Life Extension Magazine®
A staggering 53 million Americans suffer from arthritis, making it the leading cause of disability in this country.1-3
A nutritional compound has demonstrated the ability to address one of the root causes of joint pain—reducing pain and improving flexibility—with none of the side effects of typical drug treatments.4-9
Called “un-denatured type II collagen,” or “UC-II®,” this protein compound acts against the autoimmune reactions that can lead to joint pain and degeneration.5,6 UC-II® has been shown effective in previous animal and human studies of arthritis.4,7-9
A groundbreaking new study shows that UC-II® can reduce joint pain and improve joint flexibility even in healthy people who experience painful joints after exercise.4
An Underlying Cause of Osteoarthritis
While the term “arthritis” can be used to describe several different conditions, the two most common forms are osteoarthritis and rheumatoid arthritis.10
Rheumatoid arthritis is an autoimmune disease in which the body reacts to components in joint tissue (mainly collagen) to produce inflammation, pain, and disability. 10
Osteoarthritis was traditionally thought to be the result of wear and tear on the joints.10 Recent discoveries, however, have determined that osteoarthritis is accompanied by the same pro-inflammatory immune factors involved in rheumatoid arthritis.10
In both conditions, an autoimmune response is caused when the body launches an attack against collagen, the substance that makes up the bulk of the cartilage that lines your joints.10,11 Collagen is a protein critical to reducing friction and keeping joints youthful. The problem occurs when microscopic bits of collagen find their way into the bloodstream, at which point immune cells mistakenly identify them as invasive, foreign molecules.11,12
In response to this perceived “threat,” inflammatory cytokines are released that draw in more“killer” T-cells.13 Those cells bombard exposed cartilage with toxic chemicals in order to destroy it, creating oxidative stress and further inflammation in the process.
Over time, these continuous attacks erode and disintegrate the cartilage that lubricates and functions as a shock-absorber in joints.
The resulting pain can become chronic and debilitating, and can include sensations of friction or grinding involved in joint movement. While less acute at rest, this pain is exacerbated by walking, standing, or any form of weight-bearing.9,14 Osteoarthritis sufferers often experience joint stiffness or immobility after periods of inactivity.9
Fortunately, scientists have discovered a substance called un-denatured type II collagen, or UC-II®, that retrains killer T-cells so that they recognize collagen as a harmless substance—preventing the joint damage seen in osteoarthritis. 5,10
Reducing Joint Pain and Swelling
UC-II® was discovered when a team of scientists at the University of Nebraska found that chicken soup prevented the mobilization of immune system cells to sites of inflammation.15 Upon further analysis, they discovered that it was not vegetables, but a component of the chicken broth itself that exerted this anti-inflammatory activity.15
The researchers showed that chicken-derived type II collagen functions to regulate the immune system so that it stops attacking proteins normally found in healthy joint cartilage.10
The results have been remarkable.
In a pilot study of people with severe joint pain, a dose of 10 mg/day of this type II collagen (UC-II®) for 42 days was shown to significantly reduce joint pain and swelling, along with morning stiffness, stiffness following periods of rest, pain that worsens with use of the affected joint, and loss of range of motion and function.10
Follow-up studies show UC-II® reduces joint pain and stiffness that can follow as a result of exercise.4 Even normal exercise puts stress on joints, which causes the release of collagen fragments into the bloodstream.16-19 Since these fragments are partly to blame for post-exercise pain and stiffness,16-19 supplementing with UC-II® can prevent post-exercise pain.
Laboratory Studies
Extensive animal studies have been carried out on the effects of UC-II® in various kinds of arthritis—especially in horses and dogs, two species in which arthritis is common. After 90 days on a 10 mg dose of UC-II®, obese arthritic dogs showed significant decreases in overall pain, in pain during manipulation of a limb, and in lameness after exertion.7
Longer-term studies have shown that after taking UC-II® for 120 days, animals experienced a 62% reduction in overall pain, a 91% reduction in pain caused by limb manipulation, and a 78% reduction in exercise-associated lameness.8 No ill effects or adverse events were seen in any of these studies.
Evaluation of UC-II® in arthritic dogs has been carried out using a high-tech, piezo-electric ground force plate that measures how much weight the animal is putting on each limb and how hard the animal is able to push against the ground as it walks. These studies showed that UC-II ®-supplemented dogs had significant improvements in both measurements, demonstrating a reduction in arthritis-related pain.20
Horses given UC-II® treatments experienced similar benefits. In one study, horses given placebo treatments showed no change in symptoms attributed to arthritis, while the horses given UC-II® treatments experienced an 88% reduction in overall pain and a 78% reduction in pain caused by limb manipulation.21 Again, the treatments were well tolerated and free of side effects.
What You Need to Know
 |
Anti-Arthritis Vaccine
- Millions of Americans suffer from arthritis, yet medications make no real change in the course of the disease.
- Scientists have now discovered that both osteoarthritis and rheumatoid arthritis are caused when the body launches an autoimmune attack against exposed fragments of collagen.
- Un-denatured type II collagen,” or “UC-II®,” is a protein supplement that acts against the autoimmune reactions that can lead to join pain and degeneration.
- Animal and human studies convincingly demonstrate that induction of oral tolerance with UC-II® reduces pain and improves joint function in osteoarthritis and, more recently, in people without arthritis but who suffer joint pain and stiffness following exercise.
- UC-II® is safe and well tolerated; it should form part of every serious joint health program.
Relief for Osteoarthritis Pain
Human clinical trials of UC-II® demonstrate similar effectiveness in adults suffering from osteoarthritis.9
In one study, patients with knee osteoarthritis received UC-II® or standard treatment for 90 days.9 The supplemented group experienced a 33% reduction in their osteoarthritis compared to standard therapy recipients. UC-II® reduced the patients’ self-determined pain scale scores by 40%, compared with just 15.4% in those receiving standard care. And UC-II ® improved joint function by 20%, compared with 6% for usual care.
Impact of Arthritis on US Population
 |
- 53.8 million adults were affected by arthritis in 20112
- 67 million adults are expected to be affected by 20301
- 22% of US adults suffer from arthritis3
- 33.8% of obese women have arthritis3
- 25.2% of obese men have arthritis3
- 30% of adults suffer from some type of joint pain36
Improving Exercise-Induced Joint Pain
Of course, arthritis is just one of many causes of joint pain, which is why researchers in California recently conducted a study of oral UC-II® in healthy adults who did not have arthritis. These subjects had no knee pain at rest, but reported significant knee pain after exercise.4 The patients underwent a similar exertion test at each of 7 visits over a 120-day period.
Compared to their performance at the beginning of the study, by days 90 and 120, the subjects that had supplemented with 40 mg UC-II® could exercise for significantly longer before experiencing joint pain; no such changes were seen in the placebo group.4 Supplemented subjects recovered from their joint discomfort significantly faster than the placebo recipients at days 60, 90, and 120.
The same new study evaluated joint flexibility and determined that the average knee extension was significantly greater in the UC-II ® group than in the placebo group at day 120.4 Importantly, UC-II® recipients had significant increases in their knee extension compared to their own baseline level, with no such changes seen among placebo recipients.
In this study published in 2013, the researchers concluded that UC-II® was “more effective than placebo in supporting joint comfort, flexibility, and mobility."4
The broad-spectrum safety of UC-II® has been evaluated by a number of toxicological assays.22 It causes no mutations in bacterial genomes, a standard screen for carcinogenicity, and is not associated with oral toxicity.
How it Works
UC-II® works through something called oral tolerance, which is the desensitization of immune response to specific agents via an orally administered intervention.10,13 In this way, UC-II® reverses T-cell attacks on exposed cartilage.10
This makes sense, considering that when researchers want to produce an animal model of human arthritis, they inject small quantities of collagen. 23 The immune system responds by ramping up production of cells that react to collagen. Those cells then attack normal, healthy joint tissue, producing symptoms and signs of arthritis.23,24
Remarkably, however, if the animals are first given a small oral dose of collagen, the incidence of experimentally induced arthritis plummets.5,6 And the severity of joint disease is reduced in the animals that do develop arthritis.25,26
This phenomenon, called “oral tolerance,” relies on what’s known as gut-associated lymphoid tissue.10,27-30 Clumps of this tissue are found in the human intestinal tract; they are instrumental in “presenting” the oral collagen fragments to the immune system, which then suppresses its response to the protein.29,31,32
Oral tolerance has other benefits as well, including fighting food allergies through careful exposure to the offending foods.28 A similar methodology is under investigation for boosting the immune response to certain cancers, especially those of the intestinal tract (mushroom extracts are used there).33
Pre-treatment with UC-II®, in other words, may be inducing immune tolerance even in healthy adults, protecting them from deleterious exposure to their own cartilage.
We don’t react to our own cartilage normally because, in intact joints, there’s a barrier between blood and cartilage so that immune system cells in the blood don’t “see” cartilage proteins.34,35 In the aging joint, this protective barrier between blood and cartilage diminishes.34
UC-II® offers a different approach to modifying joint inflammation rather than simply masking the symptoms.
FIGURE: Nearly One-Third of Americans Suffer from Joint Pain36
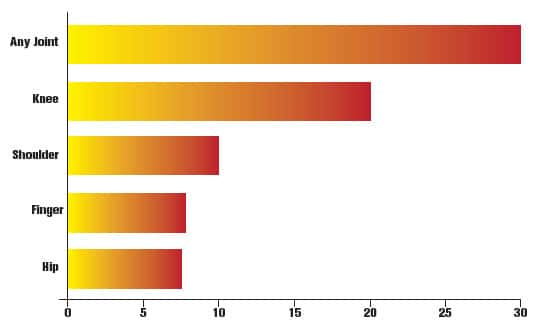
A national survey revealed that nearly one-third of US adults report suffering from joint pain of some kind within the last 30 days. The knee was by far the most common single site for pain or stiffness, though many people reported pain at more than one joint.
Summary
Arthritis leads the list of conditions that cause disability among American adults.
Standard medical treatment consists mainly of treating the symptoms, with few tolerable drugs that modify the course of the disease.
A low-cost nutritional supplement has the ability to address the root cause of joint pain—reducing joint pain and improving flexibility. Called “un-denatured type II collagen,” or “UC-II®,” this natural protein supplement acts against the autoimmune reactions that can lead to join pain and degeneration.
UC-II® has demonstrated efficacy in animal and human studies of arthritis—and can even reduce joint pain and improve joint flexibility in healthy people who experience painful joints after exercise.4
The implications cannot be overstated; the ability to move comfortably and engage in regular physical activity is critical to maintaining health in the face of our national epidemic of obesity, diabetes, and cardiovascular diseases.
If you have any questions on the scientific content of this article, please call a Life Extension® Health Advisor at 1-866-864-3027.
Important Note
Not all collagen is equal in its ability to fight joint pain. There are two types of collagen: denatured collagen (collagen that’s been disrupted by heat or chemical treatment) and undenatured collagen.
An experimental model of autoimmune arthritis showed that “denatured” collagen had no effect on the incidence or severity of the disease.5
But the specially-processed undenatured type II collagen (UC-II®) is more effective because it’s uniquely designed to preserve the 3-dimensional structure of type II collagen. Immune cells in the intestine rely on 3-D shapes to recognize and respond to the signals that turn them on or off. UC-II® provides the correct 3-D structures to intestinal immune cells, triggering the signaling required for the development of immune tolerance.10
Editor's Note
Science continues to evolve, and new research is published daily. As such, we have a more recent article on this topic: Ease Arthritis with Type II Collagen
References
- Prevalence of doctor-diagnosed arthritis and arthritis-attributable activity limitation--United States, 2003-2005. MMWR Morb Mortal Wkly Rep. 2006 Oct 13;55(40):1089-92.
- Available at: http://www.cdc.gov/nchs/fastats/arthrits.htm. Accessed August 10, 2011.
- Prevalence of doctor-diagnosed arthritis and arthritis-attributable activity limitation --- United States, 2007-2009. MMWR Morb Mortal Wkly Rep. 2010 Oct 8;59(39):1261-5.
- Udani JK. UC-II® for Joint Support: A randomized, double-blind, placebo-controlled study in healthy volunteers. Scripps 10th Annual Conference: Natural Supplements: An Evidence Based Update. Vol San Diego 2013.
- Nagler-Anderson C, Bober LA, Robinson ME, Siskind GW, Thorbecke GJ. Suppression of type II collagen-induced arthritis by intragastric administration of soluble type II collagen. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7443-6.
- Zhu P, Li XY, Wang HK, et al. Oral administration of type-II collagen peptide 250-270 suppresses specific cellular and humoral immune response in collagen-induced arthritis. Clin Immunol. 2007 Jan;122(1):75-84.
- Deparle LA, Gupta RC, Canerdy TD, et al. Efficacy and safety of glycosylated undenatured type-II collagen (UC-II®) in therapy of arthritic dogs. J Vet Pharmacol Ther. 2005 Aug;28(4):385-90.
- D’Altilio M, Peal A, Alvey M, et al. Therapeutic Efficacy and Safety of Undenatured type II collagen singly or in combination with glucosamine and chondroitin in arthritic dogs. Toxicol Mech Methods. 2007;17(4):189-96.
- Crowley DC, Lau FC, Sharma P, et al. Safety and efficacy of undenatured type II collagen in the treatment of osteoarthritis of the knee: a clinical trial. Int J Med Sci. 2009;6(6):312-21.
- Bagchi D, Misner B, Bagchi M, et al. Effects of orally administered undenatured type II collagen against arthritic inflammatory diseases: a mechanistic exploration. Int J Clin Pharmacol Res. 2002;22(3-4):101-10.
- Charni N, Juillet F, Garnero P. Urinary type II collagen helical peptide (HELIX-II) as a new biochemical marker of cartilage degradation in patients with osteoarthritis and rheumatoid arthritis. Arthritis Rheum. 2005 Apr;52(4):1081-90.
- Heinegard D, Saxne T. The role of the cartilage matrix in osteoarthritis. Nat Rev Rheumatol. 2011 Jan;7(1):50-6.
- Cohen ES, Bodmer HC. Cytotoxic T lymphocytes recognize and lyse chondrocytes under inflammatory, but not non-inflammatory conditions. Immunology .2003 May;109(1):8-14.
- Recommendations for the medical management of osteoarthritis of the hip and knee: 2000 update. American College of Rheumatology Subcommittee on Osteoarthritis Guidelines. Arthritis Rheum. 2000 Sep;43(9):1905-15.
- Rennard BO, Ertl RF, Gossman GL, Robbins RA, Rennard SI. Chicken soup inhibits neutrophil chemotaxis in vitro. Chest. 2000 Oct;118(4):1150-7.
- O’Kane JW, Hutchinson E, Atley LM, Eyre DR. Sport-related differences in biomarkers of bone resorption and cartilage degradation in endurance athletes. Osteoarthritis Cartilage. 2006 Jan;14(1):71-6.
- Petersen SG, Saxne T, Heinegard D, et al. Glucosamine but not ibuprofen alters cartilage turnover in osteoarthritis patients in response to physical training. Osteoarthritis Cartilage. 2010 Jan;18(1):34-40.
- Fujita T, Ohue M, Fujii Y, Miyauchi A, Takagi Y. Analgesic and chondroprotective effects of risedronate in osteoarthritis assessed by electroalgometry and measurement of collagen type II fragments in urine. J Int Med Res. 2008 Sep-Oct;36(5):932-41.
- Roos H, Dahlberg L, Hoerrner LA, et al. Markers of cartilage matrix metabolism in human joint fluid and serum: the effect of exercise. Osteoarthritis Cartilage. 1995 Mar;3(1):7-14.
- Gupta RC, Canerdy TD, Lindley J, et al. Comparative therapeutic efficacy and safety of type-II collagen (UC-II®), glucosamine and chondroitin in arthritic dogs: pain evaluation by ground force plate. J Anim Physiol Anim Nutr (Berl). 2012 Oct;96(5):770-7.
- Gupta RC, Canerdy TD, Skaggs P, et al. Therapeutic efficacy of undenatured type-II collagen (UC-II®) in comparison to glucosamine and chondroitin in arthritic horses. J Vet Pharmacol Ther. 2009 Dec;32(6):577-84.
- Marone PA, Lau FC, Gupta RC, Bagchi M, Bagchi D. Safety and toxicological evaluation of undenatured type II collagen. Toxicol Mech Methods. 2010 May;20(4):175-89.
- Cremer MA, Rosloniec EF, Kang AH. The cartilage collagens: a review of their structure, organization, and role in the pathogenesis of experimental arthritis in animals and in human rheumatic disease. J Mol Med (Berl). 1998 Mar;76(3-4):275-88.
- Corthay A, Backlund J, Broddefalk J, et al. Epitope glycosylation plays a critical role for T cell recognition of type II collagen in collagen-induced arthritis. Eur J Immunol. 1998 Aug;28(8):2580-90.
- Barnett ML, Kremer JM, St Clair EW, et al. Treatment of rheumatoid arthritis with oral type II collagen. Results of a multicenter, double-blind, placebo-controlled trial. Arthritis Rheum. 1998 Feb;41(2):290-7.
- Zhao W, Tong T, Wang L, et al. Chicken type II collagen induced immune tolerance of mesenteric lymph node lymphocytes by enhancing beta2-adrenergic receptor desensitization in rats with collagen-induced arthritis. Int Immunopharmacol. 2011 Jan;11(1):12-8.
- Weiner HL. Oral tolerance: immune mechanisms and treatment of autoimmune diseases. Immunol Today. 1997 Jul;18(7):335-43.
- Badina L, Barbi E, Berti I, et al. The dietary paradox in food allergy: yesterday’s mistakes, today’s evidence and lessons for tomorrow. Curr Pharm Des. 2012;18(35):5782-7.
- Park KS, Park MJ, Cho ML, et al. Type II collagen oral tolerance; mechanism and role in collagen-induced arthritis and rheumatoid arthritis. Mod Rheumatol. 2009;19(6):581-9.
- Peron JP, de Oliveira AP, Rizzo LV. It takes guts for tolerance: the phenomenon of oral tolerance and the regulation of autoimmune response. Autoimmun Rev. 2009 Sep;9(1):1-4.
- Meyer O. Oral immunomodulation therapy in rheumatoid arthritis. Joint Bone Spine. 2000;67(5):384-92.
- Min SY, Park KS, Cho ML, et al. Antigen-induced, tolerogenic CD11c+,CD11b+ dendritic cells are abundant in Peyer’s patches during the induction of oral tolerance to type II collagen and suppress experimental collagen-induced arthritis. Arthritis Rheum. 2006 Mar;54(3):887-98.
- Tanaka K, Matsui Y, Ishikawa S, Kawanishi T, Harada M. Oral ingestion of Lentinula edodes mycelia extract can restore the antitumor T cell response of mice inoculated with colon-26 cells into the subserosal space of the cecum. Oncol Rep. 2012 Feb;27(2):325-32.
- Suri S, Walsh DA. Osteochondral alterations in osteoarthritis. Bone. 2012 Aug;51(2):204-11.
- Shukunami C. Vascular development and cartilage formation. Clin Calcium. 2006 Apr;16(4):676- 81.
- Available at: http://www.cdc.gov/features/dsjointpain/index.html. Accessed February 11, 2013.

