand
In my 2010 review article,3 I analyzed all of the relevant literature and plotted the effects of iZn on mean duration of common colds, which is shown in the chart on page 76. From these numbers, it is clear that the more iZn present, the better the efficacy. The red dot is the estimated effect of the 18.75 mg zinc acetate lozenges being introduced this month. It is based upon the collection of all known data on zinc lozenges and the common cold. I have also plotted the effect of total zinc (iZn plus bound) on colds (data not shown) and the regression line was nearly flat, which is what classically trained physicians cling to as lack of evidence of efficacy of zinc lozenges.
Will physicians ever accept what is factual over their own medical school teachings and personal beliefs? I wonder. I personally don’t see too much hope, particularly since the new JAMA article2 doesn’t say much more than zinc is good for colds (and cuts their duration in half) and did not mention iZn. Actually, that zinc is good for colds can be surmised from many years of research on zinc and the primary immune system. Without enough dietary zinc and other nutrients, our immune systems don’t function well and diseases are much more likely to develop and become difficult to remedy. Diet and immunity have been linked for centuries.34 As we move away from meat-rich diets, our intake of zinc is becoming so low that it is threatening our immune system.35
Summary
I remain hopeful that Life Extension® readers will accept my message and try zinc acetate lozenges. If started at the very first symptom of a common cold, which usually starts with a scratchy throat, regular use each two wakeful hours will often, if not always, abort colds by the end of the first day. I hope that you, the reader, will have a bottle at home, at school, at work, and always when boarding an airliner or when confined in some other venue, such as a theater or stadium. That way you can start treatment as soon as you feel you need to do so and rapidly defeat the cold. Good luck!
If you have any questions on the scientific content of this article, please call a Life Extension® Wellness Specialist at 1-866-864-3027.
Editor's Note
Science continues to evolve, and new research is published daily. As such, we have a more recent article on this topic: How Ionic Zinc Can Stop Colds Fast
References
- Eby GA, Davis DR, Halcomb WW. Reduction in duration of common colds by zinc gluconate lozenges in a double blind study. Antimicrob Agents Chemother. 1984; 25:20-24.
- Das RR, Singh M. Oral zinc for the common cold. JAMA. 2014; 311:1440-1.
- Eby GA. Zinc lozenges as cure for the common cold – A review and hypothesis. Med Hypotheses. 2010: 74;482-92.
- Eby GA, Handbook for Curing the Common Cold, 1994.
- Bashford CL, Alder GM, Menestrina G, et al. Membrane damage by hemolytic viruses, toxins, complement, and other cytotoxic agents. A common mechanism blocked by divalent cations. J Biol Chem. 1986; 261:9300-8.
- Yanagihara AA, Shohet RV. Cubozoan venom-induced cardiovascular collapse is caused by hyperkalemia and prevented by zinc gluconate in mice. PLoS One. 2012;7(12).
- Available at http://www.britannica.com/ebchecked/topic/293007/ionization. Accessed September 19, 2014.
- Horrobin DF, Manku MS, Oka M, et al. The nutritional regulation of T lymphocyte function. Med Hypotheses. 1979;5:969-85.
- Eby GA. Treatment of acute lymphocytic leukemia using zinc adjuvant with chemotherapy and radiation – a case history and hypothesis. Med Hypotheses. 2005; 64;1124-6.
- Available at: http://george-eby-research.com/html/leukemia.html. Accessed September 19, 2014.
- Eby GA, Halcomb WW. Ineffectiveness of zinc gluconate nasal spray and zinc orotate lozenges in common-cold treatment: a double-blind, placebo-controlled clinical trial. Altern Ther Health Med. 2006;12:34-8.
- Al-Nakib W, Higgins PG, Barrow I, Batstone G, Tyrrell DA. Prophylaxis and treatment of rhinovirus colds with zinc gluconate lozenges. J Antimicrob Chemother. 1987;20:893-901.
- Godfrey JC, Conant Sloane B, Smith DS, et al. Zinc gluconate and the common cold: a controlled clinical study. J Int Med Res. 1992;20:234-46.
- McElroy BH, Miller SP. An open-label, single-center, phase IV clinical study of the effectiveness of zinc gluconate glycine lozenges (Cold-Eeze) in reducing the duration and symptoms of the common cold in school-aged subjects. Am J Ther. 2003;10:324-9.
- Macknin ML, Piedmonte M, Calendine C, et al. Zinc gluconate lozenges for treating the common cold in children: a randomized controlled trial. JAMA 1998;279:1962-7.
- Turner RB and Cetnarowski WE. Effect of treatment with zinc gluconate or zinc acetate on experimental and natural colds. Clin Infect Dis. 2000;31;1202-8.
- Farr BM, Conner EM, Betts RF et al. Two randomized controlled trials of zinc gluconate lozenge therapy of experimentally induced rhinovirus colds. Antimicrob Agents Chemother. 1987;31:1183-7.
- Douglas RM, Miles HB, Moore BW, et al. Failure of effervescent zinc acetate lozenges to alter the course of upper respiratory tract infections in Australian adults. Antimicrob Agents Chemother. 1987;31:1263-5.
- Petrus EJ, Lawson KA, Bucci LR, Blum K. Randomized, double-masked, placebo controlled, clinical study of the effectiveness of zinc acetate lozenges on common cold symptoms in allergy-tested subjects. Curr Ther Res. 1998;59:595-607.
- Prasad AS, Miale A Jr, Farid Z et al. Biochemical studies ondwarfism, hypogonadism, and anemia. Arch Intern Med. 1963;111:407-28.
- Prasad AS, Fitzgerald JT, Bao B, Beck FW, Chandrasekar PH. Duration of symptoms and plasma cytokine levels in patients with the common cold treated with zinc acetate. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 2000;133:245-52.
- Prasad AS, Beck FW, Bao B, et al. Duration and severity of symptoms and levels of plasma interleukin-1 receptor antagonist, soluble tumor necrosis factor receptor, and adhesion molecules in patients with common cold treated with zinc acetate. J Infect Dis. 2008;197:795-802.
- Available at: http://coldcure.com/html/solution-chemistry.html. Accessed September 19, 2014.
- Available at: http://coldcure.com/html/common-cold.pdf. Accessed September 19, 2014.
- Eby G. The Zinc Lozenge and Common Cold Story in Metal-Ligand Interactions in Biological Fluids: Bioinorganic Medicine. Editor, Guy Berthon. Volume 2, pages 1182-90, Marcel Dekker, Inc., New York, 1995.
- Schultz EW and Gebhardt LP. The use of zinc sulfate solutions for the prevention of poliomyelitis in man. JAMA. 1938;110:2024.
- Available at: http://george-eby-research.com/anosmia/anosmia.html. Accessed September 19, 2014.
- Hirt M, Nobel S, Barron E. Zinc nasal gel for the treatment of common cold symptoms: a double-blind, placebo-controlled trial. Ear Nose Throat J. 2000;79:778-80, 782.
- Mossad SB. Effect of zincum gluconicum nasal gel on the duration and symptom severity of the common cold in otherwise healthy adults. QJM. 2003;96:35-43.
- Nordenström Björn. Biologically Closed Electric Circuits: Clinical, Experimental and Theoretical Evidence for an Additional Circulatory System. Nordic Medical Publications, Stockholm, Sweden, 1983.
- Biologically Closed Electric Circuits. https://www.youtube.com/watch?v=AXd6B7dGT3Y
- Available at: https://www.youtube.com/watch?v=XKXH_4PNPcQ. Accessed September 19, 2014.
- Eby G. The mouth-nose biologically closed electric circuit in zinc lozenge therapy of common colds as explanation of rapid therapeutic action. Expert Rev Respir Med. 2012;6:251-2.
- Chandra RK. Nutrition and immunology: from the clinic to cellular biology and back again. Proc Nutr Soc. 1999;58:681-3.
- Sandstead HH. Zinc deficiency. A public health problem? Am J Dis Child. 1991;145:853-9.

