Life Extension Magazine®
Preamble If you wonder what results are occurring with the millions of dollars that Life Extension® donates to cancer research, this article provides a real world report of a successful clinical trial conducted outside the United States. We are still losing the war on cancer.1 While the establishment brags that more cancer patients survive than ever before, the horrific side effects inflicted by conventional therapy often leave patients partially or severely debilitated, and set the stage for deadly secondary diseases. Over 570,000 Americans are expected to die from cancer this year.2 For those people, the dangerous mix of chemotherapy, radiation, and surgery not only failed to cure the cancer, but destroyed their remaining quality of life. Meanwhile, many promising therapeutic techniques languish in laboratories and private clinics, quashed by excessive FDA regulations and greedy pharmaceutical giants eager to protect their own lucrative turf. But all of that may be about to change, thanks to pioneering work on breast cancer from a progressive clinic outside the US. There, a group of physicians and scientists have teamed up in a study that's the first of its kind: a human clinical trial using a sophisticated form of laser/immune therapy to treat advanced breast cancer.3 The technique is called laser-assisted immunotherapy. This laser treatment has the potential to destroy primary breast tumors. This unique treatment can also seek and destroy cancer cells that have spread (metastasized) from the original tumor to other parts of the body. This is especially critical as metastasized cells are the primary cause of cancer death. In addition to being a potentially effective cancer treatment, laser-assisted immunotherapy appears to be able to act as a cancer vaccine, effectively preventing the same cancer from recurring. This innovative procedure may be the life-saving therapy that cancer patients have been waiting for. Laser-Assisted Immunotherapy: An Innovative Approach to Treating Advanced Cancers
An ideal approach to treating advanced cancers would accomplish two things: 1. Eliminate the original tumor. 2. Empower the immune system to destroy the cancer cells. Then, instead of having to locate and treat every infiltrated lymph node and every hidden metastasis, the body's natural tumor surveillance system would do its work, root out the cancer, and prevent recurrence. This is the central principle behind laser-assisted immunotherapy.4,5 Laser-assisted immunotherapy involves no surgery, chemotherapy, or dangerous radiation. Instead, the technique uses a precise laser beam to destroy the primary tumor, and then delivers a powerful boost to the immune system's natural cancer defense systems.4 In this way, the entire immune system joins the fight against an individual's own specific cancer.6 Laser-assisted immunotherapy includes the following three components: 1. A laser beam operating in the "near-infrared" frequency of light.7 This beam heats tissue to a depth of several centimeters, allowing the beam to penetrate directly into a solid tumor with minimal damage to normal tissue.8 Because it can be focused directly through intact skin, no surgical incision is required. These lasers have been in use in cancer therapy for more than 20 years.9 On their own, however, they can't safely raise the temperature of tumor cells without damaging adjacent healthy tissue.9,10 That requires the use of a second component… 2. A photosensitizer, a chemical that makes cancer cells more vulnerable to destruction by the laser without the unacceptable heating of nearby normal cells.6 The technique was proven in pre-clinical studies with the use of a compound called indocyanin green (ICG) as the photosensitizer. Like the laser, ICG is safe for clinical applications and is FDA approved. The combination of the laser and photosensitizer leads to localized destruction of tumor cells. However, these two components alone may not be effective at completely ridding the body of cancer because they cannot attack distant, often hidden tumor metastases.11 To kill those, one more crucial component is required… 3. An immune system booster12, or "adjuvant," that activates the cancer patient's natural cancer-attacking killer cells.7 Growing tumors have multiple ways of evading the immune system, effectively concealing their abnormal nature from circulating immune surveillance cells. Once the tumor is partially broken up by the laser, however, the injection of the adjuvant then calls the immune system's attention to the newly revealed tumor markers.8,13 Circulating immune cells can then begin killing malignant cells anywhere they have spread in the body.14 This immune booster may soon be submitted to FDA for approval and those involved in submitting the New Drug Application have asked that we not disclose its name in print.  Cancer Cell The key aspect of this approach is that the stimulated immune system attacks not only the primary tumor, but also metastases located anywhere in the body.4,6,15 Because those metastases often bear the same abnormal signal proteins as the primary tumor, they now essentially have a mark on their back for the body's natural defense mechanism to seek out and destroy—no matter where they've moved to in the body. As an added bonus, the immune system "remembers" the identifying markers of the cancer and continuously maintains surveillance to prevent future recurrence.16 In essence, laser-assisted immunotherapy creates a tiny "vaccine factory" within the victim's body that throws the entire immune system's resources at precisely that person's own tumor (see Sidebar at the end of this article for more on this topic).14 What You Need to Know Breast Cancer Mammography Advanced Breast Cancer Treatment: Hope Is On The Horizon
|
Lab Studies Provide Dramatic Results
Laser-assisted immunotherapy has received compelling validation in the laboratory. When rats with experimentally induced breast cancer were subjected to the treatment, they experienced marked increases in survival rates.11 In addition, researchers have documented the eradication of both primary tumors and metastases.11,16 Most importantly, successfully treated rats have proven to be resistant to developing new tumors—even when cancer cells were directly injected into the animals' bodies.4,11
For the pre-clinical experiments, rats were injected with breast cancer cells, which formed localized primary tumors.3,4 After 11 days, the animals were treated with the laser therapy with the indocyanin green or laser therapy with indocyanin green and the immune booster described above.
In the group of untreated animals, the primary tumors grew rapidly within the first month, and all animals died by 40 days. The treated animals, on the other hand, had only modest growth of the primary tumor, which then proceeded to shrink in size—and 38% of the animals survived until the end of the study at 120 days (Figures 1 and 2).4 Only animals administered the three-prong combination achieved long-term survival. In other words, the 38% of the animals that survived until the end of the study all received the full laser-assisted immunotherapy that included indocyanin green and the immune enhancement adjuvant.
The most dramatic result—and the one with the greatest potential impact for people with breast cancer—was the effect on metastases. The untreated animals developed multiple metastases in the groin (inguinal) and armpit (axillary) areas.3,4 In the treated group, however, the metastases were completely destroyed within 40 days (Figure 3).
Human Breast Cancer Study Results: 80% Survival Rate for Stage IV Cancer
Energized by the striking results from the animal studies, researchers decided to bring the benefits of laser-assisted immunotherapy to women with breast cancer. This malignancy is expected to cause over 290,000 new cases this year in the US and affects millions of women who battle with recurrence and remissions.2 It is a significant cause of death, disfigurement, and disability.

After injection with breast cancer cells on day zero, untreated animals begin dying by day 30, and all are dead by day 40. But tumor-bearing animals treated with laser-assisted immunotherapy survived much longer and many were still alive by the end of the study.4

In an untreated animal, death occurred on day 32, following rapid growth of the primary breast cancer. The primary tumor grew much more slowly in a treated animal, peaked at about 30 days, and then gradually disappeared, becoming undetectable by day 65.4
The researchers were determined to evaluate the laser-assisted immunotherapy as soon as possible. Frustrated with the corrupt, inefficient, and time-consuming ways of the FDA—the all-powerful agency that regulates such research in the US—they chose a highly regarded private clinic in the Caribbean as the venue for their study.3
Because of the technique's unique ability to destroy both an advanced primary tumor and life-threatening metastases, the researchers decided to treat women with breast cancers that had already spread to the lymph nodes or to other parts of the body. Although the study was opened to 45 women, the following is a preliminary report on the first 15 subjects enrolled.
The study protocol was similar to that used in the animal studies described above. Subjects received two courses of treatment over two weeks, and were followed for one year. If needed, an additional course of treatment was available.
After a physical examination and blood testing, the women received an injection of a local anesthetic in the area of the primary breast cancer, followed by injection of the photosensitizing agent (indocyanin green) into the tumor. Next, the near-infrared laser was applied to the area in and around the tumor. During this phase, the laser destroyed cells in the primary superficial tumor, while exposing their biological markers to the subject's immune system.
After 10 to 12 minutes of laser therapy, the immune-boosting material was injected around and underneath the tumor. In this phase of the treatment, the subject's own immune system cells were drawn to the tumor area to attack the primary breast tumor, as well as the malignant cells in lymph nodes and metastases in other parts of the subject's body.
The results from this early study are encouraging, particularly in light of established rates of survival. Among all 15 subjects in the study, 80% remain alive today. Compare that with the typical survival rate in the United States for women with such advanced breast cancer, which is only 23.8% at 5 years.17 (This study is so new that no woman has yet reached the 5-year milestone.)
At the time of this writing, the women in the laser-assisted immunotherapy study had an average survival of more than 29 months after treatment, and still counting. Results of conventional chemotherapeutic or anti-hormone breast cancer treatment aren't nearly as encouraging. One study reported an average survival of just 15.4 months,18 another small study reported survival up to 23.1 months, but had no survivors by 3 years.19
Tales of Courage and Survival
Of course, compelling as they might be, numbers tell only a portion of any story about human health and longevity. The real picture of the treatment's potential comes from the real-life stories of the women who participated in this study. Here are just a few of those stories.
A 66-year-old woman enrolled in the study in August 2008, after having only a modest response to eight cycles of conventional chemotherapy. At the time of her enrollment, her disease had progressed to stage IV, with at least one metastasis as diagnosed with a PET/CT scan. She received one full laser-assisted immunotherapy treatment in December 2008. A follow-up PET/CT scan showed disappearance of the primary tumor, and no new metastases or lymph node involvement.

Left Microscopic Image of Breast Carcinoma.
Right Metastatic tumors in an untreated animal rapidly grew in size, culminating in the animal’s death on day 30. By contrast, those in the treated animal grew modestly, peaked at about day 32, and then rapidly regressed. No metastases were detected after day 40 in the treated animal, which survived to the end of the study.4
One year and nine months after the treatment, this patient received the following mammography report: "The result of your breast imaging exam shows no evidence of cancer."
She remains disease-free at more than 3 years and 4 months following treatment.
A 78 year-old woman with stage IV breast cancer, who had received no conventional treatment, enrolled in the study in January 2009. After undergoing the laser-assisted immunotherapy, a follow-up PET/CT scan revealed no visible cancer and no metastases. She did experience a brief recurrence of the tumor, but that condition resolved without further therapy.
More than 3 years later, she too remains disease-free.
Important Facts about Breast Cancer
According to the National Cancer Institute:
- There are more than 2.75 million living women with a history of breast cancer, including those with active disease and those who have been cured.24
- One in eight women (12.38%) born today will be diagnosed with breast cancer at some time in their lives.25
- 5.72% of women will develop cancer of the breast between their 50th and 70th birthdays.25
- The median age at diagnosis for breast cancer is 61 years old (Figure 4).26
- The median age of dying from breast cancer is 68 years old (Figure 4).26
- Overall 5-year survival for women with breast cancer is 90.3%. For those with cancer that has metastasized (stage IV), however, just 23.8% (or fewer) remain alive 5 years after their diagnosis.27
- There has been a fluctuating trend in breast cancer incidence since 1975 (Figure 5).
Two women with earlier stage cancers (stage II) entered the study in 2009; one had received some conventional therapy while the other had none. Following laser-assisted immunotherapy, both subjects remain alive and disease-free today.
These cases show the great potential of laser-assisted immunotherapy in treating breast cancer. No therapy is perfect, however, and it's important to note that not all of the women in the study experienced the same results as the ones mentioned above.
For example, a 65-year-old woman with stage IV breast cancer enrolled in the study in August 2008, after a number of failures of conventional therapy. She did experience some tumor recurrence requiring surgery 15 months after her initial laser treatment, but survived until 3 years and 3 months after the start of the study. The experimental treatment may have prolonged her life, but there is no way to be certain.

Breast cancer can occur as early as the late 20s, but diagnoses peak by about age 60 (blue bars). Rates of death from breast cancer rise sharply between early middle age, and remain high well into advanced years (red bars).26

Rates of death from breast cancer have fluctuated since the mid-1970s. Points above the zero line indicate periods of increased death rates; points below the line indicate periods of declining death rates.26
Summary
Laser-assisted immunotherapy is a promising new weapon in the war on cancer. The results of this study so far show 80% of the participants still alive today compared to the typical 5-year survival rate in the US of only 23.8% for advanced breast cancer. Providing a non-toxic therapy that supports and stimulates rather than destroys the body's best defense arsenal, the immune system, is a monumental gain against the tenacious and formidable opponent known as cancer.
Of particular significance is the lack of serious side effects caused by laser-assisted immunotherapy. Even lumpectomies can result in significant mutilation to the affected breast, while follow-up radiation, chemotherapy and hormone-blocking agents result in debilitating initial side effects and deadly long-term toxicities.
For example, in a report released last month that studied 12,000 breast cancer survivors, there was a 470% increased risk of developing heart failure in the group receiving chemotherapy compared to breast cancer patients who did not undergo chemotherapy.20 The cancer establishment considers those treated with chemotherapy to be successes if they survive the cancer, but conveniently ignores the fact that the treatment causes heart failure.
The objective of laser-assisted immunotherapy is to eradicate the primary tumor along with metastatic lesions without inflicting the side effects associated with conventional breast cancer treatment.
If you have any questions on the scientific content of this article, please call a Life Extension® Wellness Specialist at 1-866-864-3027.
Creating a Customized Cancer Vaccine
Developing an effective anti-cancer vaccine has been the goal of oncology for decades.21,22 The idea is to identify one or several markers, or antigens, borne only by tumor cells, for use in the manufacture of a vaccine. In theory, such a vaccine could eliminate cancer once and for all.22 The vaccine could even be beneficial for those with existing cancers, as the vaccine could expedite destruction of the existing tumor by activating the immune system.
However, like most attempts at a “one-size-fitsall” strategy, this approach has flaws that limit its appeal. Often what appears to be a single tumor actually contains multiple different cell lines, each bearing a unique marker, and each affecting patient survival differently.23 A cancer vaccine prepared in a laboratory therefore has essentially zero chance of raising an immune response to every cell type in any one person’s tumor, allowing a few to survive and produce cancer recurrences.
This situation is quite similar to the well-known annual quandary of flu vaccine manufacturers. Based on the best available information about the previous year’s flu virus, they must make an educated guess about which specific viral components to include in the upcoming year’s vaccine (e.g., H1N1, H5N1, etc). Most years they are successful, but occasionally a virus appears for which we are entirely unprotected.
Laser-assisted immunotherapy eliminates this kind of guesswork. That’s because the “vaccine” is manufactured within each individual person’s body, in response to their own unique tumor markers. This customized vaccine can then direct all of the immune system’s powerful resources to eliminating the existing cancer, at the same time producing persistent immunity against future recurrences.14
References
1. Frieden TR, Myers JE, Krauskopf MS, Farley TA. A public health approach to winning the war against cancer. Oncologist. 2008 Dec;13(12):1306-13.
2. Available at: http://www.cancer.org/acs/groups/content/ @epidemiologysurveilance/documents/ document/acspc-031941.pdf. Accessed September 4, 2012.
3. Adalsteinsson O. Laser-Assisted Immunotherapy: A Novel Autologous Vaccine Strategy for Cancers with Solid Tumors Clinical Protocol #ISCA 0001 ed.: International Strategic Cancer Alliance; 2012.
4. Chen WR, Zhu WG, Dynlacht JR, Liu H, Nordquist RE. Long-term tumor resistance induced by laser photo-immunotherapy. Int J Cancer. 1999 May 31;81(5):808-12.
5. Chen WR, Huang Z, Korbelik M, Nordquist RE, Liu H. Photoimmunotherapy for cancer treatment. J Environ Pathol Toxicol Oncol. 2006;25(1-2):281-91.
6. Chen WR, Liu H, Ritchey JW, Bartels KE, Lucroy MD, Nordquist RE. Effect of different components of laser immunotherapy in treatment of metastatic tumors in rats. Cancer Res. 2002 Aug 1;62(15):4295-9.
7. Chen WR, Liu H, Carubelli R, Nordquist RE. Synergistic effect of photothermal and photoimmunological reactions in treatment of metastatic tumors. J Xray Sci Technol. 2002 Jan 1;10(3):225-35.
8. Canti G, Calastretti A, Bevilacqua A, Reddi E, Palumbo G, Nicolin A. Combination of photodynamic therapy + immunotherapy + chemotherapy in murine leukiemia. Neoplasma. 2010;57(2):184-8.
9. Wainwright M. Photodynamic therapy: the development of new photosensitisers. Anticancer Agents Med Chem. 2008 Apr;8(3): 280-91.
10. Stefflova K, Chen J, Zheng G. Killer beacons for combined cancer imaging and therapy. Curr Med Chem. 2007;14(20):2110-25.
11. Chen WR, Adams RL, Carubelli R, Nordquist RE. Laser-photosensitizer assisted immunotherapy: a novel modality for cancer treatment. Cancer Lett. 1997 May 1;115(1):25-30.
12. St Denis TG, Aziz K, Waheed AA, et al. Combination approaches to potentiate immune response after photodynamic therapy for cancer. Photochem Photobiol Sci. 2011 May;10(5):792-801. Epub 2011 Apr 9.
13. Chen WR, Korbelik M, Bartels KE, Liu H, Sun J, Nordquist RE. Enhancement of laser cancer treatment by a chitosan-derived immunoadjuvant. Photochem Photobiol. 2005 Jan-Feb;81(1):190-5.
14. Garg AD, Nowis D, Golab J, Agostinis P. Photodynamic therapy: illuminating the road from cell death towards anti-tumour immunity. Apoptosis. 2010 Sep;15(9):1050-71.
15. Chen WR, Carubelli R, Liu H, Nordquist RE. Laser immunotherapy: a novel treatment modality for metastatic tumors. Mol Biotechnol. 2003 Sep;25(1):37-44.
16. Chen WR, Jeong SW, Lucroy MD, et al. Induced antitumor immunity against DMBA-4 metastatic mammary tumors in rats using laser immunotherapy. Int J Cancer. 2003 Dec 20;107(6): 1053-7.
17. Beinart G, Gonzalez-Angulo AM, Broglio K, et al. Clinical course of 771 patients with bilateral breast cancer: characteristics associated with overall and recurrence-free survival. Clin Breast Cancer. 2007 Dec;7(11):867-74.
18. Fields RC, Jeffe DB, Trinkaus K, et al. Surgical resection of the primary tumor is associated with increased long-term survival in patients with stage IV breast cancer after controlling for site of metastasis. Ann Surg Oncol. 2007 Dec;14(12):3345-51.
19. Cummiskey RD, Mera R, Levine EA. Preoperative chemotherapy for locally advanced breast carcinoma at Charity Hospital, New Orleans, Louisiana. Am Surg. 1998 Feb;64(2):103-6.
20. Available at: http://vitals.nbcnews.com/_news/2012/08/31/ 13573426-breast-cancer-survivors-may-face-second-threat-heart-failure?lite. Accessed September 4, 2012.
21. Tan PH, Lota AS. Interaction of current cancer treatments and the immune system: implications for breast cancer therapeutics. Expert Opin Pharmacother. 2008 Oct;9(15):2639-60.
22. Watson CJ, Gusterson BA. A prophylactic vaccine for breast cancer? Breast Cancer Res. 2010;12(4):310.
23. Shipitsin M, Campbell LL, Argani P, et al. Molecular definition of breast tumor heterogeneity. Cancer Cell. 2007 Mar;11(3):259-73.
24. Available at: http://seer.cancer.gov/statfacts/html/ breast.html#prevalence. Accessed April 5, 2012.
25. Available at: http://seer.cancer.gov/statfacts/html/ breast.html#risk. Accessed April 5, 2012.
26. Available at: http://seer.cancer.gov/statfacts/html/ breast.html#incidence-mortality. Accessed April 5, 2012.
27. Available at: http://seer.cancer.gov/statfacts/html/ breast.html#survival. Accessed April 5, 2012.


References
- Available at: http://www.cancer.org/acs/groups/ content/@epidemiologysurveilance/ documents/document/acspc-030975.pdf. Accessed August 14, 2012.
- Arun B, Dunn BK, Ford LG, Ryan A. Breast cancer prevention trials: large and small trials. Semin Oncol. 2010 Aug;37(4):367-83.
- Soerjomataram I, de Vries E, Pukkala E, Coebergh JW. Excess of cancers in Europe: a study of eleven major cancers amenable to lifestyle change. Int J Cancer. 2007 Mar 15;120(6):1336-43.
- Maclennan M, Ma DW. Role of dietary fatty acids in mammary gland development and breast cancer. Breast Cancer Res. 2010 Oct 26;12(5):211.
- Shen Q, Brown PH. Novel agents for the prevention of breast cancer: targeting transcription factors and signal transduction pathways. J Mammary Gland Biol Neoplasia. 2003 Jan;8(1):45-73.
- Decensi A, Costa A. Recent advances in cancer chemoprevention, with emphasis on breast and colorectal cancer. Eur J Cancer. 2000 Apr;36(6):694-709.
- Khan SI, Aumsuwan P, Khan IA, Walker LA, Dasmahapatra AK. Epigenetic events associated with breast cancer and their prevention by dietary components targeting the epigenome. Chem Res Toxicol. 2012 Jan 13;25(1):61-73.
- Wu X, Patterson S, Hawk E. Chemoprevention--history and general principles. Best Pract Res Clin Gastroenterol. 2011 Aug;25(4-5):445-59.
- McGowan PO, Kato T. Epigenetics in mood disorders. Environ Health Prev Med. 2008 Jan;13(1):16-24.
- Nian H, Delage B, Ho E, Dashwood RH. Modulation of histone deacetylase activity by dietary isothiocyanates and allyl sulfides: studies with sulforaphane and garlic organosulfur compounds. Environ Mol Mutagen. 2009 Apr;50(3):213-21.
- Meeran SM, Patel SN, Li Y, Shukla S, Tollefsbol TO. Bioactive dietary supplements reactivate ER expression in ER-negative breast cancer cells by active chromatin modifications. PLoS One. 2012;7(5):e37748.
- Davies E, Hiscox S. New therapeutic approaches in breast cancer. Maturitas. 2011 Feb;68(2):121-8.
- Muti P, Benassi B, Falvo E, et al. Omics underpins novel clues on VDR chemoprevention target in breast cancer. OMICS. 2011 Jun;15(6):337-46.
- Lockwood K, Moesgaard S, Folkers K. Partial and complete regression of breast cancer in patients in relation to dosage of coenzyme Q10. Biochem Biophys Res Commun. 1994 Mar 30;199(3):1504-8.
- Perumal SS, Shanthi P, Sachdanandam P. Augmented efficacy of tamoxifen in rat breast tumorigenesis when gavaged along with riboflavin, niacin, and CoQ10: effects on lipid peroxidation and antioxidants in mitochondria. Chem Biol Interact. 2005 Feb 28;152(1):49-58.
- Portakal O, Ozkaya O, Erden Inal M, Bozan B, Kosan M, Sayek I. Coenzyme Q10 concentrations and antioxidant status in tissues of breast cancer patients. Clin Biochem. 2000 Jun;33(4):279-84.
- Premkumar VG, Yuvaraj S, Sathish S, Shanthi P, Sachdanandam P. Anti-angiogenic potential of CoenzymeQ10, riboflavin and niacin in breast cancer patients undergoing tamoxifen therapy. Vascul Pharmacol. 2008 Apr-Jun;48(4-6):191-201.
- Cardenas C, Quesada AR, Medina MA. Anti-angiogenic and anti-inflammatory properties of kahweol, a coffee diterpene. PLoS One. 2011;6(8):e23407.
- Lee WJ, Zhu BT. Inhibition of DNA methylation by caffeic acid and chlorogenic acid, two common catechol-containing coffee polyphenols. Carcinogenesis. 2006 Feb;27(2):269-77.
- Rajendra Prasad N, Karthikeyan A, Karthikeyan S, Reddy BV. Inhibitory effect of caffeic acid on cancer cell proliferation by oxidative mechanism in human HT-1080 fibrosarcoma cell line. Mol Cell Biochem. 2011 Mar;349(1-2):11-9.
- Antony B, Merina B, Iyer VS, Judy N, Lennertz K, Joyal S. A Pilot Cross-Over Study to Evaluate Human Oral Bioavailability of BCM-95CG (Biocurcumax), A Novel Bioenhanced Preparation of Curcumin. Indian J Pharm Sci. 2008 Jul-Aug;70(4):445-9.
- Bachmeier BE, Mohrenz IV, Mirisola V, et al. Curcumin downregulates the inflammatory cytokines CXCL1 and -2 in breast cancer cells via NFkappaB. Carcinogenesis. 2008 Apr;29(4):779-89.
- Ramachandran C, Rodriguez S, Ramachandran R, et al. Expression profiles of apoptotic genes induced by curcumin in human breast cancer and mammary epithelial cell lines. Anticancer Res. 2005 Sep-Oct;25(5):3293-302.
- Thangapazham RL, Passi N, Maheshwari RK. Green tea polyphenol and epigallocatechin gallate induce apoptosis and inhibit invasion in human breast cancer cells. Cancer Biol Ther. 2007 Dec;6(12):1938-43.
- Michnovicz JJ, Adlercreutz H, Bradlow HL. Changes in levels of urinary estrogen metabolites after oral indole-3-carbinol treatment in humans. J Natl Cancer Inst. 1997 May 21;89(10):718-23.
- Al-Malki AL, Moselhy SS, Refai MY. Synergistic effect of lycopene and tocopherol against oxidative stress and mammary tumorigenesis induced by 7,12-dimethyl[a]benzanthracene in female rats. Toxicol Ind Health. 2012 Jul;28(6):542-8.
- Moselhy SS, Al mslmani MA. Chemopreventive effect of lycopene alone or with melatonin against the genesis of oxidative stress and mammary tumors induced by 7,12 dimethyl(a)benzanthracene in sprague dawely female rats. Mol Cell Biochem. 2008 Dec;319(1-2):175-80.
- Sanchez-Barcelo EJ, Mediavilla MD, Alonso-Gonzalez C, Reiter RJ. Melatonin uses in oncology: breast cancer prevention and reduction of the side effects of chemotherapy and radiation. Expert Opin Investig Drugs. 2012 Jun;21(6):819-31.
- Jung JW, Park SB, Lee SJ, Seo MS, Trosko JE, Kang KS. Metformin represses self-renewal of the human breast carcinoma stem cells via inhibition of estrogen receptor-mediated OCT4 expression. PLoS One. 2011;6(11):e28068.
- Agarwal A, Munoz-Najar U, Klueh U, Shih SC, Claffey KP. N-acetyl-cysteine promotes angiostatin production and vascular collapse in an orthotopic model of breast cancer. Am J Pathol. 2004 May;164(5):1683-96.
- Albini A, Morini M, D'Agostini F, et al. Inhibition of angiogenesis-driven Kaposi's sarcoma tumor growth in nude mice by oral N-acetylcysteine. Cancer Res. 2001 Nov 15;61(22):8171-8.
- Edlundh-Rose E, Kupershmidt I, Gustafsson AC, et al. Gene expression analysis of human epidermal keratinocytes after N-acetyl L-cysteine treatment demonstrates cell cycle arrest and increased differentiation. Pathobiology. 2005;72(4):203-12.
- Hara R, Inomata Y, Kawaji T, et al. Suppression of choroidal neovascularization by N-acetyl-cysteine in mice. Curr Eye Res. 2010 Nov;35(11):1012-20.
- Kubota M, Shimmura S, Kubota S, et al. Hydrogen and N-acetyl-L-cysteine rescue oxidative stress-induced angiogenesis in a mouse corneal alkali-burn model. Invest Ophthalmol Vis Sci. 2011 Jan;52(1):427-33.
- Martin KR, Saulnier MJ, Kari FW, Barrett JC, French JE. Timing of supplementation with the antioxidant N-acetyl-L-cysteine reduces tumor multiplicity in novel, cancer-prone p53 haploinsufficient Tg.AC (v-Ha-ras) transgenic mice but has no impact on malignant progression. Nutr Cancer. 2002;43(1):59-66.
- Dikmen M, Ozturk N, Ozturk Y. The antioxidant potency of Punica granatum L. Fruit peel reduces cell proliferation and induces apoptosis on breast cancer. J Med Food. 2011 Dec;14(12):1638-46.
- Grossmann ME, Mizuno NK, Schuster T, Cleary MP. Punicic acid is an omega-5 fatty acid capable of inhibiting breast cancer proliferation. Int J Oncol. 2010 Feb;36(2):421-6.
- Joseph MM, Aravind SR, Varghese S, Mini S, Sreelekha TT. Evaluation of antioxidant, antitumor and immunomodulatory properties of polysaccharide isolated from fruit rind of Punica granatum. Mol Med Report. 2012 Feb;5(2):489-96.
- Kiskova T, Ekmekcioglu C, Garajova M, et al. A combination of resveratrol and melatonin exerts chemopreventive effects in N-methyl-N-nitrosourea-induced rat mammary carcinogenesis. Eur J Cancer Prev. 2012 Mar;21(2):163-70.
- Hamdy SM, Latif AK, Drees EA, Soliman SM. Prevention of rat breast cancer by genistin and selenium. Toxicol Ind Health. 2011 Nov 16.
- Kim S, Han J, Kim JS, et al. Silibinin suppresses EGFR ligand-induced CD44 expression through inhibition of EGFR activity in breast cancer cells. Anticancer Res. 2011 Nov;31(11):3767-73.
- Kim S, Kim SH, Hur SM, et al. Silibinin prevents TPA-induced MMP-9 expression by down-regulation of COX-2 in human breast cancer cells. J Ethnopharmacol. 2009 Nov 12;126(2):252-7.
- Lin CJ, Sukarieh R, Pelletier J. Silibinin inhibits translation initiation: implications for anticancer therapy. Mol Cancer Ther. 2009 Jun;8(6):1606-12.
- Wang HJ, Jiang YY, Wei XF, et al. Silibinin induces protective superoxide generation in human breast cancer MCF-7 cells. Free Radic Res. 2010 Jan;44(1):90-100.
- Wang HJ, Wei XF, Jiang YY, et al. Silibinin induces the generation of nitric oxide in human breast cancer MCF-7 cells. Free Radic Res. 2010 May;44(5):577-84.
- Zi X, Feyes DK, Agarwal R. Anticarcinogenic effect of a flavonoid antioxidant, silymarin, in human breast cancer cells MDA-MB 468: induction of G1 arrest through an increase in Cip1/p21 concomitant with a decrease in kinase activity of cyclin-dependent kinases and associated cyclins. Clin Cancer Res. 1998 Apr;4(4):1055-64.
- Pugalendhi P, Manoharan S. Chemopreventive potential of genistein and daidzein in combination during 7,12-dimethylbenz[a]anthracene (DMBA) induced mammary carcinogenesis in Sprague-Dawley rats. Pak J Biol Sci. 2010 Mar 15;13(6):279-86.
- Jo EH, Kim SH, Ahn NS, et al. Efficacy of sulforaphane is mediated by p38 MAP kinase and caspase-7 activations in ER-positive and COX-2-expressed human breast cancer cells. Eur J Cancer Prev. 2007 Dec;16(6):505-10.
- Hsieh TC, Elangovan S, Wu JM. gamma-Tocotrienol controls proliferation, modulates expression of cell cycle regulatory proteins and up-regulates quinone reductase NQO2 in MCF-7 breast cancer cells. Anticancer Res. 2010 Jul;30(7):2869-74.
- Nesaretnam K, Gomez PA, Selvaduray KR, Razak GA. Tocotrienol levels in adipose tissue of benign and malignant breast lumps in patients in Malaysia. Asia Pac J Clin Nutr. 2007;16(3):498-504.
- Nesaretnam K, Meganathan P, Veerasenan SD, Selvaduray KR. Tocotrienols and breast cancer: the evidence to date. Genes Nutr. 2012 Jan;7(1):3-9.
- Patacsil D, Tran AT, Cho YS, et al. Gamma-tocotrienol induced apoptosis is associated with unfolded protein response in human breast cancer cells. J Nutr Biochem. 2012 Jan;23(1):93-100.
- Sylvester PW, Wali VB, Bachawal SV, Shirode AB, Ayoub NM, Akl MR. Tocotrienol combination therapy results in synergistic anticancer response. Front Biosci. 2012;17:3183-95.
- Dimri M, Bommi PV, Sahasrabuddhe AA, Khandekar JD, Dimri GP. Dietary omega-3 polyunsaturated fatty acids suppress expression of EZH2 in breast cancer cells. Carcinogenesis. 2010 Mar;31(3):489-95.
- King-Batoon A, Leszczynska JM, Klein CB. Modulation of gene methylation by genistein or lycopene in breast cancer cells. Environ Mol Mutagen. 2008 Jan;49(1):36-45.
- Lee H, Zhang P, Herrmann A, et al. Acetylated STAT3 is crucial for methylation of tumor-suppressor gene promoters and inhibition by resveratrol results in demethylation. Proc Natl Acad Sci U S A. 2012 May 15;109(20):7765-9.
- Zhu W, Qin W, Zhang K, et al. Trans-resveratrol alters mammary promoter hypermethylation in women at increased risk for breast cancer. Nutr Cancer. 2012 Apr;64(3):393-400.
- Meeran SM, Patel SN, Tollefsbol TO. Sulforaphane causes epigenetic repression of hTERT expression in human breast cancer cell lines. PLoS One. 2010;5(7):e11457.
- Hardy TM, Tollefsbol TO. Epigenetic diet: impact on the epigenome and cancer. Epigenomics. 2011 Aug;3(4):503-18.
- Degner SC, Papoutsis AJ, Selmin O, Romagnolo DF. Targeting of aryl hydrocarbon receptor-mediated activation of cyclooxygenase-2 expression by the indole-3-carbinol metabolite 3,3'-diindolylmethane in breast cancer cells. J Nutr. 2009 Jan;139(1):26-32.
- Abbadessa G, Spaccamiglio A, Sartori ML, et al. The aspirin metabolite, salicylate, inhibits 7,12-dimethylbenz[a]anthracene-DNA adduct formation in breast cancer cells. Int J Oncol. 2006 May;28(5):1131-40.
- Brasky TM, Bonner MR, Moysich KB, et al. Non-steroidal anti-inflammatory drug (NSAID) use and breast cancer risk in the Western New York Exposures and Breast Cancer (WEB) Study. Cancer Causes Control. 2010 Sep;21(9):1503-12.
- Davies G, Martin LA, Sacks N, Dowsett M. Cyclooxygenase-2 (COX-2), aromatase and breast cancer: a possible role for COX-2 inhibitors in breast cancer chemoprevention. Ann Oncol. 2002 May;13(5):669-78.
- Gardiner PS, Gilmer JF. The medicinal chemistry implications of the anticancer effects of aspirin and other NSAIDs. Mini Rev Med Chem. 2003 Aug;3(5):461-70.
- Harris RE, Kasbari S, Farrar WB. Prospective study of nonsteroidal anti-inflammatory drugs and breast cancer. Oncol Rep. 1999 Jan-Feb;6(1):71-3.
- Mangiapane S, Blettner M, Schlattmann P. Aspirin use and breast cancer risk: a meta-analysis and meta-regression of observational studies from 2001 to 2005. Pharmacoepidemiol Drug Saf. 2008 Feb;17(2):115-24.
- Kelley NS, Hubbard NE, Erickson KL. Conjugated linoleic acid isomers and cancer. J Nutr. 2007 Dec;137(12):2599-607.
- Wang LS, Huang YW, Liu S, et al. Conjugated linoleic acid (CLA) modulates prostaglandin E2 (PGE2) signaling in canine mammary cells. Anticancer Res. 2006 Mar-Apr;26(2A):889-98.
- Gierach GL, Freedman ND, Andaya A, et al. Coffee intake and breast cancer risk in the NIH-AARP diet and health study cohort. Int J Cancer. 2012 Jul 15;131(2):452-60. doi: 10.1002/ijc.26372. Epub 2011 Oct 20.
- Bachmeier BE, Mohrenz IV, Mirisola V, et al. Curcumin downregulates the inflammatory cytokines CXCL1 and -2 in breast cancer cells via NFkappaB. Carcinogenesis. 2008 Apr;29(4):779-89. Epub 2007 Nov 13.
- Labbozzetta M, Notarbartolo M, Poma P, et al. Curcumin as a possible lead compound against hormone-independent, multidrug-resistant breast cancer. Ann N Y Acad Sci. 2009 Feb;1155:278-83.
- Erickson KL, Hubbard NE. Fatty acids and breast cancer: the role of stem cells. Prostaglandins Leukot Essent Fatty Acids. 2010 Apr-Jun;82(4-6):237-41. Epub 2010 Apr 2.
- Khan GN, Gorin MA, Rosenthal D, et al. Pomegranate fruit extract impairs invasion and motility in human breast cancer. Integr Cancer Ther. 2009 Sep;8(3):242-53.
- Xiao X, Shi D, Liu L, et al. Quercetin suppresses cyclooxygenase-2 expression and angiogenesis through inactivation of P300 signaling. PLoS One. 2011;6(8):e22934.
- Krishnan AV, Feldman D. Mechanisms of the anti-cancer and anti-inflammatory actions of vitamin D. Annu Rev Pharmacol Toxicol. 2011 Feb 10;51:311-36.
- Choi EJ, Kim GH. Apigenin causes G(2)/M arrest associated with the modulation of p21(Cip1) and Cdc2 and activates p53-dependent apoptosis pathway in human breast cancer SK-BR-3 cells. J Nutr Biochem. 2009 Apr;20(4):285-90.
- Alfonso LF, Srivenugopal KS, Arumugam TV, Abbruscato TJ, Weidanz JA, Bhat GJ. Aspirin inhibits camptothecin-induced p21CIP1 levels and potentiates apoptosis in human breast cancer cells. Int J Oncol. 2009 Mar;34(3):597-608.
- Miura Y, Ono K, Okauchi R, Yagasaki K. Inhibitory effect of coffee on hepatoma proliferation and invasion in culture and on tumor growth, metastasis and abnormal lipoprotein profiles in hepatoma-bearing rats. J Nutr Sci Vitaminol (Tokyo). 2004 Feb;50(1):38-44.
- Aggarwal BB, Ichikawa H. Molecular targets and anticancer potential of indole-3-carbinol and its derivatives. Cell Cycle. 2005 Sep;4(9):1201-15.
- Moiseeva EP, Heukers R, Manson MM. EGFR and Src are involved in indole-3-carbinol-induced death and cell cycle arrest of human breast cancer cells. Carcinogenesis. 2007 Feb;28(2):435-45.
- Rogelsperger O, Wlcek K, Ekmekcioglu C, et al. Melatonin receptors, melatonin metabolizing enzymes and cyclin D1 in human breast cancer. J Recept Signal Transduct Res. 2011 Apr;31(2):180-7.
- Wang Y, Ding L, Wang X, et al. Pterostilbene simultaneously induces apoptosis, cell cycle arrest and cyto-protective autophagy in breast cancer cells. Am J Transl Res. 2012;4(1):44-51.
- Chen T, Wong YS, Zheng W. Induction of G1 cell cycle arrest and mitochondria-mediated apoptosis in MCF-7 human breast carcinoma cells by selenium-enriched Spirulina extract. Biomed Pharmacother. 2009 Oct 27.
- Jackson SJ, Singletary KW. Sulforaphane: a naturally occurring mammary carcinoma mitotic inhibitor, which disrupts tubulin polymerization. Carcinogenesis. 2004 Feb;25(2):219-27.
- Ip MM, Masso-Welch PA, Ip C. Prevention of mammary cancer with conjugated linoleic acid: role of the stroma and the epithelium. J Mammary Gland Biol Neoplasia. 2003 Jan;8(1):103-18.
- Bortman P, Folgueira MA, Katayama ML, Snitcovsky IM, Brentani MM. Antiproliferative effects of 1,25-dihydroxyvitamin D3 on breast cells: a mini review. Braz J Med Biol Res. 2002 Jan;35(1):1-9.
- Crew KD, Gammon MD, Steck SE, et al. Association between plasma 25-hydroxyvitamin D and breast cancer risk. Cancer Prev Res (Phila). 2009 Jun;2(6):598-604.
- Choi EJ, Kim GH. Apigenin Induces Apoptosis through a Mitochondria/Caspase-Pathway in Human Breast Cancer MDA-MB-453 Cells. J Clin Biochem Nutr. 2009 May;44(3):260-5.
- Seo HS, Choi HS, Kim SR, et al. Apigenin induces apoptosis via extrinsic pathway, inducing p53 and inhibiting STAT3 and NFkappaB signaling in HER2-overexpressing breast cancer cells. Mol Cell Biochem. 2012 Jul;366(1-2):319-34.
- Wang LS, Huang YW, Liu S, Yan P, Lin YC. Conjugated linoleic acid induces apoptosis through estrogen receptor alpha in human breast tissue. BMC Cancer. 2008;8:208.
- Prasad CP, Rath G, Mathur S, Bhatnagar D, Ralhan R. Potent growth suppressive activity of curcumin in human breast cancer cells: Modulation of Wnt/beta-catenin signaling. Chem Biol Interact. 2009 Oct 7;181(2):263-71.
- Xia Y, Jin L, Zhang B, Xue H, Li Q, Xu Y. The potentiation of curcumin on insulin-like growth factor-1 action in MCF-7 human breast carcinoma cells. Life Sci. 2007 May 16;80(23):2161-9.
- Rahman KM, Aranha O, Glazyrin A, Chinni SR, Sarkar FH. Translocation of Bax to mitochondria induces apoptotic cell death in indole-3-carbinol (I3C) treated breast cancer cells. Oncogene. 2000 Nov 23;19(50):5764-71.
- Proietti S, Cucina A, D'Anselmi F, et al. Melatonin and vitamin D3 synergistically down-regulate Akt and MDM2 leading to TGFbeta-1-dependent growth inhibition of breast cancer cells. J Pineal Res. 2011 Mar;50(2):150-8.
- Li J, Tu HJ, Dai G, et al. N-acetyl cysteine inhibits human signet ring cell gastric cancer cell line (SJ-89) cell growth by inducing apoptosis and DNA synthesis arrest. Eur J Gastroenterol Hepatol. 2007 Sep;19(9):769-74.
- Dai Z, Nair V, Khan M, Ciolino HP. Pomegranate extract inhibits the proliferation and viability of MMTV-Wnt-1 mouse mammary cancer stem cells in vitro. Oncol Rep. 2010 Oct;24(4):1087-91.
- Jeune MA, Kumi-Diaka J, Brown J. Anticancer activities of pomegranate extracts and genistein in human breast cancer cells. J Med Food. 2005 Winter;8(4):469-75.
- Dechsupa S, Kothan S, Vergote J, et al. Quercetin, Siamois 1 and Siamois 2 induce apoptosis in human breast cancer MDA-mB-435 cells xenograft in vivo. Cancer Biol Ther. 2007 Jan;6(1):56-61.
- Noh EM, Yi MS, Youn HJ, et al. Silibinin enhances ultraviolet B-induced apoptosis in mcf-7 human breast cancer cells. J Breast Cancer. 2011 Mar;14(1):8-13.
- Katdare M, Osborne M, Telang NT. Soy isoflavone genistein modulates cell cycle progression and induces apoptosis in HER-2/neu oncogene expressing human breast epithelial cells. Int J Oncol. 2002 Oct;21(4):809-15.
- Li Y, Bhuiyan M, Sarkar FH. Induction of apoptosis and inhibition of c-erbB-2 in MDA-MB-435 cells by genistein. Int J Oncol. 1999 Sep;15(3):525-33.
- Comitato R, Leoni G, Canali R, Ambra R, Nesaretnam K, Virgili F. Tocotrienols activity in MCF-7 breast cancer cells: involvement of ERbeta signal transduction. Mol Nutr Food Res. 2010 May;54(5):669-78.
- Park SK, Sanders BG, Kline K. Tocotrienols induce apoptosis in breast cancer cell lines via an endoplasmic reticulum stress-dependent increase in extrinsic death receptor signaling. Breast Cancer Res Treat. 2010 Nov;124(2):361-75.
- Bageman E, Ingvar C, Rose C, Jernstrom H. Coffee consumption and CYP1A2*1F genotype modify age at breast cancer diagnosis and estrogen receptor status. Cancer Epidemiol Biomarkers Prev. 2008 Apr;17(4):895-901.
- Li J, Seibold P, Chang-Claude J, et al. Coffee consumption modifies risk of estrogen-receptor negative breast cancer. Breast Cancer Res. 2011;13(3):R49.
- Flowers M, Thompson PA. t10c12 conjugated linoleic acid suppresses HER2 protein and enhances apoptosis in SKBr3 breast cancer cells: possible role of COX2. PLoS One. 2009;4(4):e5342.
- Rowe DL, Ozbay T, O'Regan RM, Nahta R. Modulation of the BRCA1 protein and induction of apoptosis in triple negative breast cancer cell lines by the polyphenolic compound curcumin. Breast Cancer (Auckl). 2009 Sep 2;3:61-75.
- Belguise K, Guo S, Sonenshein GE. Activation of FOXO3a by the green tea polyphenol epigallocatechin-3-gallate induces estrogen receptor alpha expression reversing invasive phenotype of breast cancer cells. Cancer Res. 2007 Jun 15;67(12):5763-70.
- Cos S, Gonzalez A, Martinez-Campa C, Mediavilla MD, Alonso-Gonzalez C, Sanchez-Barcelo EJ. Melatonin as a selective estrogen enzyme modulator. Curr Cancer Drug Targets. 2008 Dec;8(8):691-702.
- Vazquez-Martin A, Oliveras-Ferraros C, Menendez JA. The antidiabetic drug metformin suppresses HER2 (erbB-2) oncoprotein overexpression via inhibition of the mTOR effector p70S6K1 in human breast carcinoma cells. Cell Cycle. 2009 Jan 1;8(1):88-96.
- Yee LD, Young DC, Rosol TJ, Vanbuskirk AM, Clinton SK. Dietary (n-3) polyunsaturated fatty acids inhibit HER-2/neu-induced breast cancer in mice independently of the PPARgamma ligand rosiglitazone. J Nutr. 2005 May;135(5):983-8.
- Ramirez MC, Singletary K. Regulation of estrogen receptor alpha expression in human breast cancer cells by sulforaphane. J Nutr Biochem. 2009 Mar;20(3):195-201.
- Mohammed HA, Ba LA, Burkholz T, et al. Facile synthesis of chrysin-derivatives with promising activities as aromatase inhibitors. Nat Prod Commun. 2011 Jan;6(1):31-4.
- Adlercreutz H, Bannwart C, Wähälä K, et al. Inhibition of human aromatase by mammalian lignans and isoflavonoid phytoestrogens. J Steroid Biochem Mol Biol. 1993 Feb;44(2):147-53.
- Kijima I, Phung S, Hur G, Kwok SL, Chen S. Grape seed extract is an aromatase inhibitor and a suppressor of aromatase expression. Cancer Res. 2006 Jun 1;66(11):5960-7.
- Monteiro R, Becker H, Azevedo I, Calhau C. Effect of hop (Humulus lupulus L.) flavonoids on aromatase (estrogen synthase) activity. J Agric Food Chem. 2006 Apr 19;54(8):2938-43.
- Adams LS, Zhang Y, Seeram NP, Heber D, Chen S. Pomegranate ellagitannin-derived compounds exhibit antiproliferative and antiaromatase activity in breast cancer cells in vitro. Cancer Prev Res (Phila). 2010 Jan;3(1):108-13.
- Lee WJ, Chen WK, Wang CJ, Lin WL, Tseng TH. Apigenin inhibits HGF-promoted invasive growth and metastasis involving blocking PI3K/Akt pathway and beta 4 integrin function in MDA-MB-231 breast cancer cells. Toxicol Appl Pharmacol. 2008 Jan 15;226(2):178-91.
- Amaru DL, Field CJ. Conjugated linoleic acid decreases mcf-7 human breast cancer cell growth and insulin-like growth factor-1 receptor levels. Lipids. 2009 May;44(5):449-58.
- Ayoub NM, Bachawal SV, Sylvester PW. gamma-Tocotrienol inhibits HGF-dependent mitogenesis and Met activation in highly malignant mammary tumour cells. Cell Prolif. 2011 Dec;44(6):516-26.
- Sen T, Chatterjee A. Epigallocatechin-3-gallate (EGCG) downregulates EGF-induced MMP-9 in breast cancer cells: involvement of integrin receptor alpha5beta1 in the process. Eur J Nutr. 2011 Sep;50(6):465-78.
- Mafuvadze B, Benakanakere I, Hyder SM. Apigenin blocks induction of vascular endothelial growth factor mRNA and protein in progestin-treated human breast cancer cells. Menopause. 2010 Sep-Oct;17(5):1055-63.
- Mafuvadze B, Benakanakere I, Lopez Perez FR, Besch-Williford C, Ellersieck MR, Hyder SM. Apigenin prevents development of medroxyprogesterone acetate-accelerated 7,12-dimethylbenz(a)anthracene-induced mammary tumors in Sprague-Dawley rats. Cancer Prev Res (Phila). 2011 Aug;4(8):1316-24.
- Mafuvadze B, Liang Y, Besch-Williford C, Zhang X, Hyder SM. Apigenin Induces Apoptosis and Blocks Growth of Medroxyprogesterone Acetate-Dependent BT-474 Xenograft Tumors. Horm Cancer. 2012 Aug;3(4):160-71.
- Sachdanandam P. Antiangiogenic and hypolipidemic activity of coenzyme Q10 supplementation to breast cancer patients undergoing Tamoxifen therapy. Biofactors. 2008;32(1-4):151-9.
- Nagaraju GP, Aliya S, Zafar SF, Basha R, Diaz R, El-Rayes BF. The impact of curcumin on breast cancer. Integr Biol (Camb). 2012 Jul 6.
- Lu J, Zhang K, Chen S, Wen W. Grape seed extract inhibits VEGF expression via reducing HIF-1alpha protein expression. Carcinogenesis. 2009 Apr;30(4):636-44.
- Wen W, Lu J, Zhang K, Chen S. Grape seed extract inhibits angiogenesis via suppression of the vascular endothelial growth factor receptor signaling pathway. Cancer Prev Res (Phila). 2008 Dec;1(7):554-61.
- Leong H, Mathur PS, Greene GL. Inhibition of mammary tumorigenesis in the C3(1)/SV40 mouse model by green tea. Breast Cancer Res Treat. 2008 Feb;107(3):359-69.
- Leong H, Mathur PS, Greene GL. Green tea catechins inhibit angiogenesis through suppression of STAT3 activation. Breast Cancer Res Treat. 2009 Oct;117(3):505-15.
- Rose DP, Connolly JM. Regulation of tumor angiogenesis by dietary fatty acids and eicosanoids. Nutr Cancer. 2000;37(2):119-27.
- Toi M, Bando H, Ramachandran C, et al. Preliminary studies on the anti-angiogenic potential of pomegranate fractions in vitro and in vivo. Angiogenesis. 2003;6(2):121-8.
- Kim S, Choi JH, Lim HI, et al. Silibinin prevents TPA-induced MMP-9 expression and VEGF secretion by inactivation of the Raf/MEK/ERK pathway in MCF-7 human breast cancer cells. Phytomedicine. 2009 Jun;16(6-7):573-80.
- Rowell C, Carpenter DM, Lamartiniere CA. Chemoprevention of breast cancer, proteomic discovery of genistein action in the rat mammary gland. J Nutr. 2005 Dec;135(12 Suppl):2953S-59S.
- Shao ZM, Wu J, Shen ZZ, Barsky SH. Genistein exerts multiple suppressive effects on human breast carcinoma cells. Cancer Res. 1998 Nov 1;58(21):4851-7.
- Valachovicova T, Slivova V, Bergman H, Shuherk J, Sliva D. Soy isoflavones suppress invasiveness of breast cancer cells by the inhibition of NF-kappaB/AP-1-dependent and -independent pathways. Int J Oncol. 2004 Nov;25(5):1389-95.
- Bahar M, Khaghani S, Pasalar P, et al. Exogenous coenzyme Q10 modulates MMP-2 activity in MCF-7 cell line as a breast cancer cellular model. Nutr J. 2010;9:62.
- Jin UH, Lee JY, Kang SK, et al. A phenolic compound, 5-caffeoylquinic acid (chlorogenic acid), is a new type and strong matrix metalloproteinase-9 inhibitor: isolation and identification from methanol extract of Euonymus alatus. Life Sci. 2005 Oct 14;77(22):2760-9.
- Bocca C, Bozzo F, Cannito S, Colombatto S, Miglietta A. CLA reduces breast cancer cell growth and invasion through ERalpha and PI3K/Akt pathways. Chem Biol Interact. 2010 Jan 5;183(1):187-93.
- Rose P, Huang Q, Ong CN, Whiteman M. Broccoli and watercress suppress matrix metalloproteinase-9 activity and invasiveness of human MDA-MB-231 breast cancer cells. Toxicol Appl Pharmacol. 2005 Dec 1;209(2):105-13.
- Bachmeier B, Nerlich AG, Iancu CM, et al. The chemopreventive polyphenol Curcumin prevents hematogenous breast cancer metastases in immunodeficient mice. Cell Physiol Biochem. 2007;19(1-4):137-52.
- Mao L, Yuan L, Slakey LM, Jones FE, Burow ME, Hill SM. Inhibition of breast cancer cell invasion by melatonin is mediated through regulation of the p38 mitogen-activated protein kinase signaling pathway. Breast Cancer Res. 2010;12(6):R107.
- Nangia-Makker P, Hogan V, Honjo Y, et al. Inhibition of human cancer cell growth and metastasis in nude mice by oral intake of modified citrus pectin. J Natl Cancer Inst. 2002 Dec 18;94(24):1854-62.
- Rothwell PM, Wilson M, Price JF, Belch JF, Meade TW, Mehta Z. Effect of daily aspirin on risk of cancer metastasis: a study of incident cancers during randomised controlled trials. Lancet. 2012 Apr 28;379(9826):1591-601. Epub 2012 Mar 21.
- Bobek V, Boubelik M, Kovarík J, Taltynov O. Inhibition of adhesion breast cancer cells by anticoagulant drugs and cimetidine. Neoplasma. 2003;50(2):148-51.
- Harris RE, Chlebowski RT, Jackson RD, et al. Breast cancer and nonsteroidal anti-inflammatory drugs: prospective results from the Women's Health Initiative. Cancer Res. 2003 Sep 15;63(18):6096-101.
- Baker JA, Beehler GP, Sawant AC, Jayaprakash V, McCann SE, Moysich KB. Consumption of coffee, but not black tea, is associated with decreased risk of premenopausal breast cancer. J Nutr. 2006 Jan;136(1):166-71.
- Aro A, Mannisto S, Salminen I, Ovaskainen ML, Kataja V, Uusitupa M. Inverse association between dietary and serum conjugated linoleic acid and risk of breast cancer in postmenopausal women. Nutr Cancer. 2000;38(2):151-7.
- Kuriki K, Hirose K, Wakai K, et al. Breast cancer risk and erythrocyte compositions of n-3 highly unsaturated fatty acids in Japanese. Int J Cancer. 2007 Jul 15;121(2):377-85.
- Sun CL, Yuan JM, Koh WP, Yu MC. Green tea, black tea and breast cancer risk: a meta-analysis of epidemiological studies. Carcinogenesis. 2006 Jul;27(7):1310-5.
- Zhang M, Holman CD, Huang JP, Xie X. Green tea and the prevention of breast cancer: a case-control study in Southeast China. Carcinogenesis. 2007 May;28(5):1074-8.
- Li L, Zhang M, Holman D. Population versus hospital controls for case-control studies on cancers in Chinese hospitals. BMC Med Res Methodol. 2011;11:167.
- Zhang S, Tang G, Russell RM, et al. Measurement of retinoids and carotenoids in breast adipose tissue and a comparison of concentrations in breast cancer cases and control subjects. Am J Clin Nutr. 1997 Sep;66(3):626-32.
- Sato R, Helzlsouer KJ, Alberg AJ, Hoffman SC, Norkus EP, Comstock GW. Prospective study of carotenoids, tocopherols, and retinoid concentrations and the risk of breast cancer. Cancer Epidemiol Biomarkers Prev. 2002 May;11(5):451-7.
- Tamimi RM, Colditz GA, Hankinson SE. Circulating carotenoids, mammographic density, and subsequent risk of breast cancer. Cancer Res. 2009 Dec 15;69(24):9323-9.
- Schernhammer ES, Hankinson SE. Urinary melatonin levels and postmenopausal breast cancer risk in the Nurses' Health Study cohort. Cancer Epidemiol Biomarkers Prev. 2009 Jan;18(1):74-9.
- Bodmer M, Meier C, Krahenbuhl S, Jick SS, Meier CR. Long-term metformin use is associated with decreased risk of breast cancer. Diabetes Care. 2010 Jun;33(6):1304-8.
- Bosco JL, Antonsen S, Sorensen HT, Pedersen L, Lash TL. Metformin and incident breast cancer among diabetic women: a population-based case-control study in Denmark. Cancer Epidemiol Biomarkers Prev. 2011 Jan;20(1):101-11.
- Levi F, Pasche C, Lucchini F, Ghidoni R, Ferraroni M, La Vecchia C. Resveratrol and breast cancer risk. Eur J Cancer Prev. 2005 Apr;14(2):139-42.
- Wu AH, Koh WP, Wang R, Lee HP, Yu MC. Soy intake and breast cancer risk in Singapore Chinese Health Study. Br J Cancer. 2008 Jul 8;99(1):196-200.
- Cho YA, Kim J, Park KS, et al. Effect of dietary soy intake on breast cancer risk according to menopause and hormone receptor status. Eur J Clin Nutr. 2010 Sep;64(9):924-32.
- Zhang YF, Kang HB, Li BL, Zhang RM. Positive effects of soy isoflavone food on survival of breast cancer patients in China. Asian Pac J Cancer Prev. 2012;13(2):479-82.
- Robien K, Cutler GJ, Lazovich D. Vitamin D intake and breast cancer risk in postmenopausal women: the Iowa Women's Health Study. Cancer Causes Control. 2007 Sep;18(7):775-82.
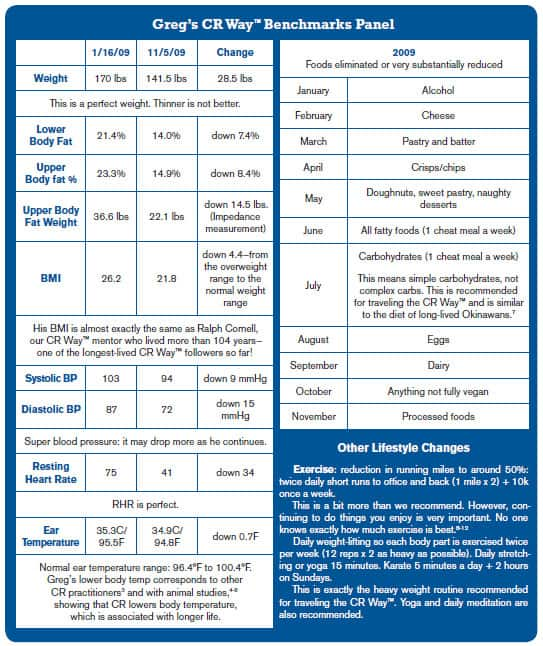 |
Motivating observations from Greg: "The positive effects of the less easily quantifiable results interests me most, e.g., mood status, libido, energy, stress management, for example. In addition, I keep waking up earlier than usual. In my "pre-CR" life this would be due to stress. Now it results from increased energy. I am sure my memory has improved as I seem able to recall and quote scientific papers, telephone numbers, names, and the like. At 46, I expected my eyesight to slowly deteriorate but it has actually IMPROVED! All these benefits cannot be coincidences."
Greg is a highly motivated, accomplished individual in his personal life. Seeing him carry that into his CR practice—achieving a great deal in a short time—is wonderful. We imagine that he will enjoy even more benefits in the years to come, amazing his friends and family with his youthful life as the years roll by.
These successes can forecast yours. The CR Way™ is about practicing calorie restriction "as you like it." It encourages molding proven CR science to individual needs.
If you have any questions on the scientific content of this article, please call a Life Extension® Health Advisor at 1-866-864-3027.
Notes:
* Body Mass Index (BMI) is a measure that relates one's weight to height: The normal range starts at 18.5; overweight at 25; and obesity at 30.
To learn more about the CR Way™ or the CR Society International, visit www.LivingTheCRWay.com or www.CRSociety.org.
References
1. McGlothin P, Averill M. The CR Way™: Using the Secrets of Calorie Restriction for a Longer, Healthier Life. New York, NY: HarperCollins; 2008:145-8.
2. McGlothin P, Averill M. The CR Way™: Using the Secrets of Calorie Restriction for a Longer, Healthier Life. New York, NY: HarperCollins; 2008:45-53.
3. Soare A, Cangemi R, Omodei D, Holloszy JO, Fontana L. Long-term calorie restriction, but not endurance exercise, lowers core body temperature in humans. Aging (Albany NY). 2011 Apr;3(4):374-9.
4. Duffy PH, Feuers RJ, Leakey JA, Nakamura K, Turturro A, Hart RW. Effect of chronic caloric restriction on physiological variables related to energy metabolism in the male Fischer 344 rat. Mech Ageing Dev. 1989 May; 48(2):117-33.
5. Duffy PH, Feuers RJ, Hart RW. Effect of chronic caloric restriction on the circadian regulation of physiological and behavioral variables in old male B6C3F1 mice. Chronobiol Int. 1990(4);7:291-303.
6. Lane MA, Baer DJ, Rumpler WV, et al. Calorie restriction lowers body temperature in rhesus monkeys, consistent with postulated antiaging mechanisms in rodents. Proc Natl Acad Sci U S A. 1996 Apr; 93(9):4159–64.
7. Willcox BJ, Willcox DC, Todoriki H, et al. Caloric restriction, the traditional Okinawan diet, and healthy aging. Ann N Y Acad Sci. 2007 Oct;1114:434-55.
8. Enea C, Boisseau N, Fargeas-Gluck MA, Diaz V, Dugué B. Circulating androgens in women: exercise-induced changes. Sports Medicine. 2011 Jan 1;41(1):1-15.
9. Archer T, Fredriksson A, Johansson B. Exercise alleviates Parkinsonism: clinical and laboratory evidence. Acta Neurologica Scandinavica. 2011 Feb;123(2):73-84.
10. Powell KE, Paluch AE, Blair SN. Physical activity for health: What kind? How much? How intense? On top of what? Annu Rev Public Health. 2011 Apr 21;32:349-65.
11. O'Keefe JH, Vogel R, Lavie CJ, Cordain L. Exercise like a hunter-gatherer: a prescription for organic physical fitness. Prog Cardiovasc Dis. 2011 May-Jun;53(6):471-9.
12. Johnson RJ, Murray R. Fructose, exercise, and health. Curr Sports Med Rep. 2010 Jul-Aug;9(4):253-8.