Life Extension Magazine®
If you enter “mitochondria and aging” into the US Library of Medicine website, you’ll find over 351 scientific studies published in 2010. The consensus among researchers is that mitochondrial dysfunction plays a central role in the development of virtually all age-related diseases.1 Go back nearly four decades, however, and very little about mitochondria appeared in medical journals. When Life Extension® first introduced methods to enhance mitochondrial function, few physicians understood our rationale. Today’s scientific data make it loud and clear that every aging person should take personal responsibility to ensure optimal mitochondrial function and structure. The good news is that nutrients used by Life Extension members to enhance mitochondrial performance have more scientific substantiation than ever. While compounds like coenzyme Q10,2-6 carnitine,7-9 and lipoic acid10-14 support mitochondrial function, it is critical that new mitochondria are generated if we are to protect against age-related decline.
Mitochondrial biogenesis is the process of creating new mitochondria within cells. Our mitochondria can be regenerated in response to intense aerobic exercise,15 calorie restriction,16 and taking certain medications like metformin.17 In 2010, researchers at the University of California at Davis released a peer-reviewed publication showing that a natural compound called PQQ (pyrroloquinoline quinone) promotes the formation of new mitochondria within cells.18 For the first time, humans are empowered with a natural agent to reverse the deadly decline in functional mitochondria that underlies degenerative disease and premature aging. Healthy Mitochondria Essential for the BrainAbout 95% of cellular energy is produced from structures in the cell called mitochondria. Unlike other cell components, mitochondria are able to divide within healthy cells. Mitochondria are required for cellular energy production. Impaired mitochondrial metabolism is now recognized as an underlying factor of many diseases.
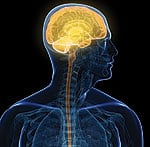
The initial interest in taking nutrients like L-carnitine and CoQ10 was to boost mitochondrial function in heart muscle cells. Overlooked was the large amount of energy required by neurons (brain cells) to carry out their specialized functions. Studies published in 2010 (and earlier) corroborate the role of abnormal mitochondria dynamics with neuronal cell death19 and the onset of Alzheimer’s,20-23 Parkinson’s,24-26 Huntington’s,27-29 and other neurodegenerative disorders. If you live to 80 years, there is a 30% chance you will develop Alzheimer’s dementia. Scientists have identified specific pathologic mechanisms that reveal the role of mitochondrial dysfunction in the initiation and progression of this hideous disease. These findings resulted in the authors of a 2010 report concluding: “We suggest that mitochondrial protection and subsequent reduction of oxidative stress are important targets for prevention and long-term treatment of early stages of Alzheimer’s disease.”30 Mitochondria Dysfunction and Heart FailureDamage to the mitochondria of endothelial cells is an underlying cause of atherosclerosis.31,32 Traditional risk factors for arterial disease such as smoking,33-36 obesity,37 high blood sugar,38,39 high cholesterol40 and high triglycerides41,42 are all linked to mitochondrial injury.
When a person suffers persistent coronary artery blockage, a severe weakening of cardiac muscle can occur that results in congestive heart failure. A study published in 2010 looked at left ventricular heart muscle tissue in patients with end-stage heart failure and normal hearts. Compared to normal hearts, mitochondrial DNA was decreased by 40% in failing hearts. This was accompanied by reductions of 25-80% in mitochondrial DNA-encoded proteins of failing hearts. The doctors who conducted this study concluded: “Mitochondrial biogenesis is severely impaired as evidenced by reduced mitochondrial DNA replication and depletion of mitochondrial DNA in the human failing heart…suggesting novel mechanisms for mitochondrial dysfunction in heart failure.”43 Nutrients that enhance mitochondrial function (like coenzyme Q10 and L-carnitine)44-46 improve clinical and symptomatic indicators of congestive heart failure. The ability of PQQ to promote mitochondrial biogenesis18 (formation of healthy new mitochondria) could lead to even greater improvements in cardiac output. Dysfunctional Mitochondria Contribute to Human CancersIn humans, it takes more than 20 years from exposure to a carcinogen before a solid tumor develops. Research published in 2010 explains the many intimate ways that dysfunctional mitochondria contribute to the development of cancer and its metastasis.47-55 Cancer is fundamentally connected to mitochondrial dysfunction. A decline in mitochondrial energy production with aging is associated with the generation of increased free radicals, which cause mitochondrial mutations.56 These mutations interfere with a normal cell-removal process known as apoptosis.57 A critical factor in protecting against cancer is the ability to eliminate damaged cells through apoptosis. Researchers are focusing on the huge energy-dependent processes required to eradicate faulty or abnormally growing cells. It turns out that dysfunctional mitochondria deny cells the ability to go through normal apoptotic removal processes, thus sowing the seeds for cancer initiation and progression. In addition, mitochondria are the source for several apoptotic proteins58 that activate cell death in the quest to eliminate damaged cells.59,60 Mitochondrial dysfunction that occurs with aging plays a major influence on carcinogenesis.61 One recent study shows that mitochondrial dysfunction predicts progression of prostate cancer in patients treated with surgery.48
Exciting research findings indicate the potential of reversing mitochondrial senescence in non-tumor cells using mitochondrial-targeted antioxidants.62-69 The benefit is that restored healthy cells are less susceptible to tumor initiation, while cancer cells that have evolved are better controlled by tumor suppressor genes activated by functional mitochondrial cell-signaling.70-72 Mitochondria Insufficiency Promotes Type 2 DiabetesType 2 diabetes is often caused by overeating, but some people lose the ability to control glucose because of hereditary factors or physical inactivity. A study looked at young, lean, sedentary children with insulin resistance whose parents had developed type 2 diabetes. Compared to similar children of non-diabetic parents, muscle biopsies showed that mitochondrial density was reduced by 38% in the offspring of diabetic parents.73 This study also showed that these insulin-resistant children exhibited increased amounts of fat content in their muscles, which also contributes to insulin resistance. These findings support the concept that hereditary mitochondrial dysfunction contributes to the development of insulin resistance and subsequent type 2 diabetes. The encouraging aspect to this study is that those genetically predisposed to type 2 diabetes may be able to avert this calamity through either rigorous physical exercise and/or supplementation with PQQ, both of which have been shown to promote mitochondrial biogenesis.15,18 (Note that certain anti-diabetic drugs like metformin and thiazolidinediones also induce mitochondrial biogenesis through additional mechanisms.)17,74-76 Stem Cells Require Healthy MitochondriaOur bodies possess remarkable ability for sustained tissue renewal throughout our lifetimes. This continuous self-renewal process is dependent on reservoirs of somatic stem cells.
A report published in 2010 describes how intact mitochondrial function is crucial for maintenance of stem cells. In response to mitochondrial impairment, there is an increase in damaging free radicals accompanied by stem cell compromise.77 Researchers have discovered that stem cell populations do not necessarily decline with advancing age, but instead lose their restorative potential.78 This functional stem cell decline is accompanied by organ malfunction and increased incidence of disease. Mitochondrial dysfunction thus underlies a degenerative cycle that robs aging humans of the renewal benefits of their own stem cells. The integration of mitochondria into the core “axis of aging” has led researchers to propose that improvements in mitochondrial health (along with other cellular modulations) could yield advanced therapeutic strategies designed to rejuvenate tissues of the aged. How Mitochondrial Structure DeterioratesAltered (glycated) proteins can bind to mitochondria and compromise their function.79,80 The accumulation of dysfunctional mitochondria results in a vicious cycle whereby increased oxidative and glycation reactions disable more mitochondria, eventually leading to a cell’s demise.81 The mitochondria in cells of elderly people are mostly dysfunctional, whereas young individuals have virtually no mitochondrial damage.82,83 A fascinating report published in 2010 describes the lethal cascade that occurs as inactive mitochondria accumulate in cells and how carnosine, acetyl-L-carnitine, and resveratrol can protect against these longevity-shortening molecular interplays.84 The Mitochondria and Human Health SpanThe aging of the American society is upon us with an accelerated impact on healthcare expenditures. Conventional medicine can affix band-aids to age-related disease, but fails to correct the major underlying cause of mitochondrial dysfunction and the severe shortage of healthy mitochondria within the cell. A search of scientific articles using the terms “mitochondria and aging” going back to the year 1980 reveals the exponential increase in our understanding of the role this cell organelle plays in sustaining healthy life span. The box below shows how many published papers discuss “mitochondria and aging” in the early 1980s compared to the last two years:
Life Extension members should be comforted in knowing that their use of nutrients to maintain mitochondrial integrity is being increasingly vindicated in the peer-reviewed scientific literature. Mitochondrial biogenesis is a term used to describe the beneficial creation of new mitochondria. With the discovery of the unique properties by which PQQ promotes mitochondrial biogenesis, a missing link to the puzzle of degenerative aging has been uncovered. PQQ is available without the requirement of a prescription. Life Extension Has No “Exit” StrategyI hope you appreciate that as we move into our fourth decade, Life Extension has not changed its scientific mission or total service commitment to members. The founders remain as dedicated today as they were in the 1970s to discovering validated scientific methods to forestall and reverse biological aging. While we use the year 1980 as our official starting date, the Life Extension Foundation received its tax-exempt status in 1978, and I set up the first Life Extension laboratory in 1977. Contrast this to companies that buy and sell themselves over and over, with new owners seeking to extract every nickel of profit at the expense of product quality and customer service. When you phone Life Extension, you never get a recording unless all of our representatives are busy assisting other members. We promise to not greet you with an automated menu that forces you to listen to endless taped messages and push many buttons before getting to talk to a live person. We are sometimes approached by Wall Street investors who ask, “What is your exit strategy?” What they mean is when do you plan to “cash out” (by selling the organization to profit-hungry investors) and retire. My response is always the same: “When we achieve biological immortality, I may consider taking some time off.” I can assure you there will never be an “exit” from our quest to achieve indefinite longevity. Advanced Nutrient Formulas at Year’s Lowest PricesLife Extension members take advantage of the annual Super Sale to acquire our most up-to-date nutrient formulations at extra discounted prices.
Virtually every year, we upgrade our formulas to provide even more effective health-sustaining nutrients. Last year we introduced a ubiquinol CoQ10 product that provides significantly higher CoQ10 levels within the cell’s mitochondria.85 This year we introduce PQQ to promote the formation of new mitochondria. PQQ is available as a standalone product, or as a component of two novel mitochondrial support formulations. In this month’s issue, you’ll learn about effective methods to maintain, protect, and improve vital mitochondrial function. The good news is that most of you have been taking proven mitochondrial-protecting nutrients for many years or decades. Members now have access to a compound (PQQ) that has been shown to induce formation of new mitochondria. Every time you purchase a Life Extension product, you contribute to research aimed at extending healthy human life span. The Life Extension Foundation continues to fund a record number of scientific projects, while battling incompetent bureaucrats who seek to suffocate medical innovation. During the traditional winter Super Sale, all Life Extension formulas are discounted so that members can obtain up-to-date versions at the lowest prices of the year. Until January 31, 2011, members take advantage of Super Sale discounts to stock up on the world’s most cutting–edge life-extending formulas. For longer life,
William Faloon | |||||||||||
| References | |||||||||||
| 1. Wallace DC. A mitochondrial paradigm of metabolic and degenerative diseases, aging, and cancer: a dawn for evolutionary medicine. Annu Rev Genet. 2005 39;359–407. 2. Ochoa JJ, Quiles JL, Huertas JR, Mataix J. Coenzyme Q10 protects from aging-related oxidative stress and improves mitochondrial function in heart of rats fed a polyunsaturated fatty acid (PUFA)-rich diet. J Gerontol A Biol Sci Med Sci. 2005 Aug;60(8):970-5. 3. Matthews RT, Yang L, Browne S, Baik M, Beal MF. Coenzyme Q10 administration increases brain mitochondrial concentrations and exerts neuroprotective effects. Proc Natl Acad Sci USA. 1998 Jul 21;95(15):8892-7. 4. Rodriguez MC, MacDonald JR, Mahoney DJ, Parise G, Beal MF, Tarnopolsky MA. Beneficial effects of creatine, CoQ10, and lipoic acid in mitochondrial disorders. Muscle Nerve. 2007 Feb;35(2):235-42. 5. Rosenfeldt FL, Pepe S, Linnane A, et al. Coenzyme Q10 protects the aging heart against stress: studies in rats, human tissues, and patients. Ann N Y Acad Sci. 2002 Apr;959:355-9; discussion 463-5. 6. Sohal RS, Forster MJ. Coenzyme Q, oxidative stress and aging. Mitochondrion. 2007 Jun;7 Suppl:S103-11. 7. Binienda ZK. Neuroprotective effects of L-carnitine in induced mitochondrial dysfunction. Ann NY Acad Sci. 2003 May;993:289-95; discussion 345-9. 8. Noland RC, Koves TR, Seiler SE, et al. Carnitine insufficiency caused by aging overnutrition compromises mitochondrial performance and metabolic control. J Biol Chem. 2009 Aug 21;284(34):22840-52. 9. Virmani A, Gaetani F, Imam S, Binienda Z, Ali S. The protective role of L-carnitine against neurotoxicity evoked by drug of abuse, methamphetamine, could be related to mitochondrial dysfunction. Ann N Y Acad Sci. 2002 Jun; 965:225–32. 10. Li CJ, Zhang QM, Li MZ, Zhang JY, Yu P, Yu DM. Attenuation of myocardial apoptosis by alpha-lipoic acid through suppression of mitochondrial oxidative stress to reduce diabetic cardiomyopathy. Chin Med J (Engl). 2009 Nov 5;122(21):2580-6. 11. Hagen TM, Moreau R, Suh JH, Visioli F. Mitochondrial decay in the aging rat heart: evidence for improvement by dietary supplementation with acetyl-L-carnitine and/or lipoic acid. Ann NY Acad Sci. 2002 Apr;959:491-507. 12. Liu J, Head E, Gharib AM, et al. Memory loss in old rats is associated with brain mitochondrial decay and RNA/DNA oxidation: partial reversal by feeding acetyl-L-carnitine and/or R-alpha-lipoic acid. Proc Natl Acad Sci USA. 2002 Feb 19;99(4):2356-61. 13. Moreira PI, Harris PL, Zhu X, et al. Lipoic acid and N-acetyl cysteine decrease mitochondrial-related oxidative stress in Alzheimer disease patient fibroblasts. J Alzheimers Dis. 2007 Sep;12(2):195-206. 14. Hagen TM, Ingersoll RT, Lykkesfeldt J, et al. (R)-alpha-lipoic acid-supplemented old rats have improved mitochondrial function, decreased oxidative damage, and increased metabolic rate. FASEB J. 1999 Feb;13(2):411-8. 15. Lanza IR, Sreekumaran Nair K. Regulation of skeletal muscle mitochondrial function: genes to proteins. Acta Physiol (Oxf). 2010 Aug;199(4):529-47. 16. Spindler SR. Caloric restriction: from soup to nuts. Ageing Res Rev. 2010 Jul;9(3):324-53. 17. Suwa M, Egashira T, Nakano H, Sasaki H, Kumagai S. Metformin increases the PGC-1alpha protein and oxidative enzyme activities possibly via AMPK phosphorylation in skeletal muscle in vivo. J Appl Physiol. 2006 Dec;101(6):1685-92. 18. Chowanadisai W, Bauerly KA, Tchaparian E, Wong A, Cortopassi GA, Rucker RB. Pyrroloquinoline quinone stimulates mitochondrial biogenesis through cAMP response element-binding protein phosphorylation and increased PGC-1alpha expression. J Biol Chem. 2010 Jan 1;285(1):142-52. 19. de Moura MB, dos Santos LS, Van Houten B. Mitochondrial dysfunction in neurodegenerative diseases and cancer. Environ Mol Mutagen. 2010 Jun;51(5):391-405. 20. Blass JP. The mitochondrial spiral. An adequate cause of dementia in the Alzheimer’s syndrome. Ann N Y Acad Sci. 2000; 924:170-83. 21. Chen JX, Yan SD. Amyloid-beta-induced mitochondrial dysfunction. J Alzheimers Dis. 2007 Sep; 12(2):177-84. 22. Lustbader JW, Cirilli M, Lin C, et al. ABAD directly links Abeta to mitochondrial toxicity in Alzheimer’s disease. Science. 2004 Apr 16; 304(5669):448–52. 23. Wang X, Su B, Lee HG, et al. Impaired balance of mitochondrial fission and fusion in Alzheimer’s disease. J Neurosci. 2009 Jul 15; 29(28):9090–103. 24. Lin TK, Liou CW, Chen SD, et al. Mitochondrial dysfunction and biogenesis in the pathogenesis of Parkinson’s disease. Chang Gung Med J. 2009 Nov-Dec;32(6):589-99. 25. Abou-Sleiman PM, Muqit MM, Wood NW. Expanding insights of mitochondrial dysfunction in Parkinson’s disease. Nat Rev Neurosci. 2006 Mar; 7(3):207–19. 26. Schapira AH, Bezard E, Brotchie J, et al. Novel pharmacological targets for the treatment of Parkinson’s disease. Nat Rev Drug Discov. 2006;5:845–54. 27. Reddy PH, Mao P, Manczak M. Mitochondrial structural and functional dynamics in Huntington’s disease. Brain Res Rev. 2009 Jun; 61(1):33–48. 28. Pandey M, Varghese M, Sindhu KM, et al. Mitochondrial NAD+-linked State 3 respiration and complex-I activity are compromised in the cerebral cortex of 3-nitropropionic acid-induced rat model of Huntington’s disease. J Neurochem. 2008 Jan; 104(2):420–34. 29. Panov AV, Gutekunst CA, Leavitt BR, et al. Early mitochondrial calcium defects in Huntington’s disease are a direct effect of polyglutamines. Nat Neurosci. 2002 Aug; 5(8):731–6. 30. Muller WE, Eckert A, Kurz C, Eckert GP, Leuner K. Mitochondrial dysfunction: common final pathway in brain aging and Alzheimer’s disease--therapeutic aspects. Mol Neurobiol. 2010 Jun;41(2-3):159-71. 31. Schleicher M, Shepherd BR, Suarez Y, et al. Prohibitin-1 maintains the angiogenic capacity of endothelial cells by regulating mitochondrial function and senescence. J Cell Biol. 2008 Jan 14;180(1):101-12. 32. Oeseburg H, Iusuf D, van der Harst P, van Gilst WH, Henning RH, Roks AJ. Bradykinin protects against oxidative stress-induced endothelial cell senescence. Hypertension. 2009 Feb;53(2):417-22. 33. Yang Z, Harrison CM, Chuang GC, Ballinger SW. The role of tobacco smoke induced mitochondrial damage in vascular dysfunction and atherosclerosis. Mutat Res. 2007 Aug 1;621(1-2):61-74. 34. Knight-Lozano CA, Young CG, Burow DL, et al. Cigarette smoke exposure and hypercholesterolemia increase mitochondrial damage in cardiovascular tissues. Circulation. 2002 Feb 19;105(7):849–54. 35. Jia L, Liu Z, Sun L, et al. Acrolein, a toxicant in cigarette smoke, causes oxidative damage and mitochondrial dysfunction in RPE cells: protection by (R)-alpha-lipoic acid. Invest Ophthalmol Vis Sci. 2007 Jan; 48(1):339-48. 36. Yang Z, Knight CA, Mamerow M, et al. Prenatal environmental tobacco smoke exposure promotes adult atherogenesis and mitochondrial damage in apoE-/- mice fed a chow diet. Circulation. 2004 Dec 14; 110(24):3715–20. 37. Bach D, Pich S, Soriano FX, et al. Mitofusin-2 determines mitochondrial network architecture and mitochondrial metabolism. A novel regulatory mechanism altered in obesity. J Biol Chem. 2003 May 9; 278(19):17190–7. 38. Li M, Absher M, Liang P, Russell JC, Sobel BE, Fukagawa NK. High glucose concentrations induce oxidative damage to mitochondrial DNA in explanted vascular smooth muscle cells. Exp Biol Med. 2001; 226:450–7. 39. Vanhorebeek I, Ellger B, De Vos R, et al. Tissue-specific glucose toxicity induces mitochondrial damage in a burn injury model of critical illness. Crit Care Med. 2009 Apr;37(4):1355-64. 40. Madamanchi NR, Runge MS. Mitochondrial dysfunction in atherosclerosis. Circ Res. 2007 Mar 2;100(4):460-73. 41. Graier WF, Malli R, Kostner GM. Mitochondrial protein phosphorylation: instigator or target of lipotoxicity? Trends Endocrinol Metab. 2009 May;20(4):186-93. 42. Maasen JA. Mitochondria, body fat and type 2 diabetes: what is the connection? Minerva Med. 2008 Jun;99(3):241-51. 43. Karamanlidis G, Nascimben L, Couper GS, Shekar PS, del Monte F, Tian R. Defective DNA replication impairs mitochondrial biogenesis in human failing hearts. Circ Res. 2010 May 14;106(9):1541-8. 44. Mortensen SA, Vadhanavikit S, Muratsu K, Folkers K. Coenzyme Q10: clinical benefits with biochemical correlates suggesting a scientific breakthrough in the management of chronic heart failure. Int J Tissue React. 1990;12(3):155-62. 45. Langsjoen PH, Langsjoen AM. Supplemental ubiquinol in patients with advanced congestive heart failure. Biofactors. 2008;32(1-4):119-28. 46. Serati AR, Motamedi MR, Emami S, Varedi P, Movahed MR. L-carnitine treatment in patients with mild diastolic heart failure is associated with improvement in diastolic function and symptoms. Cardiology. 2010;116(3):178-82. 47. Ladiges W, Wanagat J, Preston B, Loeb L, Rabinovitch P. A mitochondrial view of aging, reactive oxygen species and metastatic cancer. Aging Cell. 2010 Aug;9(4):462-5. 48. Yu JJ, Yan T, Jiang YC. Mitochondrial function score combined with Gleason score for predicting the progression of prostate cancer. Zhonghua Nan Ke Xue. 2010 Mar;16(3):220-2. 49. Ma Y, Bai RK, Trieu R, Wong LJ: Mitochondrial dysfunction in human breast cancer cells and their transmitochondrial cybrids. Biochim Biophys Acta. 2010 Jan; 1797(1):29-37. 50. Ordys BB, Launay S, Deighton RF, McCulloch J, Whittle IR. The role of mitochondria in glioma pathophysiology. Mol Neurobiol. 2010 Aug;42(1):64-75. 51. Lee HC, Chang CM, Chi CW. Somatic mutations of mitochondrial DNA in aging and cancer progression. Ageing Res Rev. 2010 Nov; 9 Suppl 1:S47-58. 52. Ralph SJ, Rodriguez-Enriquez S, Neuzil J, Saavedra E, Moreno-Sanchez R. The causes of cancer revisited: “mitochondrial malignancy” and ROS-induced oncogenic transformation - why mitochondria are targets for cancer therapy. Mol Aspects Med. 2010 Apr;31(2):145-70. 53. Kulawiec M, Owens KM, Singh KK. Cancer cell mitochondria confer apoptosis resistance and promote metastasis. Cancer Biol Ther. 2009 Jul;8(14):1378-85. 54. Ishikawa K, Takenaga K, Akimoto M, et al. ROS-generating mitochondrial DNA mutations can regulate tumor cell metastasis. Science. 2008 May 2; 320(5876):661-4. 55. Hung WY, Wu CW, Yin PH, et al. Somatic mutations in mitochondrial genome and their potential roles in the progression of human gastric cancer. Biochim Biophys Acta. 2010 Mar;1800(3):264-70. 56. Wallace DC, Shoffner JM, Trounce I, et al. Mitochondrial DNA mutations in human degenerative diseases and aging. Biochim Biophys Acta. 1995 May 24; 1271(1):141-51. 57. Shidara Y, Yamagata K, Kanamori T, et al. Positive contribution of pathogenic mutations in the mitochondrial genome to the promotion of cancer by prevention from apoptosis. Cancer Res. 2005 Mar 1;65(5):1655-63. 58. Newmeyer DD, Ferguson-Miller S. Mitochondria: releasing power for life and unleashing the machineries of death. Cell. 2003 Feb 21;112(4):481-90. 59. Ricci JE, Munoz-Pinedo C, Fitzgerald P, et al. Disruption of mitochondrial function during apoptosis is mediated by caspase cleavage of the p75 subunit of complex I of the electron transport chain. Cell. 2004 Jun 11;117(6):773-86. 60. Karbowski M, Youle RJ. Dynamics of mitochondrial morphology in healthy cells and during apoptosis. Cell Death Differ. 2003 Aug;10(8):870-80. 61. Singh KK. Mitochondrial dysfunction is a common phenotype in aging and cancer. Ann N Y Acad Sci. 2004 Jun;1019:260-4. 62. Nicolson GL, Conklin KA. Reversing mitochondrial dysfunction, fatigue and the adverse effects of chemotherapy of metastatic disease by molecular replacement therapy. Clin Exp Metastasis. 2008; 25(2):161-9. 63. Atamna H, Robinson C, Ingersoll R, Elliott H, Ames BN. N-t-Butyl hydroxylamine is an antioxidant that reverses age-related changes in mitochondria in vivo and in vitro. FASEB J. 2001 Oct;15(12):2196-204. 64. Hagen TM, Wehr CM, Ames BN. Mitochondrial decay in aging. Reversal through supplementation of acetyl-L-carnitine and N-tert-butyl-alpha-phenyl-nitrone. Ann N Y Acad Sci. 1998 Nov 20;854:214-23. 65. Smith RA, Adlam VJ, Blaikie FH, et al. Mitochondria-targeted antioxidants in the treatment of disease. Ann N Y Acad Sci. 2008 Dec;1147:105-11. 66. Murphy MP, Smith RA. Targeting antioxidants to mitochondria by conjugation to lipophilic cations. Annu Rev Pharmacol Toxicol. 2007; 47:629-56. 67. Manczak M, Mao P, Calkins MJ, et al. Mitochondria-targeted antioxidants protect against amyloid-beta toxicity in Alzheimer’s disease neurons. J Alzheimers Dis. 2010; 20 Suppl 2:S609-31. 68. Liu J, Atamna H, Kuratsune H, Ames BN. Delaying brain mitochondrial decay and aging with mitochondrial antioxidants and metabolites. Ann N Y Acad Sci. 2002 Apr;959:133-66. 69. Bagh MB, Thakurta IG, Biswas M, Behera P, Chakrabarti S. Age-related oxidative decline of mitochondrial functions in rat brain is prevented by long term oral antioxidant supplementation. Biogerontology. 2010 Sep 21. 70. Gottlieb E, Tomlinson IP. Mitochondrial tumour suppressors: a genetic and biochemical update. Nat Rev Cancer. 2005 Nov;5(11):857-66. 71. Pratheeshkumar P, Thejass P, Kutan G. Diallyl disulfide induces caspase-dependent apoptosis via mitochondria-mediated intrinsic pathway in B16F-10 melanoma cells by up-regulating p53, caspase-3 and down-regulating pro-inflammatory cytokines and nuclear factor-κβ-mediated Bcl-2 activation. J Environ Pathol Toxicol Oncol. 2010; 29(2):113-25. 72. Ip SW, Lan SH, Huang AC, et al. Capsaicin induces apoptosis in SCC-4 human tongue cancer cells through mitochondria-dependent and -independent pathways. Environ Toxicol. 2010 Oct 5. 73. Morino K, Petersen KF, Dufour S, et al. Reduced mitochondrial density and increased IRS-1 serine phosphorylation in muscle of insulin-resistant offspring of type 2 diabetic parents. J Clin Invest. 2005 Dec;115(12):3587-93. 74. Chen Y, Zhou K, Wang R, et al. Antidiabetic drug metformin (GlucophageR) increases biogenesis of Alzheimer’s amyloid peptides via up-regulating BACE1 transcription. Proc Natl Acad Sci U S A. 2009 Mar 10;106(10):3907-12. 75. Skov V, Glintborg D, Knudsen S, et al. Pioglitazone enhances mitochondrial biogenesis and ribosomal protein biosynthesis in skeletal muscle in polycystic ovary syndrome. PLoS One. 2008 Jun 18;3(6):e2466. 76. Bogacka I, Xie H, Bray GA, Smith SR. Pioglitazone induces mitochondrial biogenesis in human subcutaneous adipose tissue in vivo. Diabetes. 2005 May;54(5):1392-9. 77. Sahin E, Depinho RA. Linking functional decline of telomeres, mitochondria and stem cells during ageing. Nature. 2010 Mar 25;464(7288):520-8. 78. Conboy IM, Rando TA. Aging, stem cells and tissue regeneration: lessons from muscle. Cell Cycle. 2005 Mar;4(3):407-10. 79. Alikhani Z, Alikhani M, Boyd CM, Nagao K, Trackman PC, Graves DT. Advanced glycation end products enhance expression of pro-apoptotic genes and stimulate fibroblast apoptosis through cytoplasmic and mitochondrial pathways. J Biol Chem. 2005 Apr 1;280(13):12087-95. 80. Kil IS, Lee JH, Shin AH, Park JW. Glycation-induced inactivation of NADP(+)-dependent isocitrate dehydrogenase: implications for diabetes and aging. Free Radic Biol Med. 2004 Dec 1;37(11):1765-78. 81. Hipkiss AR. Mitochondrial dysfunction, proteotoxicity, and aging: causes or effects, and the possible impact of NAD+-controlled protein glycation. Adv Clin Chem. 2010; 50:123-50. 82. Short KR, Bigelow ML, Kahl J, et al. Decline in skeletal muscle mitochondrial function with aging in humans. Proc Natl Acad Sci U S A. 2005 Apr 12;102(15):5618-23. 83. Linnane AW, Kovalenko S, Gingold EB. The universality of bioenergetic disease: age-associated cellular bioenergetic degradation and amelioration therapy. Ann N Y Acad Sci. 1998 Nov 20;854:202-13. 84. Hipkiss AR. Aging, proteotoxicity, mitochondria, glycation, NAD and carnosine: possible inter-relationships and resolution of the oxygen paradox. Front Aging Neurosci. 2010 Mar 18;2:10. 85. Hosoe K, Kitano M, Kishida H, et al. Study on safety and bioavailability of ubiquinol (Kaneka QH) after single and 4-week multiple oral administration to healthy volunteers. Regul Toxicol Pharmacol. 2007 Feb;47(1):19-28. |