Life Extension Magazine®
The majority of the world’s population—often starting before age five—experiences a decrease in the intestinal enzyme lactase, which creates digestive problems.
Left untreated, lactose intolerance—the inability to digest lactose—can lead to nutritional deficiencies.1,2
Milk products are found in a wide range of foods. People with lactose intolerance have to carefully avoid milk products to prevent diarrhea, gas, nausea, abdominal bloating, and cramps.
To mitigate this problem, researchers in Europe have developed an advanced lactase enzyme formulation that targets the underlying cause of the problem—and helps restore the ability to digest lactose!3,4
This novel enzyme, called neutral lactase, acts in the small intestine, instead of the stomach.5 This gives the digestive system much longer exposure to lactose-degrading molecules than conventional products!3
In this article, you’ll learn how neutral lactase breaks down more lactose, helping to prevent the painful and embarrassing digestive symptoms of lactose intolerance.4
Lactose Intolerance and Nutritional Deficiencies
All humans are born with the ability to drink and digest milk, which makes the abundant nutrition in milk readily available to newborns and young children.
But as we grow older, our levels of the digestive enzyme lactase decline. The result is that some of us lose our ability to break down lactose, the major sugar in milk.6 That makes sense, since in nature, milk consumption ends at weaning.
The only human populations that continue to consume liquid, unfermented milk in sizable quantities are those from Northern Europe and the northwestern territories of the Indian subcontinent.7 But even in those groups, and people descended from them, the prevalence of lactose intolerance hovers around 25%—and in other ethnic groups, can affect up to 90% of the adult population!6,7 That’s a huge number of people who suffer significant gastrointestinal upset from milk products!
In fact, lactose intolerance and the resulting avoidance of milk products is a known risk factor for osteopenia, the steady loss of bone mineral content that leads ultimately to osteoporosis.8,9 It is regrettable that doctors don’t better advise patients with lactose intolerance to supplement with bone-protecting nutrients such as vitamin D, calcium, magnesium, and vitamin K2.
How Lactose Intolerance Develops
The biology of lactose intolerance is simple. But it is devastating in its consequences.
Milk always contains a sugar called lactose.10 In fact, a liter of milk contains about 50 grams of lactose—that’s about 12 grams per cup.11 Lactose is composed of two simple sugars: glucose, the most common sugar in the world, and galactose, a sugar found primarily in milk products.12 Each lactose molecule consists of one molecule of glucose and one of galactose, bonded together.
Lactose by itself can’t be absorbed in the human small intestine. The two component molecules, glucose and galactose, must first be split by a special enzyme—lactase.6,10
Lactase is found in the tips of the cells lining the small intestine, which are directly in contact with intestinal contents.13 As lactase goes to work, glucose and galactose are separated from one another—and then each is separately absorbed (Figure 1).6,13
FIGURE 1: Breakdown of Lactose by Lactase Enzyme
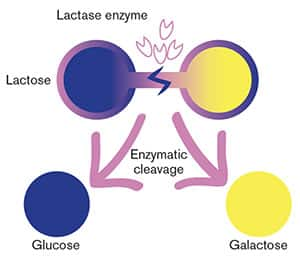 |
The two-sugar molecule lactose is broken down into its constituents, glucose and galactose, by the enzyme lactase.4
So the material that finally reaches the large intestine, or colon, is normally free of lactose, allowing colonic bacteria to live normally (Figure 2).
Newborns and toddlers have ample supplies of lactase in their intestines. That means they can readily digest lactose— along with the various other important nutrients contained in milk.6
But as they age, most children—and virtually all of those not from Northern European ancestry—lose much of their original lactase enzymes. By the time most of these children reach adulthood, lactase levels are very low.6
When a lactase-deficient person ingests lactose-containing milk products in quantities of more than about two cups per day, the small amount of lactase enzyme simply can’t deal with all of the lactose.14
And what happens next can be extremely distressing.
FIGURE 2: Normal Absorption of Glucose and Galactose
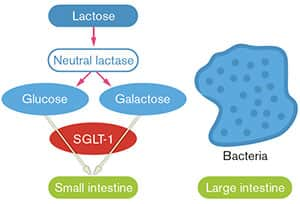 |
With normal levels of the lactase enzyme in the small intestine, lactose is rapidly broken down into glucose and galactose, which are immediately absorbed into the body. Bacteria in the large intestine never come into contact with intact lactose.4
First, the undigested lactose in the small intestine draws water and electrolytes out of the bloodstream by osmosis. All that water dilates the small intestine, causing bloating.6
The dilated small intestine speeds up the rate of peristalsis—or wavelike contractions—of the small intestines, producing cramping. This faster rate of peristalsis means that undigested food moves more rapidly through the small intestine, further impeding normal nutrient absorption. This triggers still more fluid loss as well as bloating and cramping.6
Once this mass of undigested material reaches the large intestine, colonic bacteria attack the intact lactose and digest it themselves. That process generates odorous hydrogen, methane, and carbon dioxide, along with short-chain fatty acids (Figure 3).6,13 This causes more bloating and discomfort, accompanied by the release of these gases in the form of flatulence.15
Finally, all that excess water, gas, and undigested food produces the signature symptom of lactose intolerance: watery, often “explosive” diarrhea.16
Diagnosing Lactose Intolerance
The majority of people with lactose intolerance never undergo formal testing to make the diagnosis. Physicians typically think they recognize the symptoms and often recommend a “lactose-free” diet.13
Alternatively, since lactose intolerance runs in families, many people simply assume they have the condition because of a parent or relative.13
Several laboratory tests exist that can be used to diagnose lactose intolerance. A small intestinal biopsy can allow direct measurement of the enzyme, but this test is invasive, uncomfortable, and expensive—and may not be entirely reliable.13
The “hydrogen breath test” has been developed, which is relatively cheap and quite simple. After ingesting a known amount of lactose, a person simply breathes into a device that measures exhaled hydrogen, one of the byproducts of lactose malabsorption.17 The higher the hydrogen levels in the exhaled breath, the worse the lactose intolerance is.17
Hydrogen breath testing is now used in most studies of treatment of lactose intolerance to determine treatment effectiveness. It is the gold standard in clinical practice to diagnose patients with lactose
intolerance.15
Let’s now look at standard treatment of lactose deficiency to understand what sufferers are up against.
FIGURE 3: How Lactase Deficiency Produces Symptoms of Lactose Intolerance

When there’s insufficient lactase enzyme in the small intestine, lactose can’t be broken down. But intact lactose can’t be absorbed. Instead, it passes into the large intestine, where normal bacterial action converts it into hydrogen, methane, and carbon dioxide gasses, and forms short-chain fatty acids. These, together with the excess water drawn into the intestine by osmosis, produce symptoms of bloating, flatulence, abdominal pain, and diarrhea.4
Standard Treatment of Lactose Intolerance
Most people with lactose intolerance—formally diagnosed or not—are told to “avoid dairy products.” In many cases, that’s as specific of a “treatment” as they receive.
The alternative is to replace the missing lactase enzyme, either by mixing it with milk ahead of time, or by taking a supplement containing the enzyme right before consuming milk.18
Commercial products have been available for decades that contain a form of lactase that can be taken orally before milk consumption.
But these products, derived from molds in the genus Aspergillus, are stable only at the highly acid pH of the stomach.19
The result is that standard lactase supplements have a very brief window of time during which they can attack the lactose load contained in a milk-containing meal or drink.5 After that, they are not effective.
The average time for that stomach exposure is limited to roughly 15 to 45 minutes, which may not be sufficient time for the entire lactose load to be broken down for absorption.3 Scientists have, therefore, been seeking an alternative approach, one that would allow active lactase enzymes to reach the small intestine—its normal site of action—along with the load of lactose from the stomach.
That would permit much longer contact between the lactose load and the lactase enzyme, allowing more complete digestion of lactose in a more natural and appropriate setting.4,13
Fortunately, just such an enzyme has finally been developed and packaged in a form that can survive the acid environment of the stomach.
FIGURE 4: Neutral Lactase In Action

Naturally acquired lactase deficiency can be treated by supplying sufficient neutral lactase to the small intestine, the site of its normal action. This restores the small intestine’s ability to break down lactose into glucose and galactose, which are absorbed. Just as in the normal situation (Figure 2), no intact lactose reaches the bacteria in the large intestine, and no symptoms result.4
New Neutral Lactase Works in the Small Intestine!
Neutral lactase is a novel form of the lactase enzyme that was developed and tested in Europe. It is derived not from the molds in the Aspergillus genus but from a simple yeast commonly used to make cheese, Kluyveromyces lactis.3
Unlike the mold-derived enzyme, which is active only at the acid pH of the stomach, K. lactis-derived lactose is active at the nearly neutral pH of the small intestine.19,20
That range of activity gives neutral lactase a much longer amount of time to work on ingested lactose, so more of that lactose can be broken down and absorbed.13 Neutral lactase, derived from yeast, has been shown to be more effective than mold-derived lactase at digesting lactose in milk.13
This has been evidenced by the significant decrease in the excretion of hydrogen in the breath to near-normal levels, and by the suppression of symptoms when added to milk before consumption.18,21-23
Neutral lactase has passed stringent safety testing, and no safety concerns have been identified.24 It has a long history of use in the food industry to rid milk products of lactose.19,24,25
Lactose Intolerance or Milk Allergy?
There’s a world of difference between the very common condition of lactose intolerance and the much rarer, and more dangerous, true allergy to milk.
Lactose intolerance is caused by low levels of the enzyme lactase that your body uses to break down lactose (milk sugar).3,4 While it can produce many distressing and uncomfortable signals, lactose intolerance is not a true allergy because it does not result in the body developing an antibody-mediated immune reaction.
True milk allergy, on the other hand, involves a potent immune reaction to specific proteins in milk.27 It causes an elevation in various markers of inflammation and heightened immunity. Though rare, some life-threatening allergic reactions to milk proteins have been reported.27 Lactose intolerance causes symptoms of bloating, flatulence, abdominal pain, and watery diarrhea.13 Milk protein allergy generally afflicts children and produces symptoms more commonly associated with allergic reactions, including itching, rash, and wheezing.27
Lactose intolerance is common, but a true milk allergy is rare in adults.27 While neutral lactase has been shown to be effective in decreasing the symptoms of lactose intolerance,3 it will have no effect on milk allergy.
Originally, the major barrier to the use of neutral lactase was the highly acidic, protein-digesting environment of the stomach. Unprotected, neutral lactase would simply be digested itself in the stomach, leaving no activity for the small intestine.
But scientists in Europe have developed a means of protecting neutral lactase by incorporating it into tiny acid-resistant pellets.4
The pellets are packaged in a vegetarian capsule that dissolves in the stomach within minutes of swallowing. Once released in the stomach, the pellets are transferred—without digestion—into the small intestine.3 There, the acid-resistant covering of the pellet is dissolved, releasing 100% of the enzyme for breakdown of lactose, working in the small intestine—just like your body’s own natural lactase enzyme.4
The result?
As shown in Figure 4, the complete digestion of lactose occurs within the small intestine, as neutral lactase immediately releases its glucose and galactose components for rapid absorption. This leaves no intact lactose to pass into the large intestine and trigger the painful and often embarrassing symptoms of lactose intolerance.
A human study of the new formulation showed it to be easily acceptable to patients, and highly effective.3 Among 64 individuals with lactose intolerance, scientists observed that 58% had reduction in abdominal pain, 75% had reduced bloating, 67% had less diarrhea, and nausea was reduced in 58% of subjects.3
The results of a laboratory study graphically demonstrate the difference in performance between traditional acid lactase and the new acid-stable, neutral lactase. You can see the difference for yourself in Figure 5, which illustrates the short duration of action of the acid lactase in the stomach, compared with the much longer period of time the neutral lactase has to work in the small intestine.4
What You Need to Know
 |
Next Generation Support for Lactose-Induced Digestive Disturbance
- Lactose intolerance—the inability to digest milk sugar—afflicts more than 50 million Americans with gas, cramps, bloating, and diarrhea following ingestion of milk products. Its cause is the loss of the lactase enzyme, responsible for breaking down lactose for absorption in the intestine.
- Standard treatment for lactose intolerance includes either avoidance of milk products—which raises the risk of serious nutritional deficiencies—or supplementation with a short-acting lactase enzyme derived from molds.
- Mold-derived lactase works only in the stomach, limiting the amount of time the enzyme has to work on ingested lactose.
- Neutral lactase, derived from a common yeast, can work in the small intestine—the natural site of lactase activity—resulting in a large increase in the amount of time available for the lactase to digest lactose and help prevent symptoms.
- Neutral lactase is manufactured in a novel, acid-resistant form that rapidly passes through the stomach to deliver the active enzyme to the small intestine, the natural site of lactose metabolism.
- Studies show that neutral lactase is more effective than mold-derived acid lactase at reducing signs and symptoms of lactose intolerance.
Summary
Lactose intolerance is one of the most common forms of food intolerance, afflicting upwards of 50 million people in America alone with gas, bloating, cramping, and diarrhea. Its cause is often an age-related loss—frequently beginning before age five26—of the enzyme lactase that’s required for breaking down the unique milk sugar, lactose.
Many sufferers simply avoid milk products.
Another option is replacing the lactase enzyme, but current lactase formulations work only in the acidic environment of the stomach. That limits their usefulness to the 15-45 minutes that a load of lactose remains in the stomach. A substantial amount of undigested lactose, therefore, still reaches the intestinal tract to cause symptoms.
A superior form of the lactase enzyme, neutral lactase, works in the small intestine instead, allowing it to continue breaking down lactose for up to 4 hours! Neutral lactase is packaged in a unique, acid-resistant form that allows it to pass through the stomach rapidly, and reach the small intestine intact.
Treatment with neutral lactase has been shown to reduce symptoms of lactose intolerance more effectively than treatment with standard, acid lactase supplements.
If you suffer from lactose intolerance, you owe it to yourself to use neutral lactase as a means of reducing—or even eliminating—your symptoms.
FIGURE 5: Greater Lactose-Digesting Capacity of Neutral Lactase
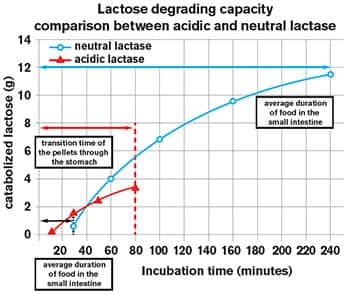 |
Standard acid lactase (red) can act only in the acid environment of the stomach. But gastrointestinal contents are present in the stomach for only a relatively short period. Neutral lactase (blue) acts in the neutral pH of the small intestine, where digesting food remains for up to 4 hours. This allows neutral lactase to break down substantially more lactose than acid lactase. The size of this effect is shown in grams on the vertical axis, while the horizontal axis indicates time.4
Warning
If one suffers lactose intolerance and can avoid milk in their diet, they may be better off than taking a product like neutral lactase. The reason is that most people already have excess amounts of glucose in their bloodstream. A lactose intolerant individual who starts consuming milk products because they take neutral lactase may be unwittingly spiking after-meal glucose blood levels, which increases risk of every disease and may accelerate aging.
While lactose intolerance is associated with loss of bone mass, this problem can be solved by supplementing with calcium, magnesium, vitamins D and K2, along with nutrients one would find in their multi-vitamin/mineral formula.
The only bone mineral not commonly found in supplements is phosphorous. A typical adult needs about 700 mg a day. Those who eat meat (or lots of whole grain cereals and breads) should be assured of adequate phosphorous. Those who avoid dairy, meat, and even grains should consider a 500 mg a day phosphorous supplement to ensure they get enough of this critical component of bone. Please know that most people get enough phosphorous from their diet and don’t need to supplement with any more.
If you have any questions on the scientific content of this article, please call a Life Extension® Health Advisor at 1-866-864-3027.
Editor's Note
Science continues to evolve, and new research is published daily. As such, we have a more recent article on this topic: Who Needs Digestive Enzymes?
References
- Obermayer-Pietsch BM, Gugatschka M, Reitter S, et al. Adult-type hypolactasia and calcium availability: decreased calcium intake or impaired calcium absorption? Osteoporos Int. 2007 Apr;18(4):445-51. Epub 2006 Nov 14.
- Savaiano D. Lactose intolerance: an unnecessary risk for low bone density. Nestle Nutr Workshop Ser Pediatr Program. 2011;67:161-71.
- Fraissl L, Leitner R, Missbichler A. Novel formulation of neutral lactase improves digestion of dairy products in case of lactose intolerance. Clinical and Translational Allergy. 2011 August 12;1(Suppl 1):104.
- SCIOTEC Diagnostic Technologies GmbH. Scientific Information on lactose intolerance and Lactosolv Tulln an der Donau, Austria 2012.
- Bayless TM, Rothfeld B, Massa C, Wise L, Paige D, Bedine MS. Lactose and milk intolerance: clinical implications. N Engl J Med. 1975 May 29;292(22):1156-9.
- Available at: http://emedicine.medscape.com/article/187249-overview#showall. Accessed June 18, 2012.
- Beja-Pereira A, Luikart G, England PR, et al. Gene-culture coevolution between cattle milk protein genes and human lactase genes. Nat Genet. 2003 Dec;35(4):311-3.
- Di Stefano M, Veneto G, Malservisi S, et al. Lactose malabsorption and intolerance and peak bone mass. Gastroenterology. 2002 Jun;122(7):1793-9.
- Kudlacek S, Freudenthaler O, Weissboeck H, Schneider B, Willvonseder R. Lactose intolerance: a risk factor for reduced bone mineral density and vertebral fractures? J Gastroenterol. 2002;37(12):1014-9.
- Lomer MC, Parkes GC, Sanderson JD. Review article: lactose intolerance in clinical practice--myths and realities. Aliment Pharmacol Ther. 2008 Jan 15;27(2):93-103.
- Suarez FL, Savaiano DA, Levitt MD. A comparison of symptoms after the consumption of milk or lactose-hydrolyzed milk by people with self-reported severe lactose intolerance. N Engl J Med. 1995 Jul 6;333(1):1-4.
- Sieber R, Stransky M, de Vrese M. Lactose intolerance and consumption of milk and milk products. Z Ernahrungswiss. 1997 Dec;36(4):375-93.
- Montalto M, Curigliano V, Santoro L, et al. Management and treatment of lactose malabsorption. World J Gastroenterol. 2006 Jan 14;12(2):187-91.
- Vonk RJ, Priebe MG, Koetse HA, et al. Lactose intolerance: analysis of underlying factors. Eur J Clin Invest. 2003 Jan;33(1):70-5.
- Beyerlein L, Pohl D, Delco F, Stutz B, Fried M, Tutuian R. Correlation between symptoms developed after the oral ingestion of 50 g lactose and results of hydrogen breath testing for lactose intolerance. Aliment Pharmacol Ther. 2008 Apr;27(8):659-65.
- Uchida N, Sakamoto O, Irie M, et al. Two novel mutations in the lactase gene in a Japanese infant with congenital lactase deficiency. Tohoku J Exp Med. 2012;227(1):69-72.
- Hermans MM, Brummer RJ, Ruijgers AM, Stockbrugger RW. The relationship between lactose tolerance test results and symptoms of lactose intolerance. Am J Gastroenterol. 1997 Jun;92(6):981-4.
- Rosado JL, Solomons NW, Lisker R, Bourges H. Enzyme replacement therapy for primary adult lactase deficiency. Effective reduction of lactose malabsorption and milk intolerance by direct addition of beta-galactosidase to milk at mealtime. Gastroenterology. 1984 Nov;87(5):1072-82.
- Garcia-Garibay M, Gomez-Ruiz L. Uses of microbial beta-galactosidases to reduce lactose content in milk and dairy products. Rev Invest Clin. 1996 Nov;48 Suppl:51-61.
- Tello-Solis SR, Jimenez-Guzman J, Sarabia-Leos C, et al. Determination of the secondary structure of Kluyveromyces lactis beta-galactosidase by circular dichroism and its structure-activity relationship as a function of the pH. J Agric Food Chem. 2005 Dec 28;53(26):10200-4.
- Solomons NW, Guerrero AM, Torun B. Effective in vivo hydrolysis of milk lactose by beta-galactosidases in the presence of solid foods. Am J Clin Nutr. 1985 Feb;41(2):222-7.
- Montalto M, Nucera G, Santoro L, et al. Effect of exogenous beta-galactosidase in patients with lactose malabsorption and intolerance: a crossover double-blind placebo-controlled study. Eur J Clin Nutr. 2005 Apr;59(4):489-93.
- Solomons NW, Guerrero AM, Torun B. Dietary manipulation of postprandial colonic lactose fermentation: II. Addition of exogenous, microbial beta-galactosidases at mealtime. Am J Clin Nutr. 1985 Feb;41(2):209-21.
- Coenen TM, Bertens AM, de Hoog SC, Verspeek-Rip CM. Safety evaluation of a lactase enzyme preparation derived from Kluyveromyces lactis. Food Chem Toxicol. 2000 Aug;38(8):671-7.
- Pereira-Rodriguez A, Fernandez-Leiro R, Gonzalez-Siso MI, Cerdan ME, Becerra M, Sanz-Aparicio J. Structural basis of specificity in tetrameric Kluyveromyces lactis beta-galactosidase. J Struct Biol. 2012 Feb;177(2):392-401.
- Wang Y, Harvey CB, Hollox EJ, et al. The genetically programmed down-regulation of lactase in children. Gastroenterology. 1998 Jun;114(6):1230-6.
- Bahna SL. Cow’s milk allergy versus cow milk intolerance. Ann Allergy Asthma Immunol. 2002 Dec;89(6 Suppl 1):56-60.

