Life Extension Magazine®
Americans with life-threatening illnesses are denied access to promising therapies, but the federal government does nothing to prohibit high-risk activities that result in people being killed for no worthwhile purpose. In early December 2006, the number one news topic related to three mountaineers who were lost while attempting to summit Mount Hood in Oregon despite dangerous weather conditions. Huge amounts of public resources were expended in an attempt to rescue the climbers, but to no avail: one was found dead, while the other two are still missing.1 During the very same week in December 2006, about 600 American men with advanced prostate cancer were on the verge of death.2 The federal government prohibited a drug from reaching these prostate cancer victims, even though this drug had demonstrated efficacy in a carefully controlled clinical study. In fact, from the time of the release of the data on this drug in 2002, tens of thousands of American prostate cancer patients have died in the FDA’s waiting room. The government argues that advanced prostate cancer patients should not risk taking a drug that could cause side effects, even though all these patients face imminent death. Parachuting to Death off a BridgeThe third Saturday of October in Fayette County, West Virginia is called “Bridge Day.” The highlight of the event involves hundreds of people parachuting off a high bridge. While a handful of minor injuries occur each year, last October’s “Bridge Day” had one person die when his parachute did not open. This is the fourth fatality since this local tradition started.3 There is no talk about banning the bridge jumps, but the federal government holds steadfast that promising drugs cannot be sold to terminally ill cancer patients until the FDA deems them as safe and effective. No government restrictions on mountain climbing, bridge jumping, or other risky activities that contribute nothing to society, but an armada of federal officers, prosecutors, and FDA investigators are ready to pounce on anyone who dares offer a cancer therapy before it receives official approval.
Drug Shown to Save Lives—But Not Approved!In mid-2004, I reported in this column about a randomized study involving men with late stage, metastatic prostate cancer who received either an immune-boosting vaccine called Provenge® or a placebo.4 Patients were randomized to receive three vaccinations of Provenge® or placebo over a four-week period. Analysis of the data revealed that the single most important predictor of response to Provenge® was a patient’s Gleason score, a measure of the aggressiveness of a patient’s tumor.5,6
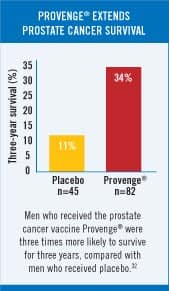
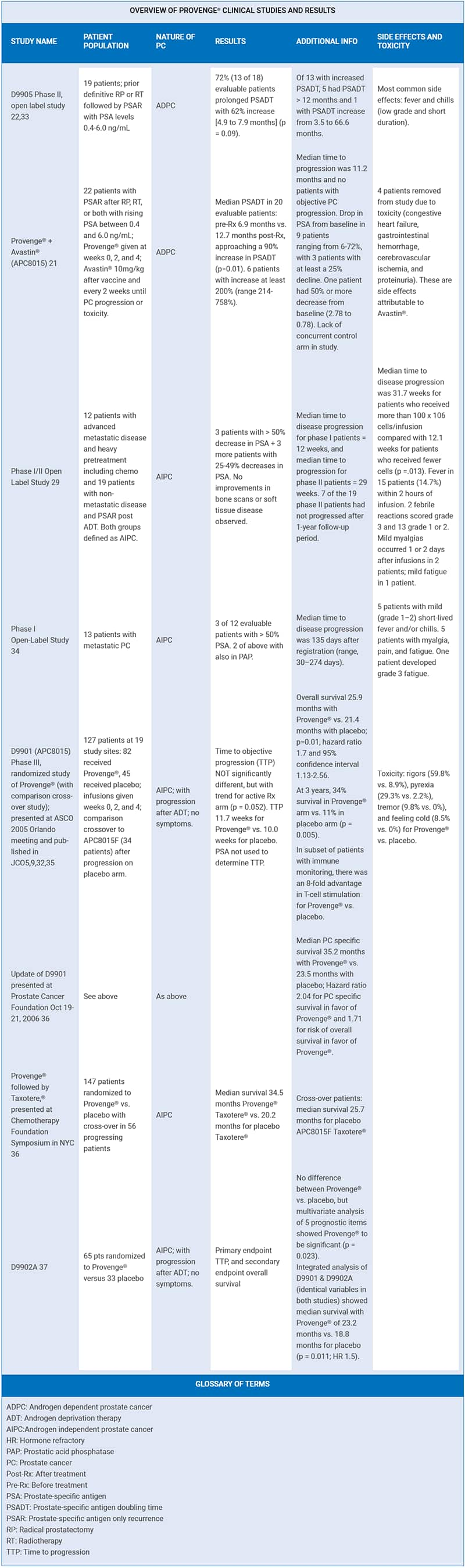
In patients with a Gleason score less than or equal to 7, the placebo group had an average time to cancer progression of 9.0 weeks, compared with 16.0 weeks for the Provenge® group. This finding was statistically significant with a p-value of 0.002 (indicating a two in 1000 probability of this occurring by chance alone). In addition, patients receiving Provenge® whose disease had not progressed six months after randomization had more than an eight-fold advantage in progression-free survival compared with those patients who received placebo (34.7% versus 4%). However, in the formal protocol of this government-approved study, the primary endpoint was the time to objective disease progression. Comparison of the Provenge®-treated group with the placebo group (using Kaplan-Meier survival curves) revealed a clinical benefit in the Provenge® treated patients (p-value=0.085) that approached but did not achieve the pre-specified primary endpoint of the study (p-value=0.05). Provenge® was rejected by the FDA because the agency does not accept a retrospective subgroup analysis to show benefit of an experimental drug. To gain FDA approval, the company who created Provenge® planned a new study on men with Gleason scores of 7 or less. However, the company continued to follow patients in the original study, and the results continue to be impressive. Of the 75 patients who entered the trial with a Gleason score of 7 or less, those receiving Provenge® were 3.7 times more likely to be alive after 30 months; this translates into 53% of the Provenge® group staying alive compared with only 14% of the placebo group. The Provenge® group also remained pain-free twice as long on average as the placebo group.7 A Wall Street Journal editorial commented on the FDA’s deplorable delay by stating: “We know that it works, and we know why it works. In any rational regulatory environment, that would be reason to speed Provenge® to market. But this is the FDA we are talking about.” 8 Fast forward to 2005, and the results of a new clinical study on Provenge® showed that three times as many advanced prostate cancer patients who received Provenge® were alive compared with patients receiving a placebo.9-11 This study evaluated patients with prostate cancer who had progressive disease during androgen-deprivation therapy and who were categorized as having androgen-independent prostate cancer. This patient subset has a highly adverse prognosis, with most dying of the disease within a few years. In the Provenge® study, 34% of the patients receiving Provenge® were still alive after three years, compared with only 11% of men who were randomly assigned a placebo. FDA Condemns Placebo Group to DeathUnder FDA regulations, end stage prostate cancer patients had to risk receiving no therapy (the placebo) in the hope that they might be lucky enough to be in the study arm that received the promising drug (Provenge®). Life Extension has long advocated that cancer patients with advanced disease should not have to risk receiving a worthless placebo. Historical controls could be used instead of placebos to spare such patients almost certain death. Prostate cancer kills more than 30,000 American men every year.2 Provenge® has clearly demonstrated that it improves survival rates, yet the FDA still has not approved it. Considering that the FDA could have approved Provenge® as an experimental therapy as early as 2002, the agency’s delay in approving this one drug alone may have resulted in the premature death of tens of thousands of men. FDA Advisory Panel Recommends Approval of Provenge®On March 30, 2007, an FDA advisory panel reviewing all data on Provenge® recommended by a 13-4 vote that the agency approve it.12-14 The approval of Provenge® would open a new front in the war on cancer, because its mode of action is different from that of existing chemotherapy drugs and radiation. Provenge® is a personalized therapy in which some of a patient’s white blood cells are removed, programmed outside the body to attack prostate cancer cells, and then infused back into the body three or four days later. FDA Ignores Its Own Advisory PanelThe clinical trials provided direct evidence that those who received Provenge® lived longer compared to control groups.9,15-29 According to the FDA, however, these “survival advantages” had “issues.” When these same “issues” were discussed in the Provenge® public meeting, the majority of the FDA Advisory Panel (in a 13-4 vote) thought that the issues, while relevant and important, were superseded by the solid immunology science behind the product. Despite irrefutable data showing Provenge® extends life span, FDA bureaucrats succumbed to intense cancer establishment lobbying, and they denied approval. The FDA now insists on more data, which could take several years. This leaves those with advanced prostate cancer without a treatment that has the possibility of extending their lives.
| |||||||||||||
How Provenge® Was DerailedProvenge® was sabotaged by a minority of the FDA Advisory Panel who voted against it. The campaign to discredit Provenge® by those in the cancer establishment was “unprecedented,” according to Mark Thornton, MD, a former medical officer in the FDA Office of Oncology Products and volunteer president of the Sarcoma Foundation of America. Dr. Thornton authored an editorial titled “Black Day at the FDA” that opened by stating: “Within an eight-hour period that day, the FDA succeeded in killing not one but two safe, promising therapies designed and developed to act by stimulating a patient’s immune system against cancer.” 30 —Wall Street Journal, May 14, 2007. The other drug Dr. Thornton described is called Junovan™, which was shown to extend the lives of children afflicted with osteosarcoma.30,31 Despite clear survival advantages, the FDA denied approval of Junovan™ the same black day it denied Provenge®. (We discuss Junovan™ in detail in an article that appears later in this issue of Life Extension magazine.) How Many Needless Deaths?It is difficult to calculate exactly how many American men perished because the federal government would not allow them to risk trying Provenge®, even though they faced probable death from their advanced prostate cancer. One way of measuring the number of human life years lost is to look at one of the Provenge® studies showing how many months of average life were added to Provenge® recipients compared with placebo. Based on this analysis, for each year Provenge® was delayed, 11,250 human life years have been lost. Since the delay has been almost five years, the total loss caused by this bureaucratic delay in approving this one drug amounts to a startling 56,000 years of human life. Even more appalling, the efficacy of Provenge® was shown in men who had already failed all conventional therapies. These advanced stages of cancer are difficult to treat, because the cancer cells have already developed survival mechanisms that make them extremely difficult to eradicate. The fact that Provenge® demonstrated such impressive survival benefits in men with these advanced forms of prostate cancer hints that it could be even more effective if administered in earlier stages of the disease—perhaps at the first sign of metastasis or in those men with highly adverse risk factors associated with short survival times. Life Extension versus Federal GovernmentFor the past three decades, Life Extension has sought to expose an insidious drug approval process that causes human beings to die, even though effective therapies to treat their diseases already exist. We have revealed many medications that the FDA dragged its feet in approving, resulting in a severe magnitude of needless suffering and death.4 There are no federal prohibitions against Americans engaging in all kinds of risky behaviors that provide no benefit to society or opportunity of extending human life. That the government pretends it has to protect citizens against drugs that it has not approved, yet has no restrictions against people engaging in dangerous activities, is somewhat hypocritical. What it all boils down to, however, is defining the term “risk.” We at Life Extension have long contended that any person with a serious illness should have the individual right to choose any therapy that they think may work. Therapies not FDA-approved should have a large warning label stating “not approved by the FDA for safety or effectiveness.” This would let each individual determine what “risk” they are willing to assume in order to be given a chance to continue enjoying the very basic right to life. The article in this issue titled “Lifesaving Cancer Drugs Not Approved by the FDA” reveals additional effective medications that are being denied to seriously ill Americans by our own government. On page 13 we describe the specific action that each one of you should take to encourage Congress to change the law, so that no American with a serious disease is ever again denied a promising therapy. For the benefit of yourself and your loved ones, please use the convenient system we have developed to contact your Congressional members about the critical need to amend the law, so that those with serious disease can take full advantage of the latest medical technologies. For longer life,
William Faloon
| |
| References | |
| 1. Available at: http://www.newwest.net/index.php/city/article/hard_lessons_learned_from_oregon_deaths/C509/L509/. Accessed April 7, 2007. 2. Available at: www.prostatecancer foundation.org/site/c.itIWK2OSG/b.97763/k.3BA8/PR_June_18_2004.htm. Accessed April 10, 2007. 3. Available at: www.fayettetribune.com/local/local_story_296115637html?keyword=topstory. Accessed April 6, 2007. 4. Available at: http://www.lifeextension.com/magazine/mag2004/jun2004_awsi_01.htm. Accessed April 7, 2007. 5. Available at: http://findarticles.com/p/articles/mi_m0EIN/is_2002_August_9/ai_90233563/pg_1. Accessed June 21, 2007. 6. Available at: http://investor.dendreon.com/ReleaseDetail.cfm?ReleaseID=146750&Header=News. Accessed April 7, 2007. 7. Available at: http://www.asco.org/portal/site/ASCO/enuitem.34d60f5624ba07fd506fe310ee37a01d/vgnextoid= 8. New cancer drugs. Wall Street Journal. January 26, 2004. 9. Small EJ, Schellhammer PF, Higano CS, et al. Placebo-controlled phase III trial of immunologic therapy with sipuleucel-T (APC8015) 10. Available at: www.investor.dendreon.com/ReleaseDetail.cfm?ReleaseID=152362&Header=News. Accessed April 7, 2007. 11. Available at: www.prnewswire.com/cgi-bin/stories.pl?ACCT=104&STORY=/www/story/10-31-2005/0004203756&EDATE=. Accessed May 21, 2007. 12. Available at: www.marketwatch.com/news/story/dendreon-shares-skyrocket-fda-panels/story.px?guid= 13. Available at: http://today.reuters.com/news/articleinvestingview=CN&WTmodLOC=C3-News-4&symbol=DNDN.O&storyID= 14. Available at: www.investor.dendreon.com/ReleaseDetail.cfm?ReleaseID=156146&Header=News. Accessed April 10, 2007. 15. Basler M, Groettrup M. Advances in prostate cancer immunotherapies. Drugs Aging. 2007;24(3):197-221. 16. Brand TC, Tolcher AW. Management of high risk metastatic prostate cancer: the case for novel therapies. J Urol. 2006 Dec;176(6 Pt 2):S76-S80. 17. So-Rosillo R, Small EJ. Sipuleucel-T (APC8015) for prostate cancer. Expert Rev Anticancer Ther. 2006 Sep;6(9):1163-7. 18. Lin AM, Hershberg RM, Small EJ. Immunotherapy for prostate cancer using prostatic acid phosphatase loaded antigen presenting cells. Urol Oncol. 2006 Sep;24(5):434-41. 19. Dawson NA. New molecular targets in advanced prostate cancer. Expert Rev Anticancer Ther. 2006 Jul;6(7):993-1002. 20. Anon. Sipuleucel-T: APC 8015, APC-8015, prostate cancer vaccine—Dendreon. Drugs RD. 2006;7(3):197-201. 21. Rini BI, Weinberg V, Fong L, et al. Combination immunotherapy with prostatic acid phosphatase pulsed antigen-presenting 22. Beinart G, Rini BI, Weinberg V, Small EJ. Antigen-presenting cells 8015 (Provenge) in patients with androgen-dependent, biochemically relapsed prostate cancer. 23. Kantoff P. Recent progress in management of advanced prostate cancer. Oncology (Williston.Park). 2005 Apr;19(5):631-6. 24. Schellhammer PF, Hershberg RM. Immunotherapy with autologous antigen presenting cells for the treatment of androgen independent prostate cancer. World J Urol. 2005 Feb;23(1):47-9. 25. Burch PA, Croghan GA, Gastineau DA, et al. Immunotherapy (APC8015, Provenge) targeting prostatic acid phosphatase can induce durable remission of 26. Eymard JC, Bernard J. Cell therapy and prostate cancer. Bull Cancer. 2003 Aug;90(8-9):734-43. 27. Rini BI. Technology evaluation: APC-8015, Dendreon. Curr Opin Mol Ther. 2002 Feb;4(1):76-9. 28. Valone FH, Small E, MacKenzie M, et al. Dendritic cell-based treatment of cancer: closing in on a cellular therapy. Cancer J. 2001 Nov;7 Suppl 2S53-S61. 29. Small EJ, Fratesi P, Reese DM, et al. Immunotherapy of hormone-refractory prostate cancer with antigen-loaded dendritic cells. J Clin Oncol. 2000 Dec 1;18(23):3894-903. 30. Available at: http://online.wsj.com/article/SB117911315709601659.html. Accessed June 21, 2007. 31. Available at: http://www.drugs.com/nda/junovan_070509.html. Accessed June 21, 2007. 32. Available at: www.hemonctoday.com/200503/frameset.asp?article=autologous.asp. Accessed June 25, 2007. 33. Available at: www.investor.dendreon.com/ReleaseDetail.cfm?ReleaseID=164087&Header=IR. Accessed June 22, 2007. 34. Burch PA, Breen JK, Buckner JC, et al. Priming tissue-specific cellular immunity in a phase I trial of autologous dendritic cells for prostate cancer. Clin Cancer Res. 2000 Jun;6(6):2175-82. 35. Available at: www.hemonctoday.com/scorecards/APC8015.asp. Accessed June 22, 2007. 36. Available at: http://professional.cancerconsultants.com/oncology_main_news.aspx?id=38681. Accessed June 22, 2007. 37. Higano C, Burch PA, Small EJ, et al. Immunotherapy (APC8015) for androgen independent prostate cancer (AIPC): final progression and survival data from a secon(+) Phase 111 trial. ECCO 13th European Conference, Oct 2005. |
Scientists have identified novel ways of treating cancer, but too little of this new technology is being used in clinical practice. When new discoveries are made, drug companies spend years seeking a patent, and then more years carrying it through the cumbersome bureaucratic approval process. A major reason so many cancer patients die today is an antiquated regulatory system that causes effective therapies to be delayed (or suppressed altogether). This system must be changed, if the 1,500 American cancer patients who perish each day are to have a realistic chance of being saved. Our long-standing proposal has been to change the law so that anyone can opt out of the FDA’s umbrella of “protection.” This approach will allow companies to sell drugs that have demonstrated safety and a reasonable likelihood of effectiveness, which are clearly labeled “Not Approved by the FDA”. Patients who wish can still use only FDA-approved drugs, while those willing to take a risk, in consultation with their doctors, will be allowed to try drugs shown to be safe that are still not approved. We believe that this initiative will result in a renaissance in the practice of medicine similar to the computer technology revolution of the past three decades. In this environment, many lethal diseases will succumb to cures that are less expensive than is presently the case. And greater competition will help eliminate the health care cost crisis that exists today. Today’s broken system results in terminally ill people learning of scientific discoveries that might well cure their disease, but are quickly advised by the newscaster that the therapy is years away from FDA approval. We think that seriously ill people, in consultation with their doctors, should be able to make up their own minds about what drugs they are willing to try. What Cancer Patients and Their Families Must DoThere are millions of cancer patients alive right now who face possible or probable death in the next twelve months. If you add their family members and friends, there are tens of millions of Americans who should be outraged by an outdated regulatory system that bans access to potentially life-saving therapies. The FDA continues to suppress innovative therapies because the public has failed to demand that our elected officials rein in the FDA’s arbitrary authority. The first step in changing today’s outmoded system is for those who understand the magnitude of this problem to communicate the urgent need for change to Congress. Those concerned about this serious issue should log on to www.lifeextension.com/lac to insist that their Representative and two Senators help enact legislation that will enable cancer patients to obtain therapies far enough along in the clinical trials process to be deemed safe, but not yet approved by the FDA. Those without computer access can photocopy the next page and mail it to their Representative at The US House of Representatives, Washington, DC 20515 and two Senators at The US Senate, Washington, DC 20510. We also ask that you phone your Congress-ional members at 1-202-224-3121 to let them know how disgusted you are that doctors and patients are not allowed to choose drugs that may be effective against an often fatal disease. The Honorable: I am writing to ask that you sponsor or co-sponsor legislation to enable cancer patients (and those with other serious diseases) to purchase medications while they are pending final approval by the FDA. This approach will allow companies to sell novel drugs with a label clearly stating that they are “Not Approved by the FDA”. Consumers who wish to rely on the FDA can limit their choices to fully approved drugs only, while those willing to take a risk (in consultation with their doctors) will be allowed to try what they choose. (Companies that make fraudulent claims for products can be prosecuted under the laws that exist today.) This initiative can result in a renaissance in the practice of medicine, similar to the computer technology revolution that has occurred over the past three decades. In this environment free of regulatory burden, many inexpensive cures will very likely be found for lethal diseases. And greater competition will help eliminate the health care cost crisis that exists today. I am tired about reading about medical breakthroughs, only to be told that I will have to wait years before the therapy might become available. As 1,500 Americans die of cancer each day, I consider the introduction and passage of such a law an extremely high priority. Seriously ill people have the fundamental right to make up their own minds about what drugs they are allowed to try, in consultation with their physicians. Please let me know that you will sponsor or co-sponsor such legislation, which will provide us with quicker access to drugs that the FDA has found safe and potentially effective, but have not yet received final approval. Sincerely, Name: Address: City: ST: Zip: |