Life Extension Magazine®
Despite regular brushing, flossing, and professional cleaning, it is challenging to optimally suppress plaque buildup. In an intriguing development, researchers have discovered two unique strains of bacteria that can prevent the buildup of plaque and biofilm on our teeth. As we have come to learn, plaque-induced gum disease causes more than just halitosis (bad breath). Chronically inflamed gums lead to a host of degenerative disorders including atherosclerosis, diabetes, and cancer. After years of study, a new oral probiotic lozenge may change how millions can achieve optimal oral health. Poor Oral Health Increases Disease RiskThe human mouth is teeming with all kinds of bacteria. Many of those bacteria are harmless or even beneficial, but a sizable handful can cause diseases such as dental caries (cavities) and the much more serious gingivitis and periodontitis.
These conditions tend to be chronic and produce a steady elevation of inflammation in a part of the body that receives a very high blood flow (the mouth). The result of this bacterial “seeding” is the promotion of a host of destructive cytokines.1 These circulating cytokines produce inflammatory responses in tissues far distant from the mouth, including the vascular lining known as the endothelium, and dramatically increase the risk of atherosclerosis, heart attack, and stroke.2,3 Sadly, there is strong evidence that gum disease can also increase the risk of pre-term births and low birth weight.4 Periodontitis has also been linked with diabetes,2,5,6 and periodontal treatment may improve blood sugar control in diabetic patients.7 In short, the mouth is a dangerous place if not kept clean through meticulous hygiene. The cardiovascular health impact of periodontitis is particularly troubling; people with chronic periodontitis may face an increased risk of heart attacks8 as well as kidney dysfunction related to atherosclerosis.9 Disturbingly, there is direct evidence that some pathogenic oral microbes make it into the bloodstream of people with gum inflammation, and even wind up forming part of the atherosclerotic plaque that causes vascular disease.10-12 Other mouth germs make platelets more sensitive to factors that increase the risk for abnormal blood clot formation.13 The good news is that we may be able to prevent or at least control the growth of those dangerous microorganisms in the first place through the use of a probiotic lozenge in the oral cavity. Scientists have recently discovered two complementary probiotics that are producing dramatic benefits for oral and periodontal health. By optimizing the health of the oral cavity, these new oral probiotics may provide protection against a broad spectrum of disease issues, ranging from cardiovascular disease to diabetes. Traditional Oral Health Care: Flossing, Brushing, and RinsingDentists despair that most people fail to engage in the proper daily oral hygiene practices such as brushing, flossing, and mouth rinsing that are currently recommended for the prevention of so many conditions.14,15 Thanks to the work of pioneering scientists, probiotics can now make the mouth itself a contributor to health rather than disease.16-21 Probiotics Fight Plaque, GingivitisProbiotics have been defined as “living microorganisms which upon ingestion in certain numbers exert health benefits beyond inherent general nutrition.”22 Scientists have been interested in the makeup of the microbes that live in the mouth (the “oral flora”) for decades, seeking to identify factors that promote the growth of healthy organisms and reduce the growth of those implicated in disease and inflammation.23-26 Probiotics not only improve oral health but can help to change the stubborn composition of dental biofilm and plaque.27,28 While reducing the total amount of plaque through tooth brushing is always a desirable goal, its complete elimination is not possible. Therefore, changing the actual composition of plaque from an inflammatory cytokine-rich environment into a more benign environment dominated by neutral or even helpful organisms can contribute to overall systemic health.29-31
It was a leap of scientific imagination that allowed researchers to realize that the simple use of pro-biotics could make a significant difference in protecting oral health. A powerful demonstration of probiotics’ oral benefits came in 2001, with the publication of a study on tooth decay in children aged one to six.32 The researchers supplemented the children’s milk with a common probiotic bacterium, comparing their rate of cavities with those of kids given normal milk. Researchers examined the children’s oral health status at the beginning and end of a seven-month period. The probiotic group had fewer cavities and lower counts of a hostile bacterium implicated in dental cavities, compared with the control subjects. In a more compelling study, Swedish scientists showed in 2006 that they could reverse symptoms of gingivitis through the use of another probiotic species in adults with moderate-to-severe inflammation.33 After just two weeks, subjects who received pro-biotics demonstrated a reduced amount of plaque and inflammation, compared with the placebo group. Numerous clinical and laboratory studies have confirmed and clarified the ways in which probiotic organisms contribute to a reduction in plaque and hostile organism colonization.34-38 Discovering Advanced Forms of ProbioticsA microbiologist from New Zealand, Dr. John R. Tagg, is credited with advancing the science of using probiotics to enhance oral health. Dr. Tagg began his work with the 1981 publication of a paper on the role of mysterious compounds called bacteriocins on the formation of dental plaque.39 Dr. Tagg and his colleagues found that certain “friendly” oral bacteria were producing substances that inhibit the growth of other microbes. Because these microbes had no known use as drugs in humans they were given the name bacteriocins.40 What was remarkable about Tagg’s work was that the species of friendly Streptococcus salivarius bacteria that produced the bacteriocins can also be found to occur naturally in the mouth. Unlike other probiotic species, these strep organisms lived in benign symbiosis with the human oral cavity.18 Even more remarkable, the bacteriocin produced by Streptococcus salivarius (S. salivarius) bacteria had powerful inhibitory actions against other strep species, most notably those that cause dental cavities as well as serious human infections such as strep throat, deep tissue infections, and even rheumatic fever.18,19,41,42 By the end of the 1980s, Tagg’s group knew enough about their molecules to name them bacteriocin-like inhibitory substances, or BLIS.20,43
Dr. Tagg and his investigative team studied these bacteriocins intensively over the subsequent decade, determining their structures, mechanisms of action, and the means by which the S. salivarius germs were controlling bacteriocin production.44-47 Their work culminated in the 2003 publication of a landmark paper entitled “Bacterial replacement therapy: adapting ‘germ warfare’ to infection prevention.”17 In this article, Tagg and colleague Karen P. Dierksen review what they describe as an “ongoing battle” between oral microbes, some of which may pose an infectious threat to the host, and others of which confer protection. They propose further exploration of the use of probiotic organisms to colonize human tissues with health-promoting effector strains of beneficial bacteria that can out-compete disease-causing germs. Finally, they focus on recent progress in application of so-called avirulent (non-dangerous) S. salivarius species in the control of dental cavities, strep throats, and even childhood ear infections. Let’s take our own look at the evidence for these oral probiotics. Dental HealthBeneficial microorganisms such as those studied by Dr. Tagg may contribute to improved dental health. In laboratory studies, the probiotic S. salivarius helped inhibit the formation of the sticky biofilm that can contribute to oral disease.41 Building on these results, a study in animals showed that the S. salivarius probiotic helped displace biofilm from the teeth, displacing cavity-causing bacteria and inhibiting tooth decay.42
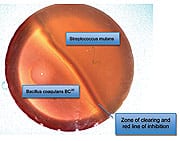
Another in vitro experiment demonstrates how effectively a second oral probiotic protects oral health.48 In this experiment, a form of Bacillus coagulans (known as GanedenBC30™) was shown to competitively inhibit the cariogenic (cavity-inducing) bacterium Streptococcus mutans, which contributes to significant tooth decay. The photo below shows a Petri dish that was incubated at body temperature for two days after the left side was streaked with GanedenBC30 and the right side with S. mutans. You can see a large zone of inhibition between the two cultures, indicating that the S. mutans culture died as it approached the GanedenBC30 culture. This is indicated by the solid red line at the edge of the GanedenBC30 growth. The final zone of inhibition was 6 mm, which showed GanedenBC30 to be effective at inhibiting the growth of S. mutans. Together, these findings suggest that both S. salivarius and B. coagulans probiotics can help prevent harmful bacteria from colonizing teeth and contributing to dental cavities. | ||||||
Aphthous Stomatitis (Canker Sores)Compelling evidence suggests that probiotics may offer relief from the most common disease affecting the oral mucosa in the US—aphthous stomatitis, also known as canker sores. These painful ulcerations, which can affect the inside lining of the lips and cheek, underside of the tongue, and the floor of the mouth, represent a significant source of suffering and distress.49 Although the precise causes of canker sores are not yet fully understood, scientists believe that immune system dysregulation may be a critical causative factor, noting elevated levels of TNF-alpha and reduced cellular expression of shock protein 27 and interleukin-10 in association with the ulcers.50-52 The occurrence of canker sores has also been linked with increased anxiety and elevated levels of salivary cortisol, a manifestation of stress that can set the stage for a host of illnesses.53 Conventional medical and dental management focuses on relieving pain and inflammation until the ulcers heal, which typically occurs in 7-10 days. Impressive reports suggest that the probiotic Bacillus coagulans (previously known as Lactobacillus sporogenes) helps dramatically speed healing of aphthous ulcers. In fact, reports suggest that supplementing with the probiotic Bacillus coagulans may help canker sores heal in as little as two to three days, providing much needed relief for those plagued with these troublesome outbreaks.54,55 Halitosis (Bad Breath)While bad breath is not dangerous physically, it can be a sign of deteriorating oral health. Intriguingly, the S. salivarius probiotic (known as BLIS K12™) is showing beneficial effects against this challenging and embarrassing problem. That makes sense to dentists, who know that the best treatment for oral malodor is the reduction of bacterial populations, especially those on the tongue—but the problem is that the germs quickly grow back after standard treatment.56 A research group led by Dr. Tagg’s colleague Jeremy P. Burton at BLIS Technologies Center for Innovation in New Zealand achieved impressive results using BLIS K12™ lozenges.57 The researchers studied 23 subjects with halitosis, giving them all a three-day regimen of antibacterial mouth rinses followed by either BLIS K12™ or placebo. They measured the odor-producing sulfur compounds in subjects’ breath a week later, finding that 85% of the BLIS group, but only 30% of the placebo group, experienced substantial reductions in the noxious chemical. The BLIS group also had lower counts of bacteria implicated in halitosis compared with the placebo recipients. Burton’s researchers concluded that BLIS K12™ “may provide an effective strategy to reduce the severity of halitosis.” Upper Respiratory Tract InfectionsPoor oral health does not just produce cavities. It can contribute to a range of other diseases such as strep throats which, if left untreated, can lead to consequences at sites far remote from the throat, including a devastating form of kidney disease and the once-deadly rheumatic fever. These former scourges of childhood and young adulthood have been largely eradicated in the modern industrialized world, but that eradication has come at the expense of growing levels of antibiotic resistance. A superior approach is to prevent the offending organism—Streptococcus pyogenes (S. pyogenes)—from setting up housekeeping in the throat to begin with. Studies from Dr. Tagg’s research group have demonstrated that beneficial probiotics hold the potential to do just that. Tagg and others studied children in New Zealand, culturing the germs that lived in their mouths, and identifying the inhibitory BLIS agents they produced.21 They were able to identify one particular strain of the beneficial organism, S. salivarius, that when present prevented the children from acquiring the dangerous S. pyogenes bacteria. This strain produced two highly potent BLIS molecules that inhibit the detrimental strep organisms efficiently. Now known as BLIS K12™, this strain of S. salivarius is the active probiotic organism in a new line of oral probiotic products. Ear infections are another cause of substantial misery, particularly in young children. The middle ear is actually a branch of the upper respiratory tract, connected to the throat by the Eustachian tube, a mucus-lined channel that normally allows equalization of air pressure between the delicate chambers of the ear and the outside world. When swollen closed, however, as so often happens following a viral illness like a cold, the tube fails to ventilate the ear, and fluid builds up. That moist, dark, and warm environment can provide a haven for disease-producing organisms—if they take hold and grow, the result is a middle ear infection. Since it is impossible to culture the germs from that space without invasive procedures, doctors tend to treat with antibiotics—often indiscriminately and with the attendant risk of antibiotic resistance. As with strep throat, a preventive approach would be superior, and BLIS K12™ is showing promise in this arena. Another of Dr. Tagg’s protégés, D.A. Power of the University of Otago in New Zealand, has just published an intriguing study of BLIS K12™ in 19 young children prone to ear infections.58 Power and colleagues gave the children the probiotic following a three-day course of an antibiotic in advance of ear surgery. The scientists found that the BLIS K12™ organisms readily colonized not only the mouth, but also the back portion of the nose, where the Eustachian tube is located. This study suggests that BLIS K12™ might be effective in preventing the growth of the organisms that can cause bacterial ear infections. A German research group has now established that BLIS K12™ goes to work immediately in the mouth after ingestion, ramping up its production of the bacteria-inhibiting compound salivaricin A.59 The researchers found high levels of this compound in saliva and on all mucous membranes, peaking between days 4 and 8 after ingestion, and remaining detectable for as long as three weeks. These researchers stressed the importance, then, of repeated uptake of the probiotic to maintain good levels in the mouth needed to confer protection against bacterial infection. Immune ModulationWe know by now that most of the debilitating and deadly conditions associated with aging are established by chronic inflammation. Intense research both in basic science and drug therapy focuses on the development of new products that limit inflammation without shutting down the beneficial parts of the immune system. This is another completely unexpected area in which the probiotics BLIS K12™ and Bacillus coagulans (GanedenBC30) may offer some relief. Their capacity to precisely modulate our inflammatory responses makes them astonishingly appropriate candidates for preventing a host of acute and chronic inflammatory conditions, reducing cytokine levels that contribute to conditions as diverse as atherosclerosis and pre-term labor.2,60 Canadian immunologists have explored the ability of the BLIS K12™ organisms to regulate immunity in a remarkable new study.61 They cultured the pro-biotic germs together with cells from the lining of the human respiratory tract, and measured the production of inflammatory cytokines in the human cells. Astonishingly, the BLIS K12™ organisms specifically altered the activities of 565 human genes, particularly those involved in immune system pathways. BLIS K12™ inhibited production of inflammatory cytokines, largely by inhibiting the activity of the powerful intracellular immune regulator called NF-kappaB, which is also a key link between infection, inflammation, and cancer. The researchers observe that BLIS K12™ organisms therefore assure their own survival in human tissue while protecting the host from inflammation and cell destruction caused by dangerous germs. Promising research likewise suggests a role for Bacillus coagulans in modulating inflammation. In laboratory studies, scientists studied the effects of Bacillus coagulans preparations on white blood cells known as polymorphonuclear cells. They found that a preparation derived from Bacillus coagulans cell walls inhibited the migration of polymorphonuclear cells toward the inflammatory chemoattractant known as leukotriene B4, indicating a powerful anti-inflammatory effect.48 Leukotriene B4 is believed to play a central role in numerous pathological conditions related to aberrant inflammation, such as rheumatoid arthritis and inflammatory bowel disease.62 The ability to dampen cellular responses to leukotriene B4 suggests a powerful disease-modulating effect for Bacillus coagulans. Preliminary findings suggest a range of applications for Bacillus coagulans in supporting optimal immune function. In the laboratory, scientists examined the effects of two preparations derived from Bacillus coagulans on immune system cells. They found that the Bacillus coagulans preparations enhanced white blood cells’ surveillance for bacterial invaders and increased the immune cells’ ability to respond to a simulated bacterial attack. Additionally, the two Bacillus coagulans preparations increased a process called phagocytosis, or the ingestion of invaders like bacteria, by polymorphonuclear cells of the immune system.48 In addition to supporting the body’s defense against bacterial infections, scientists found evidence that Bacillus coagulans may help protect the body against cancer and viral infections by activating immune cells known as natural killer cells. Two Bacillus coagulans preparations enhanced the expression of cancer-fighting factors from natural killer cells when they were brought in contact with tumor cells. The investigators noted that the Bacillus coagulans preparations would likewise be expected to enhance natural killer cells’ ability to destroy non-malignant, virally infected cells.48 SummaryWe live in a new era of living in harmony with our environment. The use of probiotics—living bacteria that have beneficial, not harmful, characteristics—is the perfect metaphor for this new, integrative approach. Rather than aggressively seeking to eradicate all germs in the mouth, incorporation of a probiotic regimen allows us to capitalize on nature’s bounty, creating a healthier oral environment and promoting overall health and longevity. Oral probiotics, combined with vigorous and attentive oral hygiene (and of course, regular checkups), show promise for reducing not only dental cavities, halitosis, and upper respiratory infections, but also for modulating the burden of chronic inflammation that can lead to atherosclerosis, the metabolic syndrome, and cancer. Through the groundbreaking work of Dr. John Tagg and the growing numbers of oral health experts who are joining in his campaign, there is renewed hope that we may further triumph over many of the chronic threats to our health that have plagued us since time immemorial. If you have any questions on the scientific content of this article, please call a Life Extension Health Advisor at 1-800-226-2370. |
| References |
| 1. Oral Microbiol Immunol. 2009 Feb;24(1):11-7. 2. Ann NY Acad Sci. 2006 Nov;1088:251-64. 3. Arterioscler Thromb Vasc Biol. 2007 Jun;27(6):1433-9. 4. J Pak Med Assoc. 2005 Oct;55(10):448-52. 5. J Am Dent Assoc. 2008 Oct;139(Suppl):19S-24S. 6. J Clin Periodontol. 2008 Sep;35(8 Suppl):398-409. 7. Diabetes Metab. 2008 Nov;34(5):497-506. 8. Eur Heart J. 2003 Dec;24(23):2108-15. 9. Am J Kidney Dis. 2005 Apr;45(4):650-7. 10. Zhonghua Xin Xue Guan Bing Za Zhi. 2008 Mar;36(3):215-8. 11. Clin Exp Immunol. 2007 Sep;149(3):445-52. 12. Oral Microbiol Immunol. 2009 Feb;24(1):64-8. 13. Platelets. 2008 Aug;19(5):352-8. 14. J Am Dent Assoc. 2006 Nov;137(Suppl 2):7S-32S. 15. J Clin Periodontol. 2006 Sep;33(9):612-9. 16. Lik Sprava. 2008 Apr;(3-4):10-21. 17. Trends Biotechnol. 2003 May;21(5):217-23. 18. Can J Microbiol. 1981 Sep;27(9):918-23. 19. Arch Oral Biol. 1983;28(10):911-5. 20. J Appl Bacteriol. 1991 Oct;71(4):339-42. 21. Indian J Med Res. 2004 May;119(Suppl):13-6. 22. Dig Liver Dis. 2002 Sep;34(Suppl 2):S2-7. 23. Se Pu. 1999 Sep;17(5):483-5. 24. Proc Finn Dent Soc. 1991;87(4):515-25. 25. Adv Dent Res. 1994 Jul;8(2):263-71. 26. Int Dent J. 2006 Aug;56(4 Suppl 1):233-9. 27. BMC Oral Health. 2006;6(Suppl 1):S14. 28. J Periodontol. 2008 Aug;79(8 Suppl):1560-8. 29. Oral Microbiol Immunol. 2009;24:7-10. 30. Compend Contin Educ Dent. 2008 Sep;29(7):402-3. 31. Int J Immunopathol Pharmacol. 2008 Oct;21(4):993-7. 32. Caries Res. 2001 Nov;35(6):412-20. 33. Swed Dent J. 2006;30(2):55-60. 34. J Clin Periodontol. 2008 Oct;35(10):897-905. 35. Acta Odontol Scand. 2009 Feb;67(1):19-24. 36. Eur J Oral Sci. 2002 Jun;110(3):218-24. 37. Eur J Oral Sci. 2007 Aug;115(4):308-14. 38. Oral Microbiol Immunol. 2008 Apr;23(2):139-47. 39. J Appl Bacteriol. 1981 Apr;50(2):305-13. 40. Appl Microbiol Biotechnol. 2008 Dec;81(4):591-606. 41. Oral Microbiol Immunol. 2009 Apr;24(2):152-61. 42. Infect Immun. 1985 Apr;48(1):44-50. 43. J Dent Res. 1987 Aug;66(8):1321-5. 44. J Clin Microbiol. 2000 Feb;38(2):643-50. 45. Appl Environ Microbiol. 1993 Jul;59(7):2014-21. 46. Dev Biol Stand. 1995;85:639-43. 47. J Bacteriol. 2001 Jul;183(13):3931-8. 48. Ganeden. Data on file. 2009. 49. www.emedicine.medscape.com/article/1075570-overview. 50. J Oral Pathol Med. Jan 1992;21(1):21-5. 51. Clin Exp Immunol. Mar 1995;99(3):392-7. 52. J Oral Pathol Med. Sep 2008;37(8):462-7. 53. Tohoku J Exp Med. Apr 2008;214(4):291-6. 54. Uttar Pradesh State Dent J. 1970;11:7-12. 55. Uttar Pradesh State Dent J. 1980 Jan;11(1):7-12. 56. Oral Dis. 2005;11(Suppl 1):29-31. 57. J Appl Microbiol. 2006 Apr;100(4):754-64. 58. Eur J Clin Microbiol Infect Dis. 2008 Dec;27(12):1261-3. 59. Oral Microbiol Immunol. 2007 Apr;22(2):126-30. 60. Am J Obstet Gynecol. 2003 Oct;189(4):1202-8. 61. Infect Immun. 2008 Sep;76(9):4163-75. 62. Proc Natl Acad Sci USA. 1995 Jan 17;92(2):517-21. 63. Cochrane Database Syst Rev. 2007;(2):CD005097. |