Life Extension Magazine®
There are now a number of diagnostic tests to identify early stage prostate cancer and then monitor the success or failure of a wide range of treatment options.
This article succinctly describes conventional prostate gland diagnostic tests along with those that mainstream medicine often overlooks, to the detriment of their patients. All of these tests, however, are commercially available.
Tests
1. PSA (Prostate-Specific Antigen)
Perhaps the greatest breakthrough in the detection and management of prostate cancer was the approval of the prostate-specific antigen (PSA) blood test in 1986, but it was only approved for men already diagnosed with prostate cancer.1 It wasn’t until 1994 that the FDA approved the PSA test as a prostate cancer screening test for all men.1 Prostate-specific antigen is a protein produced by the cells of the prostate gland, including both cancerous cells as well as cells that are benign.1 Since very little PSA escapes into the bloodstream from a healthy prostate, an elevated PSA level in the blood indicates an abnormal condition of the prostate gland—which can be either benign or malignant. PSA test results can be used both to detect potential prostate problems and to follow the progress of prostate cancer therapy.1
Because tumor growth is essentially exponential, with one cell dividing into two, two to four, four to eight, and so on, a tumor cell product such as PSA can reflect such exponential growth—measuring the time it takes for PSA to double (PSA doubling time, or PSADT).2 Also, the PSA rate of rise (PSA velocity), although not a more specific marker, may have value in prostate cancer prognosis—because men with prostate cancer whose PSA level increased by more than 2.0 ng/mL during the year before their diagnosis showed a higher risk of death from prostate cancer.3 Additionally, though not an absolute criteria for or against malignancy, PSA velocity can serve as a gauge regarding the likelihood of a malignant condition.2 A rising PSA velocity in excess of .75 ng/mL/year relates to an increased probability of a malignant condition. 2
The reference interval provided by most conventional laboratories for the PSA test is 0.00-4.00 nanograms per milliliter (ng/mL).4 Conventional reference ranges suggest that PSA levels under 4.0 ng/mL are normal, but any reading over 2.0 ng/mL can indicate unhealthy activity, such as prostatitis, benign prostate hypertrophy, or prostate cancer.2 If PSA readings begin to elevate, there are interventions that can reduce or stabilize the production of PSA, shutting down a mechanism used by cancer cells to escape their confinement within the prostate gland.5 PSA readings can increase immediately after ejaculation, returning slowly to baseline levels within 24-48 hours.6,7
Chart of PSA Ranges with Succinct Suggestions
PSA (ng/mL) |
Concise Recommendation |
0-1.0 |
Optimal |
1.1-2.4 |
Initiate measures to support prostate health and have digital rectal exam performed |
2.5-4.0 |
Moderate concern–assess PSA velocity—have digital rectal exam—consider other tests. |
>4.0 |
Too high—additional diagnostics recommended |
2. Free PSA
Free PSA is a newer evaluation for prostate health. Most PSA in the blood is bound to serum proteins, but a small amount is not protein-bound and is called free PSA.2,8 In men with prostate cancer, the ratio of free (unbound) PSA to total PSA is decreased.2 The free PSA test measures the percentage of free PSA relative to the total amount.9 The lower the ratio, the greater the probability of prostate cancer. Measuring free PSA may help eliminate unnecessary biopsies.8 Free PSA readings increase immediately after ejaculation, returning slowly to baseline levels within about 24 hours.6 Although not used as an initial screening test, a lower percentage of free PSA might mean your doctor needs to do a further workup.
Below are the percentage of PSA ranges and what they represent as far as prostate cancer risk. Note that when the percentage of free PSA is high (over 20%), this means the risk of prostate cancer is low, whereas a low percentage of free PSA (under 11% indicates high risk).
PRoStAte CAnCeR RISk
Free PSA% |
50-64 Years |
65-75 Years |
0.00-10.00% |
56% |
55% |
10.01-15.00% |
24% |
35% |
15.01-20.00% |
17% |
23% |
20.01-25.00% |
10% |
20% |
Over 25% |
5% |
9% |
3. PCA3 Urine
PCA3 is a molecular diagnostic test performed on urine rather than blood and detects mRNA that is excreted into the urethra via the epithelial cells that line the prostatic ducts.10 Prostate cancer cells tend to produce this compound far more than normal cells do.10 The PCA3 urine test has to be done in a urologist’s, or other doctor’s, office, because it requires a digital rectal massage just prior to collection of the urine. 11
PCA3 testing is most useful when repeated over a period of time to monitor for changes in the observed value. In general, a PCA3 score of 35 is considered the optimal cut-off. A score of greater than 35 reflects an increased probability of a positive biopsy. A score of less than 35 reflects a decreased probability of a positive biopsy.
4. 25-Hydroxy Vitamin D
Research points to a connection between vitamin D levels and cancer.12,13 Experimental studies indicate that low levels of vitamin D increase prostate cancer risk.14 And further evidence shows that the active form of vitamin D promotes differentiation and inhibits proliferation, invasiveness, and metastasis of human prostate cancer cells.14,15 Detecting deficient levels allows you and your physician to implement vitamin D supplementation to help avert illnesses associated with inadequate vitamin D levels. For this nutrient, individualized dosing is of particular importance, and the only way to accomplish this is through vitamin D blood testing. Although conventional laboratory reference ranges list a reference interval of 30-100 ng/mL, Life Extension supports maintaining vitamin D in the 50-80 ng/mL range.16
5. Prolactin
Prolactin, a peptide hormone largely secreted by the anterior pituitary gland, has typically remained restricted to the fields of lactation and infertility. However, researchers discovered that prolactin plays a major role in the differentiation and development of the prostate gland.17 Both malignant and healthy prostates produce prolactin. Prostatic fluids from patients with cancer also have higher prolactin levels than controls. 17 In vitro, prolactin induces proliferation and antagonizes apoptosis in prostate organ culture and in some tumor cell lines.17 Increased levels of prolactin have significant stimulatory action on the prostate and on prostate ductal development and may lead to hyperplastic growth, independent of elevations in circulating androgen levels.18
Labcorp normal reference range - Male: 4.0-15.2 ng/mL
Optimal for Prostate Cancer- <5 ng/mL
What You Need to Know
 |
Methods of Diagnosing and Assessing Prostate Cancer
- Prostate cancer remains unique in that there are many tests to identify early stage disease and then monitor the success or failure of a wide range of treatment options.
- Perhaps the greatest breakthrough in the detection and management of prostate cancer was the approval of the prostate-specific antigen (PSA) blood test.
- Experimental studies indicate that low levels of vitamin D increase prostate cancer risk.
- Men can easily be tested for palpable prostate abnormalities with a digital rectal exam (DRE), a simple test that provides a lot of information.
- A comprehensive blood test for specific hormone levels is useful since many hormones have been shown to play a role in the proliferation of prostate cancer.
- Combining imaging tests such as ultrasound, MRI, QCT, Color Doppler, and bone scan can give the most complete picture, allowing for full physical and architectural assessment of tumors, including those that have spread beyond the prostate.
6. DRE (Digital Rectal Exam)

Men can easily be tested for palpable prostate abnormalities with a digital rectal exam (DRE), a simple test that provides a lot of information.19 It gives the physician a sense of the prostate gland volume. The bigger the prostate, the more PSA the gland is entitled to make without indicating a potential problem. A basic rule is that the prostate gland volume multiplied by the amount of PSA produced per unit of volume in benign prostate tissue is 0.067 ng.19 This means that a 50-year-old man with a normal size prostate of 30 grams (or cubic centimeters) would therefore be entitled to make approximately 2 ng of PSA. If such a man has a PSA of 4.0 ng/mL, it would indicate an excess of about 2 ng of PSA and the need for further investigation to rule out prostate cancer.
In addition to estimating prostate gland volume and calculating the benign cellular contribution to the total PSA value, the DRE can also aid in finding hard nodules or other evidence of disease. Palpable (able to be felt) abnormalities of the prostate gland relate to tumor volume, also called tumor burden.19 In the years before routine testing with PSA, most prostate cancers were already palpable via DRE by the time of diagnosis. Today, close to 70% of prostate cancers newly diagnosed in the US are no longer associated with palpable disease.19 This shows the value of PSA screening in allowing an earlier diagnosis of prostate cancer — before the cancer has had a chance to get bulkier and manifest itself as a palpable disease, known as a T2 disease. Most American men when first diagnosed with prostate cancer have non-palpable, or T1, prostate cancer.19
7. Blood Hormone Profile
A comprehensive blood test for specific hormone levels is useful. In addition to the free and total testosterone levels covered earlier, a complete blood test should include levels of estradiol, DHT (dihydrotestosterone), pregnenolone, DHEA-S (dehydroepiandrosterone sulfate), FSH (follicle-stimulating hormone, LH (luteinizing hormone), and possibly, IGF-1 (insulin-like growth factor 1). DHT plays a role in the development and exacerbation of benign prostatic hyperplasia, as well as prostate cancer.20 FSH (follicle-stimulating hormone) and LH (luteinizing hormone) regulate the reproductive processes of the body, and in aging men, a rise in FSH and LH can be indicative of andropause.21,22 Studies have shown that increased levels of IGF promote cancer growth and confer resistance to conventional treatments (chemotherapy and radiation).23,24
8. PAP (Prostatic Acid Phosphatase) Test
The PAP test is a simple blood test, used to measure the amount of an enzyme—called prostatic acid phosphatase (PAP)—that is produced by prostate epithelial cells and is abundant in seminal fluid.25 Higher levels of PAP are associated with prostate cancer.25 PAP determination, in conjunction with PSA measurements, is useful in assessing the prognosis of prostate cancer. It is an important test, because it allows identification of prostate cancer patients who have an elevation of PAP, but not of PSA. This helps monitor the course of disease and response to treatment.
Baseline PAP 2 |
Freedom from prostate cancer recurrence at 5 years after prostate cancer surgery defined as psa>0.2 ng/mL |
<0.4 U/liter |
87% |
0.4-0.5 U/liter |
79% |
>0.5 U/liter |
63% |
9. Circulating Tumor Cells Assay
This test provides a measurement of cancer cells that have separated from a solid tumor site and are circulating in the bloodstream.26 Detecting the presence of circulating tumor cells in the blood has clinical usefulness in assessing the disease status and prognosis of metastatic prostate cancer, and is predictive of overall survival.27 Fasting prior to the blood draw is not required.
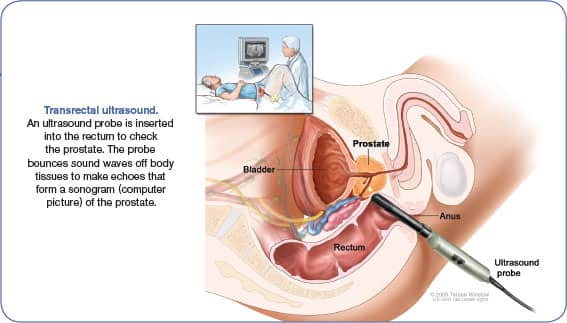
Imaging
1. Transrectal Ultrasound
Transrectal ultrasound creates an image of the organs in the pelvis, and the most common indication is for the evaluation of the prostate gland in men with elevated PSA levels, or with prostatic nodules on a digital rectal exam.28 Ultrasound clarifies the size of the prostate gland and aids in the distinction between benign prostate conditions and prostate cancer.28 This type of imaging can also be used to help guide a biopsy of the prostate.28
2. Color Doppler Ultrasound
Color Doppler ultrasound is a medical imaging technique that is used to provide visualization of blood flow, using computer processing to add color to the image to greatly clarify what is happening inside the body.29 An ultrasound transducer is used to beam sound into the area of interest, and it reads the returning sound. When the sound bounces off a moving target such as a blood vessel, the pitch changes as a result of the Doppler effect. The transducer can detect very subtle pitch changes, record them visually, and generate an image showing where blood is flowing, and in what direction. Because a simple grayscale image can be a bit difficult to read, the ultrasound machine assigns different color values, depending on whether blood is moving towards or away from the transducer. In addition to showing the direction of flow, the colors also vary in intensity depending on the velocity of the flow, allowing doctors to also see how quickly the blood is moving.28
A color Doppler ultrasound of a patient with a suspected tumor will reveal the precise areas where the velocity of blood flow is changing, mapping out the problem in full color.30 This type of imaging can map out the tumor’s blood supply to clarify exactly how far the growth has spread. 31 This can have an impact on what treatments are selected, and how surgery and other measures are approached. While color Doppler ultrasound can be done using a transducer on the outside of the body, it can also be used for transrectal procedures, in which the probe is inserted to get a better view.
3. MRI (Magnetic Resonance Imaging)
The MRI has been used for over 30 years for prostate cancer detection and evaluation.32 In contrast to ultrasound imaging, prostate MRI has superior soft tissue resolution.33 Magnetic fields are used to locate and characterize prostate cancer. To do so, radiologists use multi-parametric MRI, which includes four different types of MRI sequences.32 Currently, MRI is used to identify targets for prostate biopsy, and to make a surgical plan for men undergoing robotic prostatectomy. MRI imaging also helps surgeons decide whether to resect or spare the neurovascular bundle and assess surgical difficulty.32
4. (Nuclear) Bone Scan
Prostate cancer can cause “hot spots” to appear on a bone scan if the cancer has metastasized to the bone.34,35 A bone scan for cancer uses nuclear technology and involves administering a radioactive substance called a tracer to produce gamma radiation that can be picked up by a special camera.34 The tracer consists of radionuclides that bind to the bone and show up as dark or light spots. After the technician injects the tracer, it usually takes between one and four hours for the radioactive substance to move throughout the skeleton. During this time, patients will be asked to drink up to six glasses of water to flush any tracer material not absorbed by bone. Then, the patient must remain still on a padded table while a large camera passes over the body.
A dark spot, also called a cold spot, might indicate lack of absorption of the tracer.34 This may also indicate that cancer has spread to the bone from the prostate gland. A normal scan shows evenly distributed tracer throughout the body. Risks associated with a bone scan are considered low, with a very small level of radiation exposure.34 The radionuclides injected into the bloodstream are excreted through the urine and have a low risk of toxicity.34
5. Quantitative Computed Tomography (QCT)
Osteoporosis, or bone thinning, is associated with prostate cancer and can be a side effect of prostate cancer treatment.36 Quantitative computed tomography (QCT) is a highly sensitive test that is better able to determine bone density changes than common methods such as DEXA testing.37 Studies have shown that DEXA testing can often read degenerative changes involving bone and joint tissues and calcium deposits within blood vessels as bone density thus not suggesting osteoporosis when in fact bone loss is present.38-41 Quantitative computed tomography QCT is similar to other forms of computed tomography (CT). As with any CT scan, an X-ray tube and sensor rotate around the body area in a circular or spiral pattern, and a series of pictures are transmitted to a computer.42 The primary difference with QCT is the special analysis performed by special QCT software. While most computed tomography (CT) software produces a composite visual image to detect fractures or other symptoms in the scanned bone or soft tissue, QCT uses the data provided by the scanner to generate numerical values for the volume, mass, and density of bone.42 This allows QCT to distinguish between cortical bone, which lines the outside of the bones, and trabecular bone, the softer tissue that makes up the center of the bone.43 Trabecular bone is much more metabolically active than cortical bone—meaning, the two types of bone are replaced at different rates.43 As a result, these two bone types can show different rates of change in bone mineral density.
Genetic Testing

About half of US men diagnosed with prostate cancer are classified as low-risk by use of conventional measures such as Gleason Score (a form of tumor grading), the prostate-specific antigen test (PSA), and a physical exam.44 Nonetheless, nearly 90% of these low-risk patients will choose to undergo immediate aggressive treatment such as radical prostatectomy or radiation even though there is less than a 3% chance of deadly progression.44
A new test called Oncotype DX is now available to physicians and their patients. It measures the level of expression of 17 genes across four biological pathways to predict prostate cancer aggressiveness.44
Test results are reported as a Genomic Prostate Score (GPS) ranging from 0 to 100; this score is assessed along with other clinical factors to clarify a man’s risk prior to treatment intervention.44 This multi-gene test has been validated using the prostate needle biopsy sample taken before the prostate is removed, thereby providing the opportunity for low risk patients to avoid invasive treatments. According to the principle investigator of the validation study, individual biological information from the Oncotype DX prostate cancer test almost tripled the number of patients who can more confidently consider active surveillance and avoid unnecessary treatment and its potential side effects.44
The advantage of this test for those who choose the comprehensive surveillance program utilized by Life Extension members (which involves the use of several drugs, targeted nutrients, and adherence to healthy dietary patterns) is to provide greater assurance the right course of action is being followed.
For information about the Oncotype DX test, log on to www.genomichealth.com
Prolaris® is another genomic test developed to aid physicians in predicting prostate cancer aggressiveness in conjunction with clinical parameters such as Gleason score and PSA.45
Prolaris® measures prostate cancer tumor biology at the molecular level. By measuring and analyzing the level of expression of genes directly involved with cancer replication, Prolaris may be able to more accurately predict disease progression.45
Prolaris® is a tool designed to measure the aggressiveness of a patient’s cancers to better predict and stratify an individual’s relative risk of disease progression within ten years.45 It may enable physicians to better define a treatment/monitoring strategy for their patients.
Prolaris® claims to be significantly more prognostic than currently used variables and provides unique additional information that can be combined with other clinical factors in an attempt to make a more accurate prediction of a patient’s cancer aggressiveness and therefore disease progression.45
Prolaris® has been shown to predict clinical progression in four different clinical cohorts, in both pre and post-treatment scenarios.
In the treatment of prostate cancer, Prolaris® is prognostic at the point of diagnosis and in the post-surgery setting.45
At diagnosis, Prolaris® can help to identify patients with less aggressive cancer who may be candidates for active surveillance. In addition, Prolaris® can define patients who appear clinically low-risk but have a more aggressive disease that requires more aggressive treatment.
Prolaris® testing is also well suited for use in post-prostatectomy patients that have higher risk features after surgery to better estimate their risk of disease recurrence and therefore adjust the level of monitoring or add additional therapy.
For more information about Prolaris®, log on to www.myriad.com
Summary
Prostate cancer is the most common malignancy in US men (excluding non-melanoma skin cancer), afflicting one male in every six.46,47 A significant percentage of men have underlying prostate cancer without even knowing it.48-50
These men have access to an arsenal of tools for their doctors to diagnose and then monitor the success or failure of various treatment modalities, including “active surveillance” or “watchful waiting.”
As you’ve learned in this issue of Life Extension, one does not have to sit back and “watch” their PSA level steadily rise. Nutritional, hormonal, and drug approaches exist to help control early-stage, low-grade prostate tumors. There is data to support the efficacy of some of them as effective adjuvants in men with high-grade tumors as well.51-59
If you have any questions on the scientific content of this article, please call a Life Extension® Health Advisor at 1-866-864-3027.
References
- Available at: http://www.cancer.gov/cancertopics/factsheet/detection/psa. Accessed September 13, 2013.
- Strum SB, Pogliano D. A Primer on Prostate Cancer: An Empowered Patient’s Guide. Hollywood, FL: The Life Extension Foundation; 2005.
- D’Amico A, Chen M, Roehl K, Catalona W. Preoperative PSA velocity and the risk of death from prostate cancer after radical prostatectomy. N Engl J Med. 2004;351(2):125-35.
- Available at: https://www.labcorp.com/wps/portal/!ut/p/c1/hY1LDoIwFADP4gFMX1uosFUB-
RUrED4bQsAghj9Gg6eXC6iZ5WQyKEUrXf6sq_xR913eoBilLBMK44JwAopLdCCU7UE-
aBgMtvrkuwf4U_NT315RgtJdFmqSj02Vgmf4BEyfehInnDEFUIBikDL_DoPrzJ37Vr2zo8nc0
SavsCcIRpjfi2qDKG3rUjbNKKIqCo_VjeJiobYudCthLV4_yc_P0MYvvBWbDxFxg-
k!/dl2/d1/L0lJS2FZQSEhL3dMRUJGcUFFQWpNQy9ZSTV5bHchIS83X1VFNFMxSTkz
ME9HUzIwSVMzTzROMk42NjgwL3ZpZXdUZXN0/?testId=408050. Accessed September 13, 2013. - Webber MM, Waghray A, Bello D. Prostate-specific antigen, a serine protease, facilitates human prostate cancer cell invasion. Clin Cancer Res. 1995 Oct;1(10):1089-94.
- Tchetgen MB, Song JT, Strawderman M, Jacobsen SJ, Oesterling JE. Ejaculation increases the serum prostate-specific antigen concentration. Urology. 1996 Apr;47(4):511-6.
- Herschman JD, Smith DS, Catalona WJ. Effect of ejaculation on serum total and free prostate-specific antigen concentrations. Urology. 1997 Aug;50(2):239-43.
- Gion M, Mione R, Barioli P, et al. Percent free prostate-specific antigen in assessing the probability of prostate cancer under optimal analytical conditions. Clin Chem. 1998 Dec;44(12):2462-70.
Abrahamsson PA, Lilja H, Oesterling JE. Molecular forms of serum prostate-specific antigen. The clinical value of percent free prostate-specific antigen. Urol Clin North Am. 1997 May;24(2):353-65. - Hessels D, Schalken JA. The use of PCA3 in the diagnosis of prostate cancer. Nat Rev Urol. 2009 May;6(5):255-61.
- Day JR, Jost M, Reynolds MA, Groskopf J, Rittenhouse H. PCA3: from basic molecular science to the clinical lab. Cancer Lett. 2011 Feb 1;301(1):1-6.
- Qin W, Smith C, Jensen M, Holick MF, Sauter ER. Vitamin d favorably alters the cancer promoting prostaglandin cascade. Anticancer Res. 2013 Sep;33(9):3861-6.
- van den Bemd GJ, Chang GT. Vitamin D and vitamin D analogs in cancer treatment. Curr Drug Targets. 2002 Feb;3(1):85-94.
- Lou YR, Qiao S, Talonpoika R, Syvala H, Tuohimaa P. The role of Vitamin D3 metabolism in prostate cancer. J Steroid Biochem Mol Biol. 2004 Nov;92(4):317-25.
- John EM, Schwartz GG, Koo J, Van Den BD, Ingles SA. Sun exposure, vitamin D receptor gene polymorphisms, and risk of advanced prostate cancer. Cancer Res. 2005 Jun 15;65(12):5470-9.
- Available at: https://www.labcorp.com/wps/portal/!ut/p/c1/hY1LDoIwFADP4gFMHwUrbFVAhRZq
NXw2hKBBlD9EQ08vF1Azy8lkUIxm6vRV5OlYNHVaohDFJOE6YRwzDDrFFmCVbGC1NRWwyeyj7x7
gT832TXVDEYrXycXUhHIwVPBsgeEgVE9jmBGiAzqjELREPKCl7lBTaXi-a66Ya_Ze5vRw7mCQk-
EAvzrH07UsOx7kQejS5yAy6UvLkpBNqmPx_W68czr_op-_tgrfypIvPv02K8Q!/dl2/d1/L0lJS
2FZQSEhL3dMRUJGcUFFQWpNQy9ZSTV5bHchIS83X1VFNFMxSTkzME9HUzIwSVMzTzRO
Mk42NjgwL3ZpZXdUZXN0/?testId=408405. Accessed September 13, 2013. - Sethi BK, Chanukya GV, Nagesh VS. Prolactin and cancer: Has the orphan finally found a home? Indian J Endocrinol Metab. 2012 Dec;16(Suppl 2):S195-8.
- Available at: http://www.neurophys.gu.se/digitalAssets/1278/1278430_AVHANDLINGSRAM
__Jon_Kindblom.pdf. Accessed September 13, 2013. - Available at: http://www.prostate-cancer.org/pdfs/is4-2.pdf. Accessed September 13, 2013.
- Available at: http://www.cancer.gov/newscenter/qa/2008/pcptqanda. Accessed September 13, 2013.
- Dandona P, Rosenberg MT. A practical guide to male hypogonadism in the primary care setting. Int J Clin Pract. 2010 May;64(6):682-96.
- Miwa Y, Kaneda T, Yokoyama O. Correlation between the Aging Males’ Symptoms Scale and sex steroids, gonadotropins, dehydroepiandrosterone sulfate, and growth hormone levels in ambulatory men. J Sex Med. 2006 Jul;3(4):723-6.
- Arnaldez FI, Helman LJ. Targeting the insulin growth factor receptor 1. Hematol Oncol Clin North Am. 2012 Jun;26(3):527-42,vii-viii.
- Kojima S, Inahara M, Suzuki H, Ichikawa T, Furuya Y. Implications of insulin-like growth factor-I for prostate cancer therapies. Int J Urol. 2009 Feb;16(2):161-7.
- Kong HY, Byun J. Emerging roles of Human prostatic acid phosphatase. Biomol Ther (Seoul). 2013 Jan;21(1):10-20.
- Ligthart ST, Coumans FA, Bidard FC, et al. Circulating tumor cells count and morphological features in breast, colorectal and prostate cancer. PLoS One. 2013 Jun 27;8(6):e67148.
- Miller MC, Doyle GV, Terstappen LW. Significance of circulating tumor cells detected by the CellSearch system in patients with metastatic breast colorectal and prostate cancer. J Oncol. 2010;2010:617421.
- Available at: http://www.upmccancercenter.com/cancer/prostate/biopsyultrasound.cfm. Accessed September 17, 2013.
- Available at: http://www.webmd.com/dvt/doppler-ultrasound. Accessed September 16, 2013.
- Fleischer AC. Sonographic depiction of tumor vascularity and flow: from in vivo models to clinical applications. J Ultrasound Med. 2000 Jan;19(1):55-61.
- Available at: http://prostate-cancer.org/decision-aide/understanding-your-diagnosis/diagnostic
-imaging/#sthash.np5KXLxK.dpuf. Accessed September 16, 2013. - Gupta RT, Kauffman CR, Polascik TJ, Taneja SS, Rosenkrantz AB. The state of prostate MRI in 2013. Oncology (Williston Park). 2013 Apr;27(4):262-70.
- Available at: http://www.med.uc.edu/radiology/research/prostateimaging/mriprocedure.aspx. Accessed September 16, 2013.
- Available at: http://www.cancer.org/treatment/understandingyourdiagnosis/examsandtestdescriptions
/imagingradiologytests/imaging-radiology-tests-nuc-scan. Accessed September 16, 2013. - Tombal B, Lecouvet F. Modern detection of prostate cancer’s bone metastasis: Is the bone scan era over? Adv Urol. 2012;2012:893193.
- Available at: https://www.galileo-training.com/de-deutsch/literaturdownload.html?f=anim25.pdf. Accessed September 16, 2013.
- Tuck SP, Hanusch B, Walker J, Datta HK. Prostate cancer and osteoporosis. Curr Osteoporos Rep. 2013 Mar;11(1):11-20.
- Smith, M.R., McGovern, F.J., Fallon, M.A. et al. Low bone mineral density in hormone-naive men with prostate carcinoma. Cancer. 2001;91:2238–45.
- Bolotin HH Inaccuracies inherent in dual-energy X-ray absorptiometry in vivo bone mineral densitometry may flaw osteopenic/osteoporotic interpretations and mislead assessment of antiresorptive therapy effectiveness. Bone. 2001;28:548–55.
- Meirelles ES, Borelli A, Camargo OP. Influence of disease activity and chronicity on ankylosing spondylitis bone mass loss. Clin. Rheumatol. 1999;18:364–8.
- von der Recke P, Hansen MA, Overgaard K. et al. The impact of degenerative conditions in the spine on bone mineral density and fracture risk prediction. Osteoporos Int. 1996;6:43–9.
- Available at: http://www.medscape.com/viewarticle/738733_5. Accessed October 7, 2013.
- Harvei S. Epidemiology of prostatic cancer. Tidsskr Nor Laegeforen 1999 Oct 10;119(24):3589-94.
- Available at: http://www.idifwb.com/services/exam%20descriptions/bonedensity.htm. Accessed September 16, 2013.
- Available at: http://files.shareholder.com/downloads/GHDX/2097290524x0x661617/85f38a0e-
ccd5-47de-a348-8d0c2b5c03bd/GHDX_News_2013_5_8_General.pdf. Accessed October 1, 2013. - Available at: http://www.myriad.com/products/prolaris. Accessed October 1, 2013.
- Siegel R, Naishadham D, Jemal A. Cancer statistics 2012. CA Cancer J Clin. 2012;62:10-29.
- Available at: http://www.pcf.org/site/c.leJRIROrEpH/b.5802027/k.D271/Prostate
_Cancer_Risk_Factors.htm. Accessed October 1, 2013. - Harvei S. Epidemiology of prostatic cancer. Tidsskr Nor Laegeforen 1999 Oct 10;119(24):3589-94.
- Billis A. Latent carcinoma and atypical lesions of prostate. An autopsy study. Urology. 1986 Oct;28(4):324-9.
- Sakr WA, Grignon DJ, Haas GP, et al. Epidemiology of high grade prostatic intraepithelial neoplasia. Pathol Res Pract. 1995 Sep;191(9):838-41.
- Gallardo-Williams MT, Chapin RE, King PE, et al. Boron supplementation inhibits the growth and local expression of IGF-1 in human prostate adenocarcinoma(LNCaP) tumors in nude mice. Toxicol Pathol. 2004 Jan-Feb;32(1):73-8.
- Choi HY, Lim JE, Hong JH. Curcumin interrupts the interaction between the androgen receptor and Wnt/beta-catenin signaling pathway in LNCaP prostate cancer cells. Prostate Cancer Prostatic Dis. 2010 Dec;13(4):343-9.
- Dorai T, Cao YC, Dorai B, Buttyan R, Katz AE. Therapeutic potential of curcumin in human prostate cancer. III. Curcumin inhibits proliferation, induces apoptosis, and inhibits angiogenesis of LNCaP prostate cancer cells in vivo. Prostate. 2001;47(4):293-303.
- Fleshner, N., Fair, W.R., Huryk, R. et al. Vitamin E inhibits the high-fat diet promoted growth of established human prostate LNCaP tumors in nude mice. J Urol. 1999;161:1651-4.
- Yang Y, Ikezoe T, Zheng Z, Taguchi H, Koeffler HP, Zhu WG. Saw Palmetto induces growth arrest and apoptosis of androgen-dependent prostate cancer LNCaP cells via inactivation of STAT 3 and androgen receptor signaling. Int J Oncol. 2007 Sep;31(3):593-600.
- McCann MJ, Gill CI, Linton T, Berrar D, McGlynn H, Rowland IR. Enterolactone restricts the proliferation of the LNCaP human prostate cancer cell line in vitro. Mol Nutr Food Res. 2008 May;52(5):567-80.
- von Holtz RL, Fink CS, Awad AB. Beta-Sitosterol activates the sphingomyelin cycle and induces apoptosis in LNCaP human prostate cancer cells. Nutr Cancer. 1998;32(1):8-12.
- Zaidman BZ, Wasser SP, Nevo E, Mahajna J. Androgen receptor-dependent and -independent mechanisms mediate Ganoderma lucidum activities in LNCaP prostate cancer cells. Int J Oncol. 2007 Oct;31(4):959-67.
- Thirugnanam S, Xu L, Ramaswamy K, Gnanasekar M. Glycyrrhizin induces apoptosis in prostate cancer cell lines DU-145 and LNCaP. Oncol Rep. 2008 Dec;20(6):1387-92.

