Life Extension Magazine®
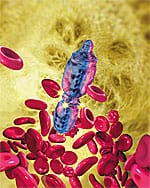 |
A revolution in medical technology looms large on the horizon. The agent of change is microscopically small and is defined in today’s nomenclature as nanotechnology.
Nanotechnology is the engineering of molecularly precise structures and, ultimately, molecular machines. The prefix “nano-” refers to the scale of these constructions. A nanometer is one-billionth of a meter, the width of about five carbon atoms nestled side by side. Nanomedicine is the application of nanotechnology to medicine. The ultimate tool of nanomedicine is the medical nanorobot—a robot the size of a bacterium, composed of molecule-size parts somewhat resembling macroscale gears, bearings, and ratchets. Medical nanorobotics holds the greatest promise for curing disease and extending health span. With diligent effort, the first fruits of medical nanorobotics could begin to appear in clinical treatment as early as the 2020s.
What is a Medical Nanorobot?
Like a regular robot, a nanorobot may be made of many thousands of mechanical parts such as bearings and gears composed of strong diamond-like material. A nanorobot will have motors to make things move, and perhaps manipulator arms or mechanical legs for mobility. It will have a power supply for energy, sensors to guide its actions, and an onboard computer to control its behavior. But unlike a regular robot, a nanorobot will be very small. A nanorobot that would travel through the bloodstream must be tiny enough to squeeze through even the narrowest capillaries in the human body. Such machines must be smaller than the red cells in our blood. A convenient measure of size is the micron, or one-millionth of a meter. A red cell is about seven microns wide. A blood-borne medical nanorobot will typically be no larger than two to three microns in its largest dimension. The parts that make up a nanorobot will be much smaller still, typically one to ten nanometers in size. For instance, the planetary gear shown in (Figure 1 below) is a proposed simple mechanism that converts one kind of rotary motion into another. It is about four nanometers wide and would comprise about 4,000 atoms arranged in an atomically precise structure.
Future Tools For Fighting Infection
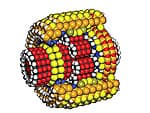
What might a typical medical nanorobot look like? The “microbivore” shown in (Figure 2 below) would act as an artificial mechanical white cell, seeking out and digesting unwanted pathogens including bacteria, viruses, or fungi in the bloodstream. A patient with a bloodborne infection might be injected with a dose of about 100 billion microbivores (about 1 cc). When a targeted bacterium bumps into a microbivore, the microbe sticks to the nanorobot’s surface like a fly caught on flypaper. Telescoping grapples emerge from the microbivore’s hull and transport the pathogen toward the front of the device, bucket-brigade style, and into the microbivore’s “mouth.” Once inside, the microbe is minced and digested into amino acids, mononucleotides, simple fatty acids and sugars. These basic molecules are then harmlessly discharged back into the bloodstream through an exhaust port at the rear of the device. The whole digestion cycle takes only 30 seconds. A complete treatment might take minutes or hours, far faster than the days or weeks often needed for antibiotics to work. When the nanorobotic treatment is finished, the doctor uses an ultrasound signal to tell the circulating microbivores that their work is done. The nanorobots then exit the body through the kidneys and are excreted with the urine in due course. Related nanorobots could be programmed to quickly recognize and digest even the tiniest aggregates of early cancer cells.
 |
 |
 |
| Figure 1. Nanoscale planetary gear. | Figure 2. Microbivore. Designer Robert A. Freitas Jr., additional design Forrest Bishop. | Figure 3. Chromallocytes. |
 |
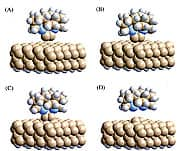 |
| Figure 4. IBM logo spelled out in atoms. | Figure 5. Mechanosynthetic tooltip deposits carbon atoms on diamond surface. |
Replacing Worn-Out or Damaged Cells
Medical nanorobots could also be used to perform surgery on individual cells. In one procedure, a nanorobot called a “chromallocyte” (Figure 3 above), controlled by a physician, would extract all existing chromosomes from a diseased cell and insert fresh new ones in their place. This process is called chromosome replacement therapy. The replacement chromosomes are manufactured earlier, outside of the patient’s body, using a desktop nanofactory that includes a molecular assembly line. The patient’s own individual genome serves as the blueprint to fabricate the new genetic material. Each chromallocyte is loaded with a single copy of a digitally corrected chromosome set. After injection, each device travels to its target tissue cell, enters the nucleus, replaces old worn-out genes with new chromosome copies, then exits the cell and is removed from the body. If the patient chooses, inherited defective genes could be replaced with non-defective base-pair sequences, permanently curing any genetic disease and even permitting cancerous cells to be reprogrammed to a healthy state. Perhaps most importantly, chromosome replacement therapy could correct the accumulating genetic damage and mutations that lead to aging in every one of our cells.
Building an Exciting Future
Right now, medical nanorobots are just theory. To actually build them, we need to create a new technology called molecular manufacturing. Molecular manufacturing is the production of complex atomically precise structures using positionally controlled fabrication and assembly of nanoparts inside a nanofactory. The first experimental proof that individual atoms could be manipulated was obtained by IBM scientists back in 1989 when they used a scanning tunneling microscope to precisely position 35 xenon atoms on a nickel surface to spell out the corporate logo “IBM” (Figure 4 above). Similarly, inside the nanofactory simple feedstock molecules such as methane (natural gas), propane, or acetylene will be manipulated by tiny probe tips to build atomically precise structures such as the nanoscale gear shown in (Figure 1 above).
Here’s how it will work. A nanoscale tool with a chemically reactive tip is brought into physical contact with a workpiece. The tip mechanically forces a chemical bond to form at a specific place between existing atoms on the workpiece and one or more feedstock atoms that are temporarily bound to the tool. Withdrawing the tool mechanically breaks the bond between feedstock and tool, leaving the feedstock atoms on the workpiece, a process called mechanosynthesis (Figure 5 above). The tool is then recharged with fresh feedstock and is ready to go again. Such a tool is conceptually similar to the more familiar case of a magnetized screwdriver that holds a screw. After the screw is rotated into a hole on a workpiece by turning the screwdriver, withdrawing the screwdriver leaves the screw in the hole because it is held there more tightly than its relatively weak magnetic attraction to the screwdriver tip.
With my scientific colleagues, we have done extensive analysis and very sophisticated quantum chemistry computer simulations (e.g., Figure 5 above) of a large number of potential tooltips and reaction sequences. We recently published the first description of a complete set of tools and positionally controlled reactions that should enable us to build small bits of perfect diamond crystal. Future extensions of these tools and reactions should let us move on to more complex nanoscale diamondoid objects such as the nanoscale gear (shown in Figure 1 above). In 2005, I published the first practical proposal for building a mechanosynthetic tooltip that was the subject of the first mechanosynthesis patent ever filed. With a colleague in 2008, I submitted the second mechanosynthesis patent ever filed, describing additional techniques for building more tooltips.
Several years ago, Ralph Merkle and I founded the Nanofactory Collaboration to coordinate a combined experimental and theoretical R&D program to design and build the first working diamondoid nanofactory. This long-term effort must start by developing the initial technology of positionally controlled mechanosynthesis of diamondoid structures using engineered tooltips and simple molecular feedstock. Our Collaboration has led to continuing efforts involving direct collaborations among 23 researchers and others, including 17 PhDs or PhD candidates at nine organizations in four countries – the US, UK, Russia, and Belgium. A dozen peer-reviewed papers are published or in progress as of 2008.
What You Need to Know: Life Extension and Medical Nanorobotics |
| • Nanotechnology is the engineering of molecularly precise structures and, ultimately, molecular machines. • Nanomedicine is the application of nanotechnology to medicine. The ultimate tool of nanomedicine is the medical nanorobot—a robot the size of a bacterium, composed of molecule-size parts. • Medical nanorobotics holds the greatest promise for curing disease and extending health span. • Current developments in nanomedicine will ultimately lead to the design and manufacture of medical nanorobots for life extension, possibly by the 2020s. |
But now it’s time to put our theories to the test. After working closely for three years with Philip Moriarty, one of the leading scanning probe microscopists in the UK, our international colleague is now undertaking direct experiments to build and validate several of our proposed mechanosynthesis tooltips in his laboratory. We are also preparing a research program proposal of our own to solicit additional funding from various US public or private sources to support further mechanosynthesis-related experimental and theory work on a greatly accelerated schedule. We expect these efforts will ultimately lead to the design and manufacture of medical nanorobots for life extension, possibly during the 2020s.
We are grateful to the Life Extension Foundation for contributing monies to help fund our research during the embryonic stages of this project’s development. © 2008 Robert A. Freitas, Jr. All Rights Reserved.
If you have any questions on the scientific content of this article, please call a Life Extension Wellness Specialist at 1-800-226-2370.
Useful Websites
-
Personal website of Robert Freitas: http://www.rfreitas.com.
-
Nanomedicine website: http://www.nanomedicine.com.
-
Nanofactory Collaboration website: http://www.molecularassembler.com/ Nanofactory/.
-
Nanomedicine Art Gallery: https://foresight.org/gallery-a-joyride-through-the-nanoscale-image-1-new-scientist/.
The Life Extension Foundation has contributed funding to support the work of Robert Freitas.
Robert Freitas is Senior Research
|
Fellow at the Institute for Molecular Manufacturing (IMM) in Palo Alto, California, and was a research scientist at Zyvex Corporation (Richardson, Texas), the first molecular nanotechnology company, during 2000-2004. He received BS degrees in Physics and Psychology from Harvey Mudd College in 1974 and a JD degree from University of Santa Clara in 1979. Freitas co-edited the 1980 NASA feasibility analysis of self-replicating space factories and in 1996, authored the first detailed technical design study of a medical nanorobot ever published in a peer-reviewed mainstream biomedical journal. His research interests include: nanomedicine, medical nanorobotics design, molecular machine systems, diamondoid mechanosynthesis (theory and experimental pathways), molecular assemblers and nanofactories, and self-replication in machine and factory systems. He has published 35 refereed journal publications, contributed book chapters, and co-founded the Nanofactory Collaboration. His home page is www.rfreitas.com.
(Figure 6 below). Robert Freitas is the author of Nanomedicine, the first book-length technical discussion of the potential medical applications of molecular nanotechnology and medical nanorobotics; the first two volumes of this four-volume series were published in 1999 and 2003 by Landes Bioscience. He has also co-authored Kinematic Self-Replicating Machines (Landes Bioscience, 2004)
 |
 |
 |
| Figure 6: Nanomedicine Volume 1 : Basic Capabilities | Nanomedicine Volume 2A : Biocompatibility | Kinematic Self-Replicating Machines |
1. First book on nanomedicine ever published: Freitas RA Jr. Nanomedicine, Volume I:
Basic Capabilities. Georgetown, TX: Landes Bioscience; 1999. Also available at: http://www.nanomedicine.comNMI.htm.
Accessed October 15, 2008.
2. Freitas RA Jr. Nanomedicine, Volume IIA: Biocompatibility. Georgetown, TX: Landes Bioscience; 2003. Also available at:
http://www.nanomedicine.comNMIIA.htm. Accessed October 15, 2008.
3. First medical nanorobot design paper ever published: Freitas RA Jr. Exploratory Design in Medical Nanotechnology:
A Mechanical Artificial Red Cell. Artif Cells Blood Substit Immobil Biotechnol. 1998;26:411-30. Also available at:
http://www.foresight.org/Nanomedicine/ Respirocytes.html. Accessed October 15, 2008.
4. Published design paper on the microbivores: Freitas RA Jr. Microbivores: Artificial Mechanical Phagocytes using Digest and
Discharge Protocol. J Evol Technol. 2005 April;14:55-106. Also available at:
http://www.jetpress.org/volume14/freitas.pdf. Accessed October 15, 2008.
5. First technical description of a cell repair nanorobot ever published: Freitas RA Jr. The ideal gene delivery vector: chromallocytes,
cell repair nanorobots for chromosome replacement therapy. J Evol Technol. 2007 June;16:1-97.
Also available at: http://jetpress.org/v16/freitas.pdf. Accessed October 15, 2008.
6. Survey book on self-replication: Freitas RA Jr, Merkle RC. Kinematic Self-Replicating Machines.
Georgetown, TX: Landes Bioscience; 2004. Also available at: http://www.MolecularAssembler.com/KSRM.htm.
Accessed October 15, 2008.
7. Freitas RA Jr. Say Ah! The Sciences. 2000 July/August;40:26-31. Also available at:
http://www.foresight.org/Nanomedicine/ SayAh/index.html. Accessed October 15, 2008.
8. Freitas RA Jr. Death is an Outrage! Invited Lecture delivered at the Fifth Alcor Conference on Extreme Life Extension,
November 16, 2002, Newport Beach, CA. Also available at: http://www.rfreitas.comNano/ DeathIsAnOutrage.htm.
Accessed October 15, 2008.
9. Freitas RA Jr. Nanomedicine. KurzweilAI.net. 2003 November 17. Also available at: http://www.kurzweilai.net/meme/frame.
html?main=/articles/art0602.html. Accessed October 15, 2008.

