Life Extension Magazine®

At least one of every two Americans over the age of 65 has atherosclerosis.1 It is so common in older people that some experts used to think that it was part of the normal aging process.2
We are going to explain in detail here how atherosclerosis develops, so you can fully understand why a modest dose of fish oil alone is not going to reverse this process in those with preexisting vascular disease.
While we understand that some members may find the material in this article overly technical, it is important we publish it so that our many physician members understand the challenges in treating aging humans with significant pre-existing atherosclerosis.
How Atherosclerotic Lesions Develop
Atherosclerosis begins with changes in endothelial cell function that cause white blood cells moving through the blood to stick to the endothelium (inner arterial wall) instead of flowing by normally.
The endothelium then becomes weakened. This allows blood cells and toxic substances circulating in the blood to pass through the endothelium and enter the artery’s sub-endothelial compartment. Lipid or fat-like substances such as LDL and triglycerides in the blood then accumulate in this area.
The lipids that accumulate in the broken endothelium become oxidized, causing the smooth muscle cells to try to “repair” the damaged endothelium. The result of this repair process is smooth muscle cell infiltration into the endothelium causing the formation of the initial atherosclerotic lesion. Depending on an individual’s risk factors—such as poor diet, lack of exercise, smoking, high blood pressure, and the aging process itself—fat accumulation continues and the atherosclerotic process accelerates.
Immune cells called macrophages then invade the damaged arterial area to digest the fat. But smooth muscle cells that have migrated to the area have already changed their nature to scavenge fat. These fat-laden white blood cells and smooth muscle cells are called “foam cells,” and provoke a chronic inflammatory attack by various immune components.
Smooth muscle cells try to curtail the injury to the endothelium by producing collagen, which forms a cap over the injury site. Calcium then accumulates over the injury site to form a material resembling bone. This is why atherosclerosis used to be referred to as “hardening of the arteries.”
This complex array of foam cells, calcification, and lipid accumulation is called an atherosclerotic plaque. The plaque grows, and if it becomes unstable, it is vulnerable to acute rupture that exposes the contents of the plaque to blood. Platelets can then rapidly accumulate around this ruptured plaque, resulting in an acute blockage (or blood clot) on the inner surface of the blood vessel wall. This clot can become very large and occlude the vessel. Even small plaques, if they rupture, can interfere with blood flow and cause an acute heart attack.
Alternatively, atherosclerotic plaques can grow to such a degree as to restrict blood flow severely. When blood flow within an artery is gravely compromised by a large plaque or blood clot, the cells of tissues that depend on blood flow from that artery become damaged or die. Coronary atherosclerosis cuts off the heart’s blood supply by occluding the heart’s arteries, which stops the oxygen supply to the heart, thus causing a heart attack. An ischemic stroke results when atherosclerotic processes cut off the oxygen supply to a portion of the brain.
As you can see, therefore, much more is involved in the development of atherosclerosis than just high cholesterol and LDL. We must emphasize, however, that maintaining optimal LDL and cholesterol levels is an important component of an atherosclerosis-prevention program.Protecting Your Arterial Walls

High blood pressure,3-7 elevated LDL-cholesterol-triglycerides,8-13 low HDL,14-17 smoking,18-20 diabetes,21-25 obesity,26-30 and lack of exercise31-33 contribute to endothelial dysfunction and the subsequent development of atherosclerosis.
Other significant artery-damaging factors are high-normal levels of glucose,34 insulin,35-39 iron,40-43 homocysteine,44-52 and fibrinogen,53-55 and any level of C-reactive protein14,56-62 that is higher than optimal.
Homocysteine can induce the initial atherosclerotic injury to the endothelium, then facilitate the oxidation of the fat and LDL that accumulate beneath the damaged endothelium, and finally contribute to the abnormal accumulation of blood components around the atherosclerotic plaque.
Fibrinogen is a clotting factor that accumulates at the site of the endothelial lesion. Fibrinogen contributes to plaque buildup and can participate in the arterial blockage after an unstable atherosclerotic plaque ruptures.
Glucose at high-normal levels may accelerate the glycation process that causes arterial stiffening, while high-normal fasting glucose and insulin inflicts direct damage to the endothelium. High levels of iron promote oxidation of LDL in the damaged endothelium, while low levels of testosterone (in men) appear to interfere with normal endothelial function.
C-reactive protein is an inflammatory marker and directly damages the endothelium. Chronic inflammation, as evidenced by persistent high levels of C-reactive protein, not only creates initial injuries to the endothelium, but also accelerates the progression of existing atherosclerotic lesions.
In response to a large number of published studies, enlightened people are taking charge of the health of their arteries. They are eating better, exercising regularly, and undergoing regular blood testing to identify the specific drugs, hormones, and dietary supplements they need to reduce their atherosclerotic risk factors.
The emphasis in treating aging humans with pre-existing arterial disease is that all risk factors should be controlled if there is to be an opportunity to reverse the occlusion of vital arteries. When only a few atherogenic factors like elevated LDL are lowered, disease progression is virtually inevitable, albeit at a slower rate.
What You Need to Know
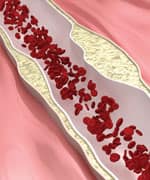 |
Pathway to the Development of Deadly Arterial Plaque
- At least one of every two Americans over the age of 65 has atherosclerosis.
- Life Extension has identified 17 major risk factors for atherosclerosis.
- Atherosclerosis begins with changes in endothelial cell function that cause white blood cells and lipids moving through the blood to stick to the endothelium (inner arterial wall) instead of flowing by normally.
- The lipids that accumulate in the broken endothelium become oxidized, causing the smooth muscle cells to try to “repair” the damaged endothelium.
- Immune cells called macrophages then invade the damaged arterial area to digest the fat.
- Smooth muscle cells try to curtail the injury to the endothelium by producing collagen, which forms a cap over the injury site.
- Fat-laden white blood cells and smooth muscle cells become “foam cells” which provoke a chronic inflammatory attack by various immune components.
- This complex array of foam cells, calcification, and lipid accumulation is called an atherosclerotic plaque.
Summary

At least one of every two Americans over the age of 65 has atherosclerosis.1 A number of biochemical factors in the blood can affect the development of atherosclerosis such as elevated LDL cholesterol, low HDL cholesterol, and elevated glucose, homocysteine or fibrinogen to name a few. Atherosclerosis begins with changes in endothelial cell function that cause white blood cells moving through the blood to stick to the endothelium (inner arterial wall) instead of flowing by normally. The endothelium then becomes weakened. This allows blood cells and toxic substances circulating in the blood to pass through the endothelium and enter the artery’s sub-endothelial compartment. Lipid or fat-like substances such as LDL and triglycerides in the blood then accumulate in this area.
Immune cells called macrophages then invade the damaged arterial area to digest the fat. But smooth muscle cells that have migrated to the area have already changed their nature to scavenge fat. These fat-laden white blood cells and smooth muscle cells are called “foam cells,” and provoke a chronic inflammatory attack by various immune components. Smooth muscle cells try to curtail the injury to the endothelium by producing collagen, which forms a cap over the injury site. Calcium then accumulates over the injury site to form a material resembling bone.
This complex array of foam cells, calcification, and lipid accumulation is called an atherosclerotic plaque. This plaque can become unstable resulting in an increased risk of rupture and clot formation. Alternatively, the plaque can continue to grow to a size so large that it impedes or completely blocks blood flow. Either path can lead to a potentially life-threatening stroke or heart attack.If you have any questions on the scientific content of this article, please call a Life Extension® Health Advisor at 1-866-864-3027.
References
- Available at: http://www.nia.nih.gov/health/publication/aging-hearts-and-arteries-scientific-quest/chapter-4-blood-vessels-and-aging-rest. Accessed December 21, 2012.
- Available at: http://www.ncbi.nlm.nih.gov/pmc/articles/pmc3209544. Accessed January 14, 2013.
- Landmesser U, Hornig B, Drexler H. Endothelial function: a critical determinant in atherosclerosis? Circulation. 2004 Jun 1;109(21 Suppl 1):II27-33.
- Endemann DH, Schiffrin EL. Endothelial dysfunction. J Am Soc Nephrol. 2004 Aug;15(8):1983-92.
- Chang HJ, Chung J, Choi SY, et al. Endothelial dysfunction in patients with exaggerated blood pressure response during treadmill test. Clin Cardiol. 2004 Jul;27(7):421-5.
- Tu L, Wei W, Liu X, Deng Y, Yu S. Endothelial function and carotid artery wall thickening in patients with early essential hypertension. J Tongji Med Univ. 1999;19(4):288-90, 303.
- Rodriguez-Porcel M, Lerman LO, Herrmann J, Sawamura T, Napoli C, Lerman A. Hypercholesterolemia and hypertension have synergistic deleterious effects on coronary endothelial function. Arterioscler Thromb Vasc Biol. 2003 May 1;23(5):885-91.
- Maggi FM, Raselli S, Grigore L, Redaelli L, Fantappie S, Catapano AL. Lipoprotein remnants and endothelial dysfunction in the postprandial phase. J Clin Endocrinol Metab. 2004 Jun;89(6):2946-50.
- Saini HK, Arneja AS, Dhalla NS. Role of cholesterol in cardiovascular dysfunction. Can J Cardiol. 2004 Mar 1;20(3):333-46.
- Dart AM, Chin-Dusting JP. Lipids and the endothelium. Cardiovasc Res. 1999 Aug 1;43(2):308-22.
- Kusterer K, Pohl T, Fortmeyer HP, et al. Chronic selective hypertriglyceridemia impairs endothelium-dependent vasodilatation in rats. Cardiovasc Res. 1999 Jun;42(3):783-93.
- Liu L, Zhao SP, Gao M. Influence of postprandial hypertriglyceridemia on the endothelial function in elderly patients with coronary heart disease. Hunan Yi Ke Da Xue Xue Bao. 2002 Jun 28;27(3):259-62.
- De Caterina R, Lenzi S. The role of LDL in the origin and progression of atherosclerosis: pathobiological concepts on the origin and development of atherosclerotic lesions and the role of the endothelium. G Ital Cardiol. 1998 Feb;28(2):158-67.
- Ridker PM, Stampfer MJ, Rifai N. Novel risk factors for systemic atherosclerosis: a comparison of C-reactive protein, fibrinogen, homocysteine, lipoprotein(a), and standard cholesterol screening as predictors of peripheral arterial disease. JAMA. 2001 May 16;285(19):2481-5.
- Toikka JO, Ahotupa M, Viikari JS, et al. Constantly low HDL-cholesterol concentration relates to endothelial dysfunction and increased in vivo LDL-oxidation in healthy young men. Atherosclerosis. 1999 Nov 1;147(1):133-8.
- Viles-Gonzalez JF, Fuster V, Corti R, Badimon JJ. Emerging importance of HDL cholesterol in developing high-risk coronary plaques in acute coronary syndromes. Curr Opin Cardiol. 2003 Jul;18(4):286-94.
- Spieker LE, Sudano I, Hurlimann D, et al. High-density lipoprotein restores endothelial function in hypercholesterolemic men. Circulation. 2002 Mar 26;105(12):1399-402.
- Ambrose JA, Barua RS. The pathophysiology of cigarette smoking and cardiovascular disease: an update. Am Coll Cardiol. 2004 May 19;43(10):1731-7.
- Poreba R, Skoczynska A, Derkacz A. Effect of tobacco smoking on endothelial function in patients with coronary arteriosclerosis. Pol Arch Med Wewn. 2004 Jan;111(1):27-36.
- Puranik R, Celermajer DS. Smoking and endothelial function. Prog Cardiovasc Dis. 2003 May-Jun;45(6):443-58.
- Furuta M, Tsunoda K, Arita M, Nanjo K, Sanke T. Endothelium-dependent vasodilation in type II diabetes mellitus. Rinsho Byori. 2003 Nov;51(11):1111-5.
- Higashi Y, Yoshizumi M. Endothelial function. Nippon Rinsho. 2003 Jul;61(7):1138-44.
- Shinozaki K, Kashiwagi A, Masada M, Okamura T. Molecular mechanisms of impaired endothelial function associated with insulin resistance. Curr Drug Targets Cardiovasc Haematol Disord. 2004 Mar;4(1):1-11.
- Najemnik C, Sinzinger H, Kritz H. Endothelial dysfunction, atherosclerosis and diabetes. Acta Med Austriaca. 1999;26(5):148-53.
- Jarvisalo MJ, Raitakari M, Toikka JO, et al. Endothelial dysfunction and increased arterial intima-media thickness in children with type 1 diabetes. Circulation. 2004 Apr 13;109(14):1750-5.
- Bakker SJ, IJzerman RG, Teerlink T, Westerhoff HV, Gans RO, Heine RJ. Cytosolic triglycerides and oxidative stress in central obesity: the missing link between excessive atherosclerosis, endothelial dysfunction, and beta-cell failure? Atherosclerosis. 2000 Jan;148(1):17-21.
- Blann AD, Bushell D, Davies A, Faragher EB, Miller JP, McCollum CN. von Willebrand factor, the endothelium and obesity. Int J Obes Relat Metab Disord. 1993 Dec;17(12):723-5.
- Yu YR, Li HL, Yu HL, Wang C, Pu S. The relationship between insulin resistance and endothelium-dependent vasodilatation in obese subjects. Zhonghua Yi Xue Za Zhi. 2003 Sep 10;83(17): 1467-70.
- Lyon CJ, Law RE, Hsueh WA. Minireview: adiposity, inflammation, and atherogenesis. Endocrinology. 2003 Jun;144(6):2195-200.
- Mitu F, Mitu M. Physical exercise and vascular endothelium. Rev Med Chir Soc Med Nat Iasi. 2003 Jul-Sep;107(3):487-93.
- Edwards DG, Schofield RS, Lennon SL, Pierce GL, Nichols WW, Braith RW. Effect of exercise training on endothelial function in men with coronary artery disease. Am J Cardiol. 2004 Mar 1;93(5):617-20.
- Higashi Y, Yoshizumi M. Exercise and endothelial function: role of endothelium-derived nitric oxide and oxidative stress in healthy subjects and hypertensive patients. Pharmacol Ther. 2004 Apr;102(1):87-96.
- Gokce N, Vita JA, Bader DS, et al. Effect of exercise on upper and lower extremity endothelial function in patients with coronary artery disease. Am J Cardiol. 2002 Jul 15;90(2):124-7.
- Bjørnholt JV, Erikssen G, Aaser E, et al. Fasting blood glucose: an underestimated risk factor for cardiovascular death. Results from a 22-year follow-up of healthy nondiabetic men. Diabetes Care. 1999 Jan;22(1):45-9.
- Bonora E, Kiechl S, Willeit J, et al. Insulin resistance as estimated by homeostasis model assessment predicts incident symptomatic cardiovascular disease in caucasian subjects from the general population: the Bruneck study. Diabetes Care. 2007 Feb;30(2):318-24.
- Sundell J, Luotolahti M. Association between insulin resistance and reduced coronary vasoreactivity in healthy subjects. Can J Cardiol. 2004 May 15;20(7):691-5
- Stochmal E, Szurkowska M, Czarnecka D, et al. Association of coronary atherosclerosis with insulin resistance in patients with impaired glucose tolerance. Acta Cardiol. 2005 Jun;60(3):325-31.
- Takahashi F, Hasebe N, Kawashima E, et al. Hyperinsulinemia is an independent predictor for complex atherosclerotic lesion of thoracic aorta in non-diabetic patients. Atherosclerosis. 2006 Aug;187(2):336-42.
- Stout RW. The relationship of abnormal circulating insulin levels to atherosclerosis. Atherosclerosis. 1977 May;27(1):1-13.
- Chau LY. Iron and atherosclerosis. Proc Natl Sci Counc Repub China B. 2000 Oct;24(4):151-5.
- Howes PS, Zacharski LR, Sullivan J, Chow B. Role of stored iron in atherosclerosis. J Vasc Nurs. 2000 Dec;18(4):109-14; quiz 115-6.
- Kraml P, Potockova J, Koprivova H, et al. Ferritin, oxidative stress and coronary atherosclerosis. Vnitr Lek. 2004 Mar;50(3):197-202.
- Minqin R, Watt F, Huat BT, Halliwell B. Correlation of iron and zinc levels with lesion depth in newly formed atherosclerotic lesions. Free Radic Biol Med. 2003 Mar 15;34(6):746-52.
- Montalescot G, Ankri A, Chadefaux-Vekemans B, et al. Plasma homocysteine and the extent of atherosclerosis in patients with coronary artery disease. Int J Cardiol. 1997 Aug 8;60(3):295-300.
- Stampfer MJ, Malinow MR, Willett WC, et al. A prospective study of plasma homocysteine and risk of myocardial infarction in US physicians. JAMA. 1992 Aug 19;268(7):877-81.
- Verhoef P, Stampfer MJ, Buring JE, et al. Homocysteine metabolism and risk of myocardial infarction: relation with vitamins B6, B12, and folate. Am J Epidemiol. 1996 May 1;143(9):845-59.
- Robinson K, Mayer EL, Miller DP, et al. Hyperhomocysteinemia and low pyridoxal phosphate. Common and independent reversible risk factors for coronary artery disease. Circulation. 1995 Nov 15;92(10):2825-30.
- Arnesen E, Refsum H, Bonaa KH, Ueland PM, Forde OH, Nordrehaug JE. Serum total homocysteine and coronary heart dis- ease. Int J Epidemiol. 1995 Aug;24(4):704-9.
- Aronow WS, Ahn C. Association between plasma homocysteine and coronary artery disease in older persons. Am J Cardiol. 1997 Nov 1;80(9):1216-8.
- Berwanger CS, Jeremy JY, Stansby G. Homocysteine and vascular disease. Br J Surg. 1995 Jun;82(6):726-31.
- Bostom AG, Rosenberg IH, Silbershatz H, et al. Nonfasting plasma total homocysteine levels and stroke incidence in elderly persons: the Framingham Study. Ann Intern Med. 1999 Sep 7;131(5):352-5.
- Bots ML, Launer LJ, Lindemans J, Hofman A, Grobbee DE. Homocysteine, atherosclerosis and prevalent cardiovascular disease in the elderly: The Rotterdam Study. J Intern Med. 1997 Oct;242(4):339-47.
- Maresca G, Di Blasio A, Marchioli R, Di Minno G. Measuring plasma fibrinogen to predict stroke and myocardial infarction: an update. Arterioscler Thromb Vasc Biol. 1999 Jun;19(6): 1368-77.
- Acevedo M, Foody JM, Pearce GL, Sprecher DL. Fibrinogen: associations with cardiovascular events in an outpatient clinic. Am Heart J. 2002 Feb;143(2):277-82.
- Thompson SG, Kienast J, Pyke SD, Haverkate F, van de Loo JC. Hemostatic factors and the risk of myocardial infarction or sudden death in patients with angina pectoris. European Concerted Action on Thrombosis and Disabilities Angina Pectoris Study Group. N Engl J Med. 1995 Mar 9;332(10):635-41.
- Jarosz A, Nowicka G. C-reactive protein and homocysteine as risk factors of atherosclerosis. Przegl Lek. 2008 65(6):268-72.
- Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of car- diovascular disease in women. N Engl J Med. 2000 Mar 23;342(12):836-43.
- Kuller LH, Tracy RP, Shaten J, Meilahn EN. Relation of C-reactive protein and coronary heart disease in the MRFIT nested case-control study. Multiple Risk Factor Intervention Trial. Am J Epidemiol. 1996 Sep 15;144(6):537-47.
- Mendall MA, Strachan DP, Butland BK, et al. C-reactive protein: relation to total mortality, cardiovascular mortality and cardio- vascular risk factors in men. Eur Heart J. 2000 Oct;21(19): 1584-90.
- Pasceri V, Willerson JT, Yeh ET. Direct proinflammatory effect of C-reactive protein on human endothelial cells. Circulation. 2000 Oct 31;102(18):2165-8.
- Ridker PM, Buring JE, Shih J, Matias M, Hennekens CH. Prospective study of C- reactive protein and the risk of future car- diovascular events among apparently healthy women. Circulation. 1998 Aug 25;98(8):731-3.
- Auer J, Berent R, Lassnig E, Eber B. C- reactive protein and coronary artery disease. Jpn Heart J. 2002 Nov;43(6):607-19.

