Life Extension Magazine®
Rapid advances in the field of stem cell therapeutics are bringing us closer to the elusive goal of harnessing the “immortal flame” that survives in all of us. Indeed, it has become clear that, in the words of internationally known gerontology researcher Dr. Michael West, “You and I are made from cells that have no dead ancestors.” Life Extension interviewed Dr. West recently, to learn about his paradigm-shattering work, which has led to new techniques related to induced pluripotent stem cells (iPS) and human embryonic progenitor cells (hEP), both of which allow researchers to leap-frog over many previously existing barriers. Shortly after our interview, Harvard researchers announced that they had used the iPS technique to produce a “robust new collection of disease-specific stem cell lines” that hold immediate hope for advances in a host of diseases including Parkinson’s disease, type 1 diabetes, Huntington’s disease, Down’s syndrome, a form of combined immunodeficiency (“bubble boy disease”), two forms of muscular dystrophy, and others.1 Based on our discussion with Dr. West, combined with the recent news from the Harvard researchers, it appears that we are truly poised at the threshold of an incredible new era in which scientists can unlock the inner workings of our cells and “mass produce” virtually any cell or tissue type in the human body in a youthful state. The implications of these advances are enormous. They include readily accessible tissue and organ transplantation, the obliteration of genetic disorders, the end of chronic age-related diseases, and perhaps even physical immortality itself. Some Background“We’re seeing a congruence between some of mankind’s oldest aspirations and where modern science is heading,” West begins. “From ancient times, we’ve recognized that, although we as individuals age and die, the species does not. Amazing as it sounds, each of us is made of cells that have been proliferating since the dawn of life on Earth. And that, really, was the beginning of what we today seek to achieve through gerontology and regenerative medicine.” The lineage of cells produced by the reproductive system has been known as “germ-line” cells since the work of 19th century German scientist August Weismann. West continues, “Weismann recognized that germ-line cells represent a lineage of cells that has continued unbroken since life began—these cells obviously never aged or you and I would not be here.” Weismann was interested in this fundamental question: why is it that the non-germ-line cells in our bodies (the somatic cells) inevitably age and die on cue. As West asks, “Why are cells in our skin, our blood, our bones, and even our brains so reproducibly programmed to age in such a short period of time, while germ-line cells have been proliferating for billions of years?” Intrigued, Weismann made an astonishing prediction in 1881, speculating that the reason individuals age is that somatic cells (what we are mostly made of) have somehow lost the capacity (still inherent in primitive single-celled creatures) for proliferating without limit. Restore that capability, Weismann claimed, and one would restore the cellular immortality still present in our germ-line cells. A series of elegant and now classic experiments by the biologist Leonard Hayflick in the 1950s showed, in fact, that there is a limit to how many times human somatic cells can reproduce in vitro before they simply stop. Hayflick had discovered the existence of what amounts to a cellular “clock” that appears to run down after a relatively short number of generations of cell replication. Telomeres: Cellular FusesWhat is the actual physical nature of the mysterious “cellular clock” that limits the life span of human somatic cells?
At the time when Dr. West began his scientific career, he became convinced that this intrinsic clock of cellular aging was somehow connected with the chromosomes. He became intrigued by a little-known theory proposed in the early 1970s by Russian scientist Alexy Olovnikov.2,3 “Olovnikov’s idea was that repeating DNA sequences at the ends of each chromosome were like a burning fuse, which acted as a clock because every time the cell replicated, the segment (called a telomere) became shorter and shorter. When it ‘ran out,’ the cellular machinery signaled a fatal error in copying the vital genetic material, which would trigger the senescence of the somatic cell.” The full-length repeating telomere sequence was maintained in germ-line cells, Olovnikov suggested, by an “immortalizing enzyme” that continuously spun fresh telomere DNA, allowing germ-line cells to reproduce forever.4 By 1986, West had read the work of biologist Howard Cook who found that telomeres shortened with each generation in white blood cells (which are somatic cells and have finite life spans), but maintained their length in germ-line cells like sperm. Seeing these puzzle pieces fall into place was so convincing that West took a leave of absence from medical school to court venture capitalists, eventually starting a company called Geron to build on what West still refers to as the “telomere gamble.” “Forty million dollars later,” West recalls, “the gamble paid off.” West’s group had in fact produced Olovnikov’s mysterious enzyme, now known as telomerase, because of its ability to continuously spin out the vital strands of telomere DNA that keep germ cells immortal. Immortalized Cells: Can They Lengthen Individual Life Spans?But would it work? Could Dr. West actually convert human somatic cells, destined to expire after a few score doublings in tissue culture, into immortal versions of themselves that could continue to grow normally? In a fitting tribute, West chose to collect actual skin cells from pioneer Leonard Hayflick and insert the telomerase gene into them. In a notebook headed “The Immortalization of Dr. Hayflick,” Dr. West recorded the astonishing findings: Hayflick’s untreated skin cells began to die off after about two months in culture, exactly as expected, but cells that had been infused with the telomerase gene continued to thrive in identical culture conditions! These “telomerized” human somatic cells had demonstrated scientists’ ability to “rewind” the clock of cellular aging.5 While the immortalization of cells was a giant leap forward, says Dr. West, it wasn’t clear what role the telomere and the telomerase enzyme played in aging at the level of a human individual. There were several intriguing directions to go in at this point. It’s now known, for example, that telomere length is related to the risk of many chronic diseases.6-12 What about simply finding a way to restore the telomerase gene to every somatic cell in the body, lengthening all the telomeres together—wouldn’t that result in a body with an essentially limitless supply of cells? “That’s exactly how I was thinking back in the mid-90s,” West responds. “Why not just package the telomerase gene up in a virus? (Viruses provide a common laboratory method of introducing specific genes into cells.) Although gene therapy hadn’t come of age at that point, it seemed that if we took that approach and succeeded, it could have a profound impact on aging. Embryonic Stem Cells and Cloning
But gene transfer using viruses was a tricky business fraught with pitfalls, especially in the 1990s. In a dramatic conceptual leap, Dr. West suggested an alternative: why not go all the way back to embryonic stem cells, which are formed immediately after a sperm fertilizes an egg? For the first several cell division cycles, all cells are identical, and capable of differentiating into every cell type in the body (in fact, so-called “identical twins” are the result of a two-cell embryo splitting apart, producing an entirely new individual from each primitive embryonic stem cell). “If we could immortalize embryonic stem cells,” West says, “we could potentially repair the human heart, for example, after a heart attack, making young heart muscle by injecting these primitive cells into the heart to regenerate damaged tissue.” That was an astonishingly prescient vision because we’re already seeing clinical trials using this technique (cardiomyoplasty) in humans.13 In 1998, West reasoned that if an aged somatic cell were returned to the germ line, such as is used in cloning, it might be possible to not only reprogram say a skin cell into embryonic stem cells, but also reset the clock of aging in the aged cell. In 1999, the team that cloned Dolly the sheep had reported that cloning did not in fact do this, that is, they reported that Dolly was born old because the egg cell could not reset telomere length.14 However, Dr. West’s group later demonstrated that the Dolly team had it wrong, and that cloning could reset the clock of aging in animal cells after all.15 This suggested that there may actually be a way to use germ-line cells like the egg cell as a cellular time machine, resetting the clock of aging in human cells, turning a skin cell into the primitive embryonic stem cells we were originally developed from and creating young cells and tissues of any kind to repair the ravages of aging. Of course, cloning and the use of human embryonic stem cells are highly controversial, to say the least. The situation has been made worse by extreme political posturing on the one hand,16,17 and instances of overt scientific fraud on the other.18-21 These procedures are also technically challenging.
Beyond Cloning: At the Threshold of Modern Regenerative Medicine
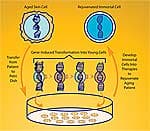
“But the really good news,” Dr. West says with obvious excitement, “is that a team at Kyoto University in Japan has now shown that it’s possible to take some molecules, called transcription factors, out of the egg cell, and use them to act just like the egg cell itself in transforming an ordinary somatic cell, say from the skin, and taking it back in time to become an embryonic stem cell!” No need for cloning and then deriving stem cells from the cloned embryo—or any of the challenging and controversial techniques that have preoccupied the media for the past decade. “The new cells are called induced pluripotent stem cells (iPS),” West explains. “They are equivalent in function to embryonic stem cells, but they don’t come from an embryo—hence the name change. The idea is simple: take a skin cell, culture it in a dish, add in three or four of these transcription factors, and, over a couple of weeks, the skin cells are transformed back into colonies of stem cells that act exactly like embryonic stem cells.22-24 It’s just spectacular, really.” “And since numerous papers on iPS have now shown switching on the telomerase gene in these cells,” continues Dr. West, “I believe that within the next 12 months, the scientific community will have documented, for the first time ever, the reversal of aging of a human cell.” |
|||||||||
iPS: From Vision to RealityDr. West’s exciting work at publicly traded BioTime, Inc. is aimed at making that vision a reality on the fast track. West’s team is working on a “reprogramming matrix.” BioTime recently licensed patents Dr. West had filed years before the publication of papers on human iPS cells that cover the use of the iPS genes in turning back the clock of human aging. “The goal is to use this material to permanently re-engineer old cells (say from a 100-year-old person), and bring them back to cells indistinguishable from those they were born with 100 years earlier. “We’re faced with a ‘tsunami’ of aging,’” West says, “and with the age wave of the baby boom generation, the timing is right for us as a nation to utilize these technologies to offer lower-cost therapies for crippling diseases like arthritis, for example. If we can rebuild the cartilage in your joints so you can walk to the store, the cost savings alone to this country would be just enormous. Many people have no idea that this emerging field of regenerative medicine was born of a desire to find a means to regenerate tissue function in the aged human and directly address this enormous human need. “Now that we can turn back the arrow of time on human cells, the next question is how to learn how to make the cell types we need in a purified form and industrial scale for a host of age-related diseases. The good news here is that we’ve made some remarkable progress already.” The challenge Dr. West refers to is that scientists still don’t understand just how stem cells “figure out” what kind of tissue they are destined to turn into. During natural embryonic development, there are specific genetic codes that seem to help cells “recognize” their environment, so that a cell developing at a location past the elbow joint turns into part of a wrist, a hand, or a finger—and never into an upper arm or a shoulder.25-27 “In some primitive animals,” West points out, “an arm amputated above the elbow regenerates with a normal elbow and everything beyond it—but if the amputation is below the elbow, the limb grows back without producing a second elbow.” So today’s stem cell researchers must “decode” the cells’ intricate and complex position-finding mechanism—a daunting task considering the thousands of cell types and subtypes in the body, which not only have to form properly, but then must work together correctly. Or is that arduous decoding actually necessary? According to West, absolutely not. Using a bold new approach, West’s lab simply exposes stem cells to a “shotgun” mix of conditions, and then uses modern genetic techniques to figure out what they’ve produced. “Instead of looking up at the apple tree and saying, ‘we want to pick that particular apple right there,’ West says, “we just lay out a tarp on the ground and shake the tree. It’s a random approach, but it is turning out to be very powerful—we produced 140 different cell types in just our first experiment alone.” Each of those 140 types is known as an embryonic progenitor cell—more advanced than a stem cell, but still capable of developing into many cells of a particular tissue type—say cells in the musculoskeletal system, or blood cells, or even nervous system tissue.28 These cells may have the capacity to “recognize” their environment when injected into injured or aged tissue, and proceed to develop into appropriate cell types and even organize themselves into functioning tissue based on the environment in which they find themselves. “The bottom line,” Dr. West says, “is that for the first time, medicine has an all-powerful stem cell to make everything in the human body, and young cells of any kind can be generated that are genetic matches to the patient who needs them. The hope—I would even say the anticipation—is that we’ll be able to fulfill the vision of regenerative medicine, which is to make cells for an old person just like those they were born from decades earlier. They can be used to regenerate at least some aspects of the human body’s function, whether it be the heart or the hair cell of the inner ear to restore hearing, and so on.” The Bright Future of Stem Cell TherapyIn wrapping up, we ask Dr. West to speculate cautiously which of the major age-related diseases might be addressed with the new technology within the lifetime of people reading this article. “First I think it’s important to understand that we’re very early in the dawn of this phase of medicine. The Bush administration’s highly ill-advised restrictions on funding in this area continue to hamper progress, though California has stepped up to the plate with three billion dollars to advance the field. But with those caveats, I’m still terribly excited about how we can potentially affect some of the largest aspects of aging.” “There’s an old adage in gerontology,” West continues, “that ‘you’re only as old as your arteries.” In fact, children afflicted with catastrophic premature aging diseases such as progeria and Werner’s syndrome have the identical, short-telomere-induced cardiovascular changes as people literally 10 times their age.29 “We can actually see vascular endothelial cells getting old by measuring their telomeres—the cells “know” in a sense that there’s something terribly wrong, and then send out signals that arrest normal blood flow, accelerate clotting, and produce inflammatory signals that cause immune cells to invade the vessel wall—that’s what produces cardiovascular disease.” “Well, could we fix that? At my previous company, we showed that it is possible to make young circulating precursors that can become either blood vessel cells or actual blood cells. We could actually label those cells and watch them traffic through the blood and see them land in damaged vessels and patch the damage. So we believe that if you could infuse the aged human with these cells, we could not only give older people a younger immune system, but we could potentially repair their vasculature as well.” In fact, human studies now bear out these cells’ potential for stimulating vessel growth in ischemic tissues.30,31
So is the implication that one can not only stop further aging with this technology, but perhaps actually reverse some existing changes? “There are animal data to suggest that,” Dr. West responds. In fact, a quick search of the medical literature for 2008 alone reveals the following astonishing discoveries: iPS cells from patients with the crippling paralytic disease, amyotrophic lateral sclerosis (ALS, or Lou Gehrig’s disease), can be directed to develop into functioning motor nerve cells;32 injection of stem cells into humans has already been shown to be safe and effective at slowing ALS disease progression.33 Laboratory and animal studies have shown that musculoskeletal stem cells can be directed to develop into functional bone and cartilage cells with physical characteristics similar to young healthy bones and joints.34-37 Neural-derived stem cells have improved status of mice with Huntington’s disease, promoted recovery in rats with ischemic stroke, and are being explored as potential therapy for patients with brain tumors.38-40 Summary
“We are always accused of hyping all this,” Dr. West says wryly as we end our conversation. “I believe that with time, people will begin to understand the basis of our excitement. The problem is that so many people still believe that aging is inevitable—what they don’t understand is the immortality of the species. I know this defies common wisdom, but common wisdom is perhaps wrong: the reality is that we’re born from cells that have been proliferating since the dawn of life on Earth. That’s just the way it is.” “As scientists, we’ve got this duty to find ways to clarify truth and dispel myth. We have to explain that aside from the innumerable circus shows, snake oil salesmen, and all that, there’s really serious gerontology at work. Molecular biology today is able to do experiments a thousand times faster and better than it could even 10 years ago, and it’s leading to some really dramatic breakthroughs. Our tools are just so powerful these days. I conceive of the day when we can engineer embryonic stem cells at a molecular level and create new kinds of cells that never existed even in nature.” Dr. West readily acknowledges that it’s impossible to tell just how far this technology can take us. But with more than 6,000 studies on stem cells published in 2008 alone, it’s clear we are privileged witnesses to a genuine scientific revolution. Will BioTime succeed in reversing the aging of human cells? Must we continue to succumb to aging-related disease and death? Or can we harness the power of our undying cell lineages to achieve a taste of immortality? Only time will tell.
In the meantime, the Life Extension Foundation continues to contribute funding to Dr. West’s pioneering research.
Dr. West is the Chief Executive Officer of BioTime, Inc. and Embryome Sciences, Inc. of Emeryville, California and Adjunct Professor of Bioengineering at the University of California, Berkeley. You can contact Dr. West at mwest@biotimemail.com. If you have any questions on the scientific content of this article, please call a Life Extension Wellness Specialist at 1-800-226-2370. | |||||||
| References | |||||||
1. Park IH, Arora N, Huo H, et al. Disease-specific induced pluripotent stem cells. Cell. 2008 Aug 6. 2. Olovnikov AM. Telomeres, telomerase, and aging: origin of the theory. Exp Gerontol. 1996 Jul;31(4):443-8. 3. Olovnikov AM. Aging is a result of a shortening of the “differotene” in the telomere due to end under-replication and under-repair of DNA. Izv Akad Nauk SSSR Biol. 1992 Jul;(4):641-3. 4. Olovnikov AM. Hypothesis: life span is regulated by chronomere DNA of the hypothalamus. J Alzheimers Dis. 2007 May;11(2):241-52. 5. West M. The Immortal Cell. New York: Doubleday; 2003. 6. Farzaneh-Far R, Cawthon RM, Na B, et al. Prognostic value of leukocyte telomere length in patients with stable coronary artery disease: data from the Heart and Soul Study. Arterioscler Thromb Vasc Biol. 2008 Jul;28(7):1379-84. 7. Han J, Qureshi AA, Prescott J, et al. A Prospective Study of Telomere Length and the Risk of Skin Cancer. J Invest Dermatol. 2008 Jul 31. 8. Huzen J, van Veldhuisen DJ, van Gilst WH, van der Harst P. Telomeres and biological ageing in cardiovascular disease. Ned Tijdschr Geneeskd. 2008 May 31;152(22):1265-70. 9. Kimura M, Hjelmborg JV, Gardner JP, et al. Telomere length and mortality: a study of leukocytes in elderly Danish twins. Am J Epidemiol. 2008 Apr 1;167(7):799-806. 10. Richards JB, Valdes AM, Gardner JP, et al. Homocysteine levels and leukocyte telomere length. Atherosclerosis. 2008 Feb 14. 11. Yu WY, Chang HW, Lin CH, Cho CL. Short telomeres in patients with chronic schizophrenia who show a poor response to treatment. J Psychiatry Neurosci. 2008 May;33(3):244-7. 12. Zhang J, Kong Q, Zhang Z, et al. Telomere dysfunction of lymphocytes in patients with Alzheimer disease. Cogn Behav Neurol. 2003 Sep;16(3):170-6. 13. Schuldt AJ, Rosen MR, Gaudette GR, Cohen IS. Repairing damaged myocardium: evaluating cells used for cardiac regeneration. Curr Treat Options Cardiovasc Med. 2008 Feb;10(1):59-72. 14. Shiels PG, Kind AJ, Campbell KH, et al. Analysis of telomere lengths in cloned sheep. Nature. 1999 May 27;399(6734):316-7. 15. Lanza RP, Cibelli JB, Blackwell C, et al. Extension of cell life-span and telomere length in animals cloned from senescent somatic cells. Science. 2000 Apr 28;288(5466):665-9. 16. Doerflinger RM. The problem of deception in embryonic stem cell research. Cell Prolif. 2008 Feb;41 Suppl 165-70. 17. Skene L. Human cloning and stem cell research: engaging in the political process. (Legislation review: prohibition of Human Cloning Act 2002 and the research involving Human Embryos Act). Med Law. 2008 Mar;27(1):119-30. 18. Saunders R, Savulescu J. Research ethics and lessons from Hwanggate: what can we learn from the Korean cloning fraud? J Med Ethics. 2008 Mar;34(3):214-21. 19. Rusnak AJ, Chudley AE. Stem cell research: cloning, therapy and scientific fraud. Clin Genet. 2006 Oct;70(4):302-5. 20. Disgraced Korean cloning scientist indicted. NY Times (Print). 2006;A12. 21. Wohn DY, Normile D. Korean cloning scandal. Prosecutors allege elaborate deception and missing funds. Science. 2006 May 19;312(5776):980-1. 22. Narazaki G, Uosaki H, Teranishi M, et al. Directed and systematic differentiation of cardiovascular cells from mouse induced pluripotent stem cells. Circulation. 2008 Jul 29;118(5):498-506. 23. Park IH, Lerou PH, Zhao R, Huo H, Daley GQ. Generation of human-induced pluripotent stem cells. Nat Protoc. 2008;3(7):1180-6. 24. Zhao R, Daley GQ. From fibroblasts to iPS cells: Induced pluripotency by defined factors. J Cell Biochem. 2008 Jul 30. 25. Degnan BM, Degnan SM, Giusti A, Morse DE. A hox/hom homeobox gene in sponges. Gene. 1995 Apr 3;155(2):175-7. 26. Thomas BL, Tucker AS, Ferguson C, et al. Molecular control of odontogenic patterning: positional dependent initiation and morphogenesis. Eur J Oral Sci. 1998 Jan;106 Suppl 1:44-7. 27. Yin Z, Frasch M. Regulation and function of tinman during dorsal mesoderm induction and heart specification in Drosophila. Dev Genet. 1998;22(3):187-200. 28. West MD, Sargent RG, Long J, et al. The ACTCellerate initiative: large-scale combinatorial cloning of novel human embryonic stem cell derivatives. Regen Med. 2008 May;3(3):287-308. 29. Erusalimsky JD, Kurz DJ. Endothelial cell senescence. Handb Exp Pharmacol. 2006;(176 Pt 2):213-48. 30. Cogle CR, Madlambayan GJ, Hubsher G, et al. Marrow cell therapies for cardiovascular diseases. Exp Hematol. 2008 Jun;36(6):687-94. 31. Ribatti D, Nico B, Crivellato E, Vacca A. Endothelial progenitor cells in health and disease. Histol Histopathol. 2005 Oct;20(4):1351-8. 32. Dimos JT, Rodolfa KT, Niakan KK, et al. Induced pluripotent stem cells generated from patients with ALS can be differentiated into motor neurons. Science. 2008 Aug 29;321(5893):1218-21. 33. Mazzini L, Mareschi K, Ferrero I, et al. Stem cell treatment in Amyotrophic Lateral Sclerosis. J Neurol Sci. 2008 Feb 15;265(1-2):78-83. 34. Ando W, Tateishi K, Katakai D, et al. In Vitro Generation of a Scaffold-Free Tissue-Engineered Construct (TEC) Derived from Human Synovial Mesenchymal Stem Cells: Biological and Mechanical Properties, and Further Chondrogenic Potential. Tissue Eng Part A. 2008 Jul 17. 35. Chang CH, Lin HY, Fang HW, et al. Chondrogenesis from immortalized human mesenchymal stem cells: comparison between collagen gel and pellet culture methods. Artif Organs. 2008 Jul;32(7):561-6. 36. Pei M, He F, Vunjak-Novakovic G. Synovium-derived stem cell-based chondrogenesis. Differentiation. 2008 Jul 2. 37. Yamaza T, Miura Y, Bi Y, et al. Pharmacologic stem cell based intervention as a new approach to osteoporosis treatment in rodents. PLoS ONE. 2008;3(7):e2615. 38. Yang CR, Yu RK. Intracerebral transplantation of neural stem cells combined with trehalose ingestion alleviates pathology in a mouse model of Huntington’s disease. J Neurosci Res. 2008 Aug 5. 39. Yip S, Shah K. Stem-cell based therapies for brain tumors. Curr Opin Mol Ther. 2008 Aug;10(4):334-42. 40. Zhao Y, Xie P, Zhu XF, Cai ZY. Neural stem cell transplantation and nerve growth factor promote neurological recovery in rats with ischemic stroke. Nan Fang Yi Ke Da Xue Xue Bao. 2008 Jul;28(7):1123-6. |