Life Extension Magazine®
It may surprise you that of the ten leading causes of death in this country—including heart disease, cancer, and diabetes—seven are linked to a single enzyme in your body.1 It's called 5-lipoxygenase or 5-LOX and in excess can create a cascade of dangerous inflammatory conditions throughout your body. Ironically, the same systemic inflammation triggered by 5-LOX once defended early humans against exposure to infectious disease. With these diseases now largely eradicated, the pro-inflammatory action of 5-LOX is not only unnecessary, but lethal to aging humans.2-5 Long known to researchers for its role in arthritis, pharmaceutical companies are trying to develop safe drugs to defuse 5-LOX, but the few "5-LOX inhibitors" currently available are proving no safer than other anti-inflammatory drugs.6,7 The good news is that a superior form of a natural 5-LOX inhibitor has recently been developed. This highly purified extract of boswellia has been shown to effectively inhibit excess 5-LOX at the molecular level. This article describes recent data on the anti-inflammatory mechanisms of boswellia extract and its multimodal protective effects against age-related problems such as cancer, heart disease, and neurodegenerative disorders.
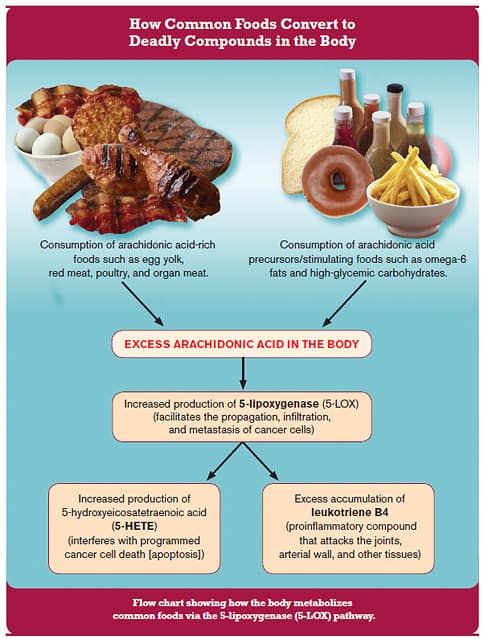
Readers will be encouraged to discover a new extract that absorbs into the blood 52% better than previously available boswellia. Excess 5-LOX Cripples and Kills Millions of AmericansIn response to poor dietary choices, our bodies suffer an overload of arachidonic acid. To remove arachidonic acid, the body increases production of enzymes like 5-lipoxygenase (5-LOX). Elevated 5-LOX and end products formed from degradation of arachidonic acid result in a constellation of age-related disorders. The graphic below (Figure 1) is re-printed from the February 2007 issue of Life Extension Magazine®. It clearly shows common foods in the Western diet that cause excess arachidonic acid to accumulate and the deadly cascade caused by elevated 5-LOX.
5-LOX stimulates the manufacture in the body of pro-inflammatory molecules called leukotrienes.8 Hundreds of published studies connect leukotrienes to cardiovascular disease,9 cancer,10-16 arthritis,17-19 and breathing disorders such as asthma and chronic obstructive pulmonary disease (COPD).8 They have also been implicated in Alzheimer's,20-25 inflammatory bowel diseases,26-28 and osteoporosis.29,30 Following healthier dietary patterns reduces 5-LOX levels, yet typical foods consumed by Americans today make it challenging to optimally suppress 5-LOX and its deadly metabolites. Fortunately, an Indian plant extract has demonstrated profound inhibiting effects against the 5-lipoxygenase (5-LOX) enzyme. Clinical Validation for a Traditional TreatmentFor millennia, traditional cultures prized the fragrant leaves of the boswellia tree both as aromatics and medicinals capable of reducing fever, rheumatism, and gastrointestinal disorders.
Modern researchers first validated boswellia's medical relevance with the discovery of boswellic acids. One in particular, AKBA (3-acetyl-11-keto-betaboswellic acid), was shown to bind directly to the 5-LOX enzyme in our bodies, preventing it from facilitating production of pro-inflammatory leukotrienes.39,40 Subsequent animal experiments confirmed that boswellia extracts exert a powerful anti-inflammatory effect, particularly in alleviating joint swelling in arthritis.41 Early trials in humans yielded similarly compelling results that paralleled traditional uses. Boswellia extracts proved to be an effective treatment in patients suffering from rheumatoid arthritis, Crohn's disease, ulcerative colitis, and asthma.39,42 Detailed safety and toxicological testing of boswellia extracts demonstrated their broad safety spectrum with both internal and external use.43 A Superior Form of BoswelliaThe limitation in using boswellia extracts as 5-LOX inhibitors is that they are difficult to absorb into the bloodstream (scientists call this "bioavailability").44 Recently, a novel boswellia extract was developed in which the plant's bioactive components were suspended in non-volatile oil also derived from boswellia. Researchers found this extract to be 52% more bioavailable compared to standard boswellia extracts.44 It offered superior protection from damage to joint cartilage induced by pro-inflammatory molecules and provided 15% better inhibition of certain pro-inflammatory molecules, including TNF-alpha and MMP3, compared with standard boswellia extracts.44
Clinical studies of patients with osteoarthritis are already confirming the important improvements in effectiveness offered by the new formulation.45 This makes sense based on a wealth of data showing life-enhancing benefits that boswellia extracts can afford through their blockade of dangerous 5-LOX and other mediators of inflammation. Cardiovascular ProtectionFor more than 20 years, we have known of the major role played by inflammation in the development of atherosclerosis, which is the occlusion and hardening of the arteries. In 1991, it was discovered that end products of 5-LOX were involved in the formation of atherosclerosis.52,53
Within a decade, the hunt was on for 5-LOX inhibitors that could prevent or even reverse cardiovascular aging.54 Boswellia extracts emerged as the most prominent natural sources of 5-LOX-inhibiting, anti-atherosclerotic compounds.55 In vascular tissue, boswellia extracts exert multiple complementary effects. They possess powerful free radical–quenching effects.56 Boswellia extracts lower total cholesterol by up to 48% and boost artery-cleansing high-density lipoprotein (HDL) by as much as 30%.57 It is boswellia extracts' inhibition of 5-LOX and other inflammatory factors, however, which is generating intense interest among heart specialists. In human capillary lining (endothelial) cells, the extracts significantly prevented expression of specific molecules central to forming atherosclerotic lesions.43,58 Boswellia exerted this effect by favorably modulating 113 genes, all of which were involved in producing blood vessel inflammation.58,59 Extracts rich in AKBA, a key bioactive agent in boswellia, caused a 50% reduction in the size of atherosclerotic lesions.60 They also significantly reduced activity of several different coagulation (clotting) factors in the blood.56 Neuroprotection and Brain HealthConstituents of a resin from a boswellia species have been shown to be potent stimulators of a brain receptor system called TRPV3 that lowers anxiety and exerts antidepressant-like effects.61
A major component of Boswellia carterri (called Incensole acetate) inhibits inflammatory mediators in brain tissue, an effect that offers significant hope in treatment of brain injury.62 That's because a dangerous inflammatory response follows brain injury within hours, contributing to a substantial portion of the long-lasting neurological deficit. Incensole acetate specifically inhibits degeneration of brain cells in the hippocampus, the region responsible for memory processing.62 Animal models confirm this effect. Laboratory rats treated with a drug to impair memory, along with boswellia extract, performed significantly better on tests involving spatial orientation and learning than did animals treated with the drug alone.63 A similar result was shown in healthy animals not treated with a memory-destroying drug. Extracts of Boswellia papyrifera—one of five species of boswellia used in the making of frankincense—increase spatial memory retention, enabling the animals to quickly find their way out of a maze.64 | |||||||||||||||||||||||||||||||||||||||||||||
Chemoprevention
AKBA, a principal component of boswellia, acts by numerous mechanisms to prevent cancer cells from proliferating. It induces apoptosis, or programmed cell death, by switching on the aptly named "death receptor" on cancer cell surfaces, an effect accompanied by activation of so-called "suicide" pathways inside cancer cells.65 Boswellic acid also blocks important signaling molecules required for cancer cell replication.66 And AKBA further inhibits tumor growth by suppressing a growth factor, VEGF (vascular endothelial growth factor), required by cancers to grow necessary new blood vessels.67 Boswellia oil extracts can distinguish between healthy and cancerous tissue, suppressing tumor cell viability but sparing normal cells.68 Finally, AKBA has recently been shown to suppress activity of a cellular receptor, CXCR4, that cancer cells use to trigger the metastases that account for 90% of cancer deaths. To date, boswellia extracts have also shown promise in reducing malignant cell growth, replication, or metastases in cancers of the prostate, bladder, cervix, colon, as well as in multiple myeloma, a cancer of the bone marrow. 66-73
Boswellia Extracts Relieve Arthritis SufferingEarly studies involved combinations of boswellia extracts with other known natural anti-inflammatory agents such as Withania somnifera (ashwagandha), Curcuma longa (curcumin), and zinc. In one such study, 42 patients were randomly assigned to receive this combination of nutrients or a placebo for 3 months.85 The treated group, but not the placebo recipients, demonstrated a significant drop in pain severity and disability scores. A similar study included the widely used WOMAC score of physical function difficulty and demonstrated significant reductions in that parameter as well as in pain and disability.86 No significant side effects were noted in either study.
More recently, studies have been conducted using boswellia extracts alone in double-blind, placebo-controlled trials, with doses up to 3,600 mg per day. Again, significant reductions are routinely reported in all parameters, including joint pain and swelling, ability to flex the joint, and increased walking distances.87
A 2008 study using a purified extract containing 30% of the vital AKBA was conducted among 70 patients with osteoarthritis.88 Subjects received the extract at doses of either 100 or 250 mg per day, or a placebo, for 90 days. Both doses of the extract, but not the placebo, resulted in clinically and statistically significant improvements in pain scores and physical function.88 Several other features of this study are worthy of notice. First, samples of joint fluid revealed a significant reduction in MMP-3 (matrix metalloproteinase-3), the inflammatory enzyme responsible for much of the cartilage destruction seen in osteoarthritis. Secondly, improvements in pain and functional ability were evident as early as 7 days into the 90-day treatment program. A follow-up study was conducted in 2010 to evaluate the efficacy of both the standard 30% AKBA extract and the newer, more bioavailable extract.45 Patients received just 100 mg of either supplement, or placebo, again for 90 days. In this case, both supplements produced significant improvements in pain scores and function and a reduction in MMP-3 levels. In both groups, improvements were evident in only 7 days, although the highly bioavailable preparation was more effective in providing a meaningful level of joint protection.
SummaryThe enzyme 5-lipoxygenase or 5-LOX triggers seven of the top ten leading causes of death in the United States, including cancer and heart disease. Excess 5-LOX leads to deadly release of pro-inflammatory molecules, including leukotrienes. While few safe drugs exist to neutralize 5-LOX, an exciting natural 5-LOX inhibitor with greater absorbability was recently developed. An abundance of compelling data indicates that the bioactive compounds in boswellia block the inflammatory response induced by 5-LOX that accelerates the onset of multiple killer diseases of aging. This new form of boswellia has been shown to absorb 52% better into the bloodstream, thus providing more of this natural 5-lipoxygenase inhibitor to cells throughout the body. If you have any questions on the scientific content of this article, please call a Life Extension® Health Advisor at 1-866-864-3027.
| ||||||||||
| Reference | ||||||||||
| 1. Available at: http://www.cdc.gov/nchs/fastats/lcod.htm. Accessed July 29, 2011. 2. Chinnici CM, Yao Y, Pratico D. The 5-lipoxygenase enzymatic pathway in the mouse brain: young versus old. Neurobiol Aging. 2007 Sep;28(9):1457-62. 3. Chu J, Pratico D. The 5-lipoxygenase as a common pathway for pathological brain and vascular aging. Cardiovasc Psychiatry Neurol. 2009;2009:174657. 4. Graham FD, Erlemann KR, Gravel S, Rokach J, Powell WS. Oxidative stress-induced changes in pyridine nucleotides and chemoattractant 5-lipoxygenase products in aging neutrophils. Free Radic Biol Med. 2009 Jul 1;47(1):62-71. 5. Zou Y, Kim DH, Jung KJ, et al. Lysophosphatidylcholine enhances oxidative stress via the 5-lipoxygenase pathway in rat aorta during aging. Rejuvenation Res. 2009 Feb;12(1):15-24. 6. Abe M, Yoshimoto T. Leukotriene-lipoxygenase pathway and drug discovery. Nihon Yakurigaku Zasshi. 2004 Dec;124(6):415-25. 7. Chohnabayashi N, Sugiura R, Nishimura N. Adverse effects of leukotriene-antagonists. Nihon Rinsho. 2007 Oct 28;65 Suppl 8:272-6. 8. Sampson AP. FLAP inhibitors for the treatment of inflammatory diseases. Curr Opin Investig Drugs. 2009 Nov;10(11):1163-72. 9. Jawien J, Korbut R. The current view on the role of leukotrienes in atherogenesis. J Physiol Pharmacol. 2010 Dec;61(6):647-50. 10. Goodman LA, Jarrett CL, Krunkosky TM, et al. 5-Lipoxygenase expression in benign and malignant canine prostate tissues. Vet Comp Oncol. 2011 Jun;9(2):149-57. 11. Angelucci A, Garofalo S, Speca S, et al. Arachidonic acid modulates the crosstalk between prostate carcinoma and bone stromal cells. Endocr Relat Cancer. 2008 Mar;15(1):91-100. 12. Faronato M, Muzzonigro G, Milanese G, et al. Increased expression of 5-lipoxygenase is common in clear cell renal cell carcinoma. Histol Histopathol. 2007 Oct;22(10):1109-18. 13. Grant GE, Rubino S, Gravel S, et al. Enhanced formation of 5-oxo-6,8,11,14-eicosatetraenoic acid by cancer cells in response to oxidative stress, docosahexaenoic acid and neutrophil-derived 5-hydroxy-6,8,11,14-eicosatetraenoic acid. Carcinogenesis. 2011 Jun;32(6):822-8. 14. Kim EY, Seo JM, Cho KJ, Kim JH. Ras-induced invasion and metastasis are regulated by a leukotriene B4 receptor BLT2-linked pathway. Oncogene. 2010 Feb 25;29(8):1167-78. 15. Radmark O, Samuelsson B. Microsomal prostaglandin E synthase-1 and 5-lipoxygenase: potential drug targets in cancer. J Intern Med. 2010 Jul;268(1):5-14. 16. Ye YN, Liu ES, Shin VY, Wu WK, Cho CH. Contributory role of 5-lipoxygenase and its association with angiogenesis in the promotion of inflammation-associated colonic tumorigenesis by cigarette smoking. Toxicology. 2004 Oct 15;203(1-3):179-88. 17. Chen M, Lam BK, Luster AD, et al. Joint tissues amplify inflammation and alter their invasive behavior via leukotriene B4 in experimental inflammatory arthritis. J Immunol. 2010 Nov 1;185(9):5503-11. 18. Chen SH, Fahmi H, Shi Q, Benderdour M. Regulation of microsomal prostaglandin E2 synthase-1 and 5-lipoxygenase-activating protein/5-lipoxygenase by 4-hydroxynonenal in human osteoarthritic chondrocytes. Arthritis Res Ther. 2010;12(1):R21. 19. Mathis SP, Jala VR, Lee DM, Haribabu B. Nonredundant roles for leukotriene B4 receptors BLT1 and BLT2 in inflammatory arthritis. J Immunol. 2010 Sep 1;185(5):3049-56. 20. Chu J, Pratico D. 5-lipoxygenase as an endogenous modulator of amyloid beta formation in vivo. Ann Neurol. 2011 Jan;69(1):34-46. 21. Chu J, Pratico D. Pharmacologic blockade of 5-lipoxygenase improves the amyloidotic phenotype of an Alzheimer's disease transgenic mouse model involvement of gamma-secretase. Am J Pathol. 2011 Apr;178(4):1762-9. 22. Firuzi O, Zhuo J, Chinnici CM, Wisniewski T, Pratico D. 5-Lipoxygenase gene disruption reduces amyloid-beta pathology in a mouse model of Alzheimer's disease. FASEB J. 2008 Apr;22(4):1169-78. 23. Ikonomovic MD, Abrahamson EE, Uz T, Manev H, Dekosky ST. Increased 5-lipoxygenase immunoreactivity in the hippocampus of patients with Alzheimer's disease. J Histochem Cytochem. 2008 Dec;56(12):1065-73. 24. Klegeris A, McGeer PL. Cyclooxygenase and 5-lipoxygenase inhibitors protect against mononuclear phagocyte neurotoxicity. Neurobiol Aging. 2002 Sep-Oct;23(5):787-94. 25. Qu T, Manev R, Manev H. 5-Lipoxygenase (5-LOX) promoter polymorphism in patients with early-onset and late-onset Alzheimer's disease. J Neuropsychiatry Clin Neurosci. 2001 Spring;13(2):304-5. 26. Cuzzocrea S, Rossi A, Mazzon E, et al. 5-Lipoxygenase modulates colitis through the regulation of adhesion molecule expression and neutrophil migration. Lab Invest. 2005 Jun;85(6):808-22. 27. Mazzon E, Sautebin L, Caputi AP, Cuzzocrea S. 5-lipoxygenase modulates the alteration of paracellular barrier function in mice ileum during experimental colitis. Shock. 2006 Apr;25(4):377-83. 28. Rask-Madsen J, Bukhave K, Laursen LS, Lauritsen K. 5-Lipoxygenase inhibitors for the treatment of inflammatory bowel disease. Agents Actions. 1992;Spec No:C37-46. 29. Boyce BF, Hughes DE, Wright KR, Xing L, Dai A. Recent advances in bone biology provide insight into the pathogenesis of bone diseases. Lab Invest. 1999 Feb;79(2):83-94. 30. Mundy GR. Cytokines and growth factors in the regulation of bone remodeling. J Bone Miner Res. 1993 Dec;8 Suppl 2:S505-10. 31. Bell RL, Harris RR, Malo PE, et al. ABT-761 attenuates bronchoconstriction and pulmonary inflammation in rodents. J Pharmacol Exp Ther. 1997 Mar;280(3):1366-73. 32. Cuzzocrea S, Rossi A, Serraino I, et al. 5-Lipoxygenase knockout mice exhibit a resistance to pleurisy and lung injury caused by carrageenan. J Leukoc Biol. 2003 Jun;73(6):739-46. 33. Doi K, Hamasaki Y, Noiri E, et al. Role of leukotriene B4 in accelerated hyperlipidaemic renal injury. Nephrology (Carlton). 2011 Mar;16(3):304-9. 34. Gubitosi-Klug RA, Talahalli R, Du Y, Nadler JL, Kern TS. 5-Lipoxygenase, but not 12/15-lipoxygenase, contributes to degeneration of retinal capillaries in a mouse model of diabetic retinopathy. Diabetes. 2008 May;57(5):1387-93. 35. Kim DC, Hsu FI, Barrett NA, et al. Cysteinyl leukotrienes regulate Th2 cell-dependent pulmonary inflammation. J Immunol. 2006 Apr 1;176(7):4440-8. 36. Martinez-Clemente M, Claria J, Titos E. The 5-lipoxygenase/leukotriene pathway in obesity, insulin resistance, and fatty liver disease. Curr Opin Clin Nutr Metab Care. 2011 Jul;14(4):347-53. 37. Pace E, Profita M, Melis M, et al. LTB4 is present in exudative pleural effusions and contributes actively to neutrophil recruitment in the inflamed pleural space. Clin Exp Immunol. 2004 Mar;135(3):519-27. 38. Zhou YJ, Wang JH, Li L, Yang HW, Wen de L, He QC. Expanding expression of the 5-lipoxygenase/leukotriene B4 pathway in atherosclerotic lesions of diabetic patients promotes plaque instability. Biochem Biophys Res Commun. 2007 Nov 9;363(1):30-6. 39. Ammon HP. Boswellic acids (components of frankincense) as the active principle in treatment of chronic inflammatory diseases. Wien Med Wochenschr. 2002;152(15-16):373-8. 40. Sailer ER, Subramanian LR, Rall B, Hoernlein RF, Ammon HP, Safayhi H. Acetyl-11-keto-beta-boswellic acid (AKBA): structure requirements for binding and 5-lipoxygenase inhibitory activity. Br J Pharmacol. 1996 Feb;117(4):615-8. 41. Fan AY, Lao L, Zhang RX, et al. Effects of an acetone extract of Boswellia carterii Birdw. (Burseraceae) gum resin on adjuvant-induced arthritis in lewis rats. J Ethnopharmacol. 2005 Oct 3;101(1-3):104-9. 42. Ammon HP. Boswellic acids in chronic inflammatory diseases. Planta Med. 2006 Oct;72(12):1100-16. 43. Lalithakumari K, Krishnaraju AV, Sengupta K, Subbaraju GV, Chatterjee A. Safety and Toxicological Evaluation of a Novel, Standardized 3-O-Acetyl-11-keto-beta-Boswellic Acid (AKBA)-Enriched Boswellia serrata Extract (5-Loxin(R)). Toxicol Mech Methods. 2006;16(4):199-226. 44. Sengupta K, Kolla JN, Krishnaraju AV, et al. Cellular and molecular mechanisms of anti-inflammatory effect of Aflapin: a novel Boswellia serrata extract. Mol Cell Biochem. 2011 Aug;354(1-2):189-97. 45. Sengupta K, Krishnaraju AV, Vishal AA, et al. Comparative efficacy and tolerability of 5-Loxin and AflapinAgainst osteoarthritis of the knee: a double blind, randomized, placebo controlled clinical study. Int J Med Sci. 2010;7(6):366-77. 46. Available at:http://www.cdc.gov/nchs/fastats/arthrits.htm. Accessed July 29, 2011. 47. Barnes DE, Yaffe K. The projected effect of risk factor reduction on Alzheimer's disease prevalence. Lancet Neurol. 2011 Jul 18. 48. Available at:http://www.cdc.gov/nchs/fastats/asthma.htm. Accessed July 29, 2011. 49. Available at:http://www.cdc.gov/nchs/fastats/cancer.htm. Accessed July 29, 2011. 50. Available at:http://www.cdc.gov/nchs/fastats/heart.htm. Accessed July 29, 2011. 51. Available at:http://www.cdc.gov/nchs/fastats/kidbladd.htm. Accessed July 29, 2011. 52. Hagihara H, Nomoto A, Mutoh S, Yamaguchi I, Ono T. Role of inflammatory responses in initiation of atherosclerosis: effects of anti-inflammatory drugs on cuff-induced leukocyte accumulation and intimal thickening of rabbit carotid artery. Atherosclerosis. 1991 Nov;91(1-2):107-16. 53. Hatmi M, Samama MM, Elalamy I. Prevention of thrombosis and vascular inflammation: importance of combined cyclooxygenase and 5-lipoxygenase inhibitors. J Mal Vasc. 2006 Feb;31(1):4-9. 54. Zweifel BS, Hardy MM, Anderson GD, Dufield DR, Pufahl RA, Masferrer JL. A rat air pouch model for evaluating the efficacy and selectivity of 5-lipoxygenase inhibitors. Eur J Pharmacol. 2008 Apr 14;584(1):166-74. 55. Tripathi YB, Reddy MM, Pandey RS, et al. Anti-inflammatory properties of BHUx, a polyherbal formulation to prevent atherosclerosis. Inflammopharmacology. 2004;12(2):131-52. 56. Kokkiripati PK, Bhakshu LM, Marri S, et al. Gum resin of Boswellia serrata inhibited human monocytic (THP-1) cell activation and platelet aggregation. J Ethnopharmacol. 2011 Jul 8. 57. Pandey RS, Singh BK, Tripathi YB. Extract of gum resins of Boswellia serrata L. inhibits lipopolysaccharide induced nitric oxide production in rat macrophages along with hypolipidemic property. Indian J Exp Biol. 2005 Jun;43(6):509-16. 58. Roy S, Khanna S, Shah H, et al. Human genome screen to identify the genetic basis of the anti-inflammatory effects of Boswellia in microvascular endothelial cells. DNA Cell Biol. 2005 Apr;24(4):244-55. 59. Roy S, Khanna S, Krishnaraju AV, et al. Regulation of vascular responses to inflammation: inducible matrix metalloproteinase-3 expression in human microvascular endothelial cells is sensitive to antiinflammatory Boswellia. Antioxid Redox Signal. 2006 Mar-Apr;8(3-4):653-60. 60. Cuaz-Perolin C, Billiet L, Bauge E, et al. Antiinflammatory and antiatherogenic effects of the NF-kappaB inhibitor acetyl-11-keto-beta-boswellic acid in LPS-challenged ApoE-/- mice. Arterioscler Thromb Vasc Biol. 2008 Feb;28(2):272-7. 61. Moussaieff A, Rimmerman N, Bregman T, et al. Incensole acetate, an incense component, elicits psychoactivity by activating TRPV3 channels in the brain. FASEB J. 2008 Aug;22(8):3024-34. 62. Moussaieff A, Shein NA, Tsenter J, et al. Incensole acetate: a novel neuroprotective agent isolated from Boswellia carterii. J Cereb Blood Flow Metab. 2008 Jul;28(7):1341-52. 63. Hosseini M, Hadjzadeh MA, Derakhshan M, et al. The beneficial effects of olibanum on memory deficit induced by hypothyroidism in adult rats tested in Morris water maze. Arch Pharm Res. 2010 Mar;33(3):463-8. 64. Mahmoudi A, Hosseini-Sharifabad A, Monsef-Esfahani HR, et al. Evaluation of systemic administration of Boswellia papyrifera extracts on spatial memory retention in male rats. J Nat Med. 2011 Jul;65(3-4):519-25. 65. Lu M, Xia L, Hua H, Jing Y. Acetyl-keto-beta-boswellic acid induces apoptosis through a death receptor 5-mediated pathway in prostate cancer cells. Cancer Res. 2008 Feb 15;68(4):1180-6. 66. Kunnumakkara AB, Nair AS, Sung B, Pandey MK, Aggarwal BB. Boswellic acid blocks signal transducers and activators of transcription 3 signaling, proliferation, and survival of multiple myeloma via the protein tyrosine phosphatase SHP-1. Mol Cancer Res. 2009 Jan;7(1):118-28. 67. Pang X, Yi Z, Zhang X, et al. Acetyl-11-keto-beta-boswellic acid inhibits prostate tumor growth by suppressing vascular endothelial growth factor receptor 2-mediated angiogenesis. Cancer Res. 2009 Jul 15;69(14):5893-900. 68. Frank MB, Yang Q, Osban J, et al. Frankincense oil derived from Boswellia carteri induces tumor cell specific cytotoxicity. BMC Complement Altern Med. 2009;9:6. 69. Park B, Sung B, Yadav VR, Cho SG, Liu M, Aggarwal BB. Acetyl-11-keto-beta-boswellic acid suppresses invasion of pancreatic cancer cells through the downregulation of CXCR4 chemokine receptor expression. Int J Cancer. 2011 Jul 1;129(1):23-33. 70. Yuan HQ, Kong F, Wang XL, Young CY, Hu XY, Lou HX. Inhibitory effect of acetyl-11-keto-beta-boswellic acid on androgen receptor by interference of Sp1 binding activity in prostate cancer cells. Biochem Pharmacol. 2008 Jun 1;75(11):2112-21. 71. Yadav VR, Prasad S, Sung B, et al. Boswellic acid inhibits growth and metastasis of human colorectal cancer in orthotopic mouse model by downregulating inflammatory, proliferative, invasive, and angiogenic biomarkers. Int J Cancer. 2011 Jun 23. 72. Liu JJ, Duan RD. LY294002 enhances boswellic acid-induced apoptosis in colon cancer cells. Anticancer Res. 2009 Aug;29(8):2987-91. 73. Bhushan S, Malik F, Kumar A, et al. Activation of p53/p21/PUMA alliance and disruption of PI-3/Akt in multimodal targeting of apoptotic signaling cascades in cervical cancer cells by a pentacyclic triterpenediol from Boswellia serrata. Mol Carcinog. 2009 Dec;48(12):1093-108. 74. Dreikorn K. The role of phytotherapy in treating lower urinary tract symptoms and benign prostatic hyperplasia. World J Urol. 2002 Apr;19(6):426-35. 75. Paubert-Braquet M, Cave A, Hocquemiller R, et al. Effect of Pygeum africanum extract on A23187-stimulated production of lipoxygenase metabolites from human polymorphonuclear cells. J Lipid Mediat Cell Signal. 1994 May;9(3):285-90. 76. Paubert-Braquet M, Mencia Huerta JM, Cousse H, Braquet P. Effect of the lipidic lipidosterolic extract of Serenoa repens (Permixon) on the ionophore A23187-stimulated production of leukotriene B4 (LTB4) from human polymorphonuclear neutrophils. Prostaglandins Leukot Essent Fatty Acids. 1997 Sep;57(3):299-304. 77. Safarinejad MR. Urtica dioica for treatment of benign prostatic hyperplasia: a prospective, randomized, double-blind, placebo-controlled, crossover study. J Herb Pharmacother. 2005;5(4):1-11. 78. Lopatkin NA, Sivkov AV, Medvedev AA, et al. Combined extract of Sabal palm and nettle in the treatment of patients with lower urinary tract symptoms in double blind, placebo-controlled trial. Urologiia. 2006 Mar-Apr (2):12, 14-9. 79. Mantovani F. Serenoa repens in benign prostatic hypertrophy: analysis of 2 Italian studies. Minerva Urol Nefrol. 2010 Dec;62(4):335-40. 80. Pavone C, Abbadessa D, Tarantino ML, et al. Associating Serenoa repens, Urtica dioica and Pinus pinaster. Safety and efficacy in the treatment of lower urinary tract symptoms. Prospective study on 320 patients. Urologia. 2010 Jan-Mar;77(1):43-51. 81. Quiles MT, Arbos MA, Fraga A, de Torres IM, Reventos J, Morote J. Antiproliferative and apoptotic effects of the herbal agent Pygeum africanum on cultured prostate stromal cells from patients with benign prostatic hyperplasia (BPH). Prostate. 2010 Jul 1;70(10):1044-53. 82. Ejike CE, Ezeanyika LU. Inhibition of the Experimental Induction of Benign Prostatic Hyperplasia: A Possible Role for Fluted Pumpkin (Telfairia occidentalis Hook f.) Seeds. Urol Int. 2011 Jun 28. 83. Schleich S, Papaioannou M, Baniahmad A, Matusch R. Extracts from Pygeum africanum and other ethnobotanical species with antiandrogenic activity. Planta Med. 2006 Jul;72(9):807-13. 84. Papaioannou M, Schleich S, Roell D, et al. NBBS isolated from Pygeum africanum bark exhibits androgen antagonistic activity, inhibits AR nuclear translocation and prostate cancer cell growth. Invest New Drugs. 2010 Dec;28(6):729-43. 85. Kulkarni RR, Patki PS, Jog VP, Gandage SG, Patwardhan B. Treatment of osteoarthritis with a herbomineral formulation: a double-blind, placebo-controlled, cross-over study. J Ethnopharmacol. 1991 May-Jun;33(1-2):91-5. 86. Chopra A, Lavin P, Patwardhan B, Chitre D. A 32-week randomized, placebo-controlled clinical evaluation of RA-11, an Ayurvedic drug, on osteoarthritis of the knees. J Clin Rheumatol. 2004 Oct;10(5):236-45. 87. Kimmatkar N, Thawani V, Hingorani L, Khiyani R. Efficacy and tolerability of Boswellia serrata extract in treatment of osteoarthritis of knee--a randomized double blind placebo controlled trial. Phytomedicine. 2003 Jan;10(1):3-7. 88. Sengupta K, Alluri KV, Satish AR, et al. A double blind, randomized, placebo controlled study of the efficacy and safety of 5-Loxin for treatment of osteoarthritis of the knee. Arthritis Res Ther. 2008;10(4):R85. 89. Marone PA, Lau FC, Gupta RC, Bagchi M, Bagchi D. Safety and toxicological evaluation of undenatured type II collagen. Toxicol Mech Methods. 2010 May;20(4):175-89. 90. Bagchi D, Misner B, Bagchi M, et al. Effects of orally administered undenatured type II collagen against arthritic inflammatory diseases: a mechanistic exploration. Int J Clin Pharmacol Res. 2002;22(3-4):101-10. 91. Deparle LA, Gupta RC, Canerdy TD, et al. Efficacy and safety of glycosylated undenatured type-II collagen (UC-II) in therapy of arthritic dogs. J Vet Pharmacol Ther. 2005 Aug;28(4):385-90. 92. D'Altilio M, Peal A, Alvey M, et al. Therapeutic Efficacy and Safety of Undenatured Type II Collagen Singly or in Combination with Glucosamine and Chondroitin in Arthritic Dogs. Toxicol Mech Methods. 2007;17(4):189-96. 93. Trentham DE, Dynesius-Trentham RA, Orav EJ, et al. Effects of oral administration of type II collagen on rheumatoid arthritis. Science. 1993 Sep 24;261(5129):1727-30. 94. Crowley DC, Lau FC, Sharma P, et al. Safety and efficacy of undenatured type II collagen in the treatment of osteoarthritis of the knee: a clinical trial. Int J Med Sci. 2009;6(6):312-21. 95. Hunt CD. Regulation of enzymatic activity: one possible role of dietary boron in higher animals and humans. Biol Trace Elem Res. 1998 Winter;66(1-3):205-25. 96. Scorei IR. Calcium fructoborate: plant-based dietary boron as potential medicine for cancer therapy. Front Biosci (Schol Ed). 2011;3:205-15. 97. Scorei R, Cimpoiasu VM, Iordachescu D. In vitro evaluation of the antioxidant activity of calcium fructoborate. Biol Trace Elem Res. 2005 Nov;107(2):127-34. 98. Scorei R, Ciubar R, Iancu C, Mitran V, Cimpean A, Iordachescu D. In vitro effects of calcium fructoborate on fMLP-stimulated human neutrophil granulocytes. Biol Trace Elem Res. 2007 Jul;118(1):27-37. 99. Scorei R, Mitrut P, Petrisor I, Scorei I. A Double-Blind, Placebo-Controlled Pilot Study to Evaluate the Effect of Calcium Fructoborate on Systemic Inflammation and Dyslipidemia Markers for Middle-Aged People with Primary Osteoarthritis. Biol Trace Elem Res. 2011 May 24. 100. Scorei RI, Ciofrangeanu C, Ion R, et al. In vitro effects of calcium fructoborate upon production of inflammatory mediators by LPS-stimulated RAW 264.7 macrophages. Biol Trace Elem Res. 2010 Jun;135(1-3):334-44. 101. Scorei RI, Rotaru P. Calcium Fructoborate-Potential Anti-inflammatory Agent. Biol Trace Elem Res. 2011 Jan 28. 102. Wagner CC, Ferraresi Curotto V, Pis Diez R, Baran EJ. Experimental and theoretical studies of calcium fructoborate. Biol Trace Elem Res. 2008 Apr;122(1):64-72. 103. Ali AA, Lewis SM, Badgley HL, Allaben WT, Leakey JE. Oral glucosamine increases expression of transforming growth factor beta1 (TGFbeta1) and connective tissue growth factor (CTGF) mRNA in rat cartilage and kidney: implications for human efficacy and toxicity. Arch Biochem Biophys. 2011 Jun 1;510(1):11-8. 104. Black C, Clar C, Henderson R, et al. The clinical effectiveness of glucosamine and chondroitin supplements in slowing or arresting progression of osteoarthritis of the knee: a systematic review and economic evaluation. Health Technol Assess. 2009 Nov;13(52):1-148. 105. Gruenwald J, Petzold E, Busch R, Petzold HP, Graubaum HJ. Effect of glucosamine sulfate with or without omega-3 fatty acids in patients with osteoarthritis. Adv Ther. 2009 Sep;26(9):858-71. 106. Igarashi M, Kaga I, Takamori Y, Sakamoto K, Miyazawa K, Nagaoka I. Effects of glucosamine derivatives and uronic acids on the production of glycosaminoglycans by human synovial cells and chondrocytes. Int J Mol Med. 2011 Jun;27(6):821-7. 107. Igarashi M, Sakamoto K, Nagaoka I. Effect of glucosamine, a therapeutic agent for osteoarthritis, on osteoblastic cell differentiation. Int J Mol Med. 2011 Sep;28(3):373-9. 108. Ivanovska N, Dimitrova P. Bone resorption and remodeling in murine collagenase-induced osteoarthritis after administration of glucosamine. Arthritis Res Ther. 2011 Mar 16;13(2):R44. 109. Ng NT, Heesch KC, Brown WJ. Efficacy of a progressive walking program and glucosamine sulphate supplementation on osteoarthritic symptoms of the hip and knee: a feasibility trial. Arthritis Res Ther. 2010;12(1):R25. 110. Petersen SG, Saxne T, Heinegard D, et al. Glucosamine but not ibuprofen alters cartilage turnover in osteoarthritis patients in response to physical training. Osteoarthritis Cartilage. 2010 Jan;18(1):34-40. 111. Taccone-Gallucci M, Manca-di-Villahermosa S, Battistini L, et al. N-3 PUFAs reduce oxidative stress in ESRD patients on maintenance HD by inhibiting 5-lipoxygenase activity. Kidney Int. 2006 Apr;69(8):1450-4. 112. Calder PC. N-3 polyunsaturated fatty acids and inflammation: from molecular biology to the clinic. Lipids. 2003 Apr;38(4):343-52. 113. Aggarwal BB, Sung B. Pharmacological basis for the role of curcumin in chronic diseases: an age-old spice with modern targets. Trends Pharmacol Sci. 2009 Feb;30(2):85-94. 114. Safayhi H, Rall B, Sailer ER, Ammon HP. Inhibition by boswellic acids of human leukocyte elastase. J Pharmacol Exp Ther. 1997 Apr;281(1):460-3. 115. Safayhi H, Sailer ER, Ammon HP. Mechanism of 5-lipoxygenase inhibition by acetyl-11-keto-beta-boswellic acid. Mol Pharmacol. 1995 Jun;47(6):1212-6. |