Life Extension Magazine®
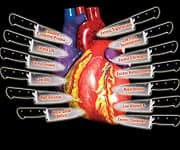
Cardiovascular disease causes 1 of every 2.7 deaths in the United States today.1 Unless a drastic change occurs in the way doctors treat vascular disorders, aging adults face even greater threats of heart disease and stroke. Mainstream doctors keep trying to find simple answers to protect against atherosclerotic disease. The problem is that scientists have identified at least 13 different factors that all conspire to destroy our aging arteries. The illustration below depicts 13 named daggers aimed at the heart. Any one of these daggers can trigger a cardiovascular event, yet conventional medicine pretends that guarding against only a few of these proven risk factors adequately prevents vascular disease. By failing to comprehend the underlying processes involved in circulatory breakdown, most doctors overlook documented methods of maintaining healthy blood flow in our maturing bodies. In my early career, I performed over 300 postmortem arterial dissections. In aged cadavers, I often found arteries that were so occluded that it was virtually impossible to insert a small catheter (tube) into them. My experience provided a vivid image of the jagged structural devastation inflicted by atherosclerosis. As you will read, many of today’s physicians seem to have forgotten what they should have observed in medical school. The result is a misconception of epidemic proportion that threatens the lives of tens of millions of aging Americans. Cardiologists Still Don’t Get ItMainstream medicine is fixated on the misconception that atherosclerosis is caused by excess levels of “lipids” in the blood. Lipids are usually defined as total cholesterol, LDL (low-density lipoprotein), and triglycerides, though other fatty substances contribute to the atherosclerotic process. What doctors fail to understand is that atherosclerosis begins when the inner arterial wall (the endothelium) sustains an injury.2 Endothelial injuries are caused by numerous and often unavoidable factors. As humans age (and engage in bad habits), the endothelium becomes progressively dysfunctional. The end results are the major structural changes clinically defined as “atherosclerosis.” People often analogize atherosclerosis as a “clogged pipe.” When a “clog” occurs in a coronary artery, the recommendation is either bypass surgery or a procedure in which a stent is inserted to keep the artery open. While these surgical procedures have become necessary for many people, the “clogged pipe” analogy is an inaccurate way to view the atherosclerosic process. The underlying reason why arteries become occluded in older people is the progressive deterioration of the endothelial lining (the inner arterial wall). If people do not take steps to correct the “endothelial dysfunction” occurring in their aging bodies, the consequence will be a worsening epidemic of arterial disease that currently kills 37% of all Americans and 30% of people worldwide.3
Daggers Aimed at Your Arterial WallIf one accepts the premise that age-related arterial disease is almost universally caused by “endothelial dysfunction,” then human studies in which a single agent is tested to prevent heart attack or stroke begin to look absurd, at least in those who seek to maintain healthy blood flow over the long term. High blood pressure,4-10 excess cholesterol-LDL-triglycerides,11-20 low HDL,21-23 cigarette smoking,24-32 diabetes,33-38 obesity,39-42 and lack of exercise43-49 all contribute to endothelial dysfunction and the subsequent development of atherosclerosis. Additional endothelium-damaging factors include high-normal levels of glucose,50-52 insulin,53,54 iron,55-57 homocysteine,58-84 and fibrinogen,85-98 low free testosterone (in men),99-102 and any level of C-reactive protein that is higher than optimal.103-125 Homocysteine causes initial injury to the endothelium, contributes to the production of pro-inflammatory cytokines, and facilitates the oxidation of the fat/LDL that accumulates beneath the damaged endothelium. Homocysteine also increases the expression of adhesion molecules and the abnormal accumulation of blood components around the atherosclerotic lesion. Fibrinogen is a clotting factor that accumulates at the site of the endothelial lesion, contributing to plaque buildup. Fibrinogen can also participate in the acute blockage (thrombosis) of an artery after unstable atherosclerotic plaque ruptures. Glucose at even high-normal levels may accelerate the glycation process that causes arterial stiffening, while high-normal fasting insulin inflicts direct damage to the endothelium and induces abnormal platelet aggregation. High levels of iron promote oxidation of LDL in the damaged endothelium, while low levels of testosterone appear to interfere with normal endothelial function. Low levels of vitamin K enable calcium to be deposited into atherosclerotic lesions, instead of into the bone where it belongs.126-129
C-reactive protein is an inflammatory byproduct that directly damages the endothelium. This not only creates initial injuries to the endothelium, but also accelerates the progression of existing atherosclerotic lesions. Nitric oxide is produced in the endothelium and regulates endothelial cell interactions, arterial wall blood flow, vascular tone, and platelet aggregation-adhesion. Abnormalities in nitric oxide production are a direct cause of endothelial dysfunction, which is the prelude to atherosclerosis (and often hypertension). The common feature of endothelial dysfunction is a decrease in the amount of nitric oxide.130 In response to overwhelming published data, health-conscious people are altering their diets, taking drugs, hormones, and dietary supplements, and even trying to exercise in order to reduce all of these known atherosclerosis risk factors. Despite these efforts, protecting against these risk factors until now has been only a partial solution. One problem is that age itself is a major risk factor for endothelial dysfunction and subsequent atherosclerosis. Fortunately, there are now proven ways to protect against the many risk factors (i.e., all 13 daggers) that are the underlying causes of today’s heart attacks and strokes. Differing Views on HomocysteineHomocysteine is one of many factors involved in the atherosclerotic process. A recent analysis of some previous studies using folic acid in patients with preexisting cardiovascular disease claimed that this vitamin provided no benefit. A major flaw in this analysis is that none of the actual studies cited in this review lowered homocysteine levels enough to meaningfully reduce cardiovascular risk.131 Based on published epidemiological data, homocysteine should ideally be below 7-8 µmol/L of blood in order for homocysteine to no longer be a risk factor for vascular disease.132,133 Table 1 below represents the before and after homocysteine blood levels in 11 studies that were used to erroneously discredit the dangers of homocysteine.133-143
As can be clearly seen in Table 1, in none of these studies was homocysteine adequately reduced to safe ranges of below 7-8 µmol/L of blood. As is also shown in the table, some groups with severely high homocysteine had levels that remained very high even after folic acid supplementation. In other groups, homocysteine levels were not particularly high to begin with, suggesting that the preexisting vascular disease in these study subjects may have been caused by one or more of the 12 other proven risk factors (daggers). These hard facts did not stop the media from proclaiming that those with preexisting vascular disease were wasting their money by taking folic acid supplements. None of these studies, by the way, evaluated the effects of folic acid supplementation on healthy people. Even if homocysteine had been sufficiently lowered, it is doubtful that significant benefits would have been observed—the reason being that people with “preexisting” cardiovascular disease already suffer arterial damage that is so severe that far more than a folic acid supplement is needed to restore healthy blood flow. Remember the 13 correctable risk factors (daggers) involved in arterial degradation and occlusion shown on the first page of this article. It is ludicrous to think that mitigating one risk factor (such as homocysteine) would result in some miraculous benefit in people already afflicted with massive damage to their inner arterial wall (i.e., endothelial dysfunction). Based on the disparaging attacks made by mainstream cardiologists against folic acid, it is obvious that these doctors remain in a pathetic state of ignorance regarding why so many of their patients suffer from chronic circulatory disorders. At the same time the negative review on homocysteine was released, another published scientific review came to very different conclusions about the role that homocysteine plays in a host of age-related disorders.144 Needless to say, the media ignored the paper that advised aging humans to lower toxic homocysteine levels. | |||||||||||||||||||||||||||||||||||
Protect Your Arteries Against Today’s Lethal MisconceptionsIf you rely on mainstream doctors to be your sole health advisor, your longevity could be in serious jeopardy. Based on their consistent bias against dietary supplements, the media appears to function as a mouthpiece for the pharmaceutical industry, whose profits are threatened when people choose low-cost supplements like folic acid. Conventional doctors routinely prescribe statin drugs that reduce cholesterol and low-density lipoprotein (LDL) while sometimes boosting beneficial high-density lipoprotein (HDL). Although more people take cardiac drugs than ever before, hundreds of thousands of Americans still perish each year from heart failure while under a doctor’s care. Many cardiac patients do require medications to stay alive. The obvious limitation of these drugs is that they address only a few of the many underlying causes of heart attack and stroke. Since the early 1980s, Life Extension has advised its members to have annual blood tests to identify disease risk factors that can be reversed before serious illness develops. The value of these blood tests in preventing future disease and premature death is incalculable. The problem people still encounter is that their doctors refuse to prescribe blood tests for important vascular markers such as fibrinogen, homocysteine, and C-reactive protein. The cost of these tests is also expensive at commercial labs. Eleven years ago, Life Extension resolved this problem by offering blood tests at discounted prices directly to its members. Once a year, we reduce our everyday low prices. Until May 31, 2007, we are discounting all blood tests so that members can obtain comprehensive blood evaluations at a fraction of the price charged by commercial laboratories. Whether you use your own doctor, a commercial laboratory, or our blood testing service, I continue to encourage members to have their blood tested at least once a year.
For longer life, | |||
| References | |||
| 1. Thom T, Haase N, Rosamond W, et al. Heart disease and stroke statistics—2006 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2006 Feb 14;113(6):e85-e151. 2. Friedman RJ, Moore S, Singal DP. Repeated endothelial injury and induction of atherosclerosis in normolipemic rabbits by human serum. Lab Invest. 1975 Mar;32(3):404-15. 3. Yusuf S, Reddy S, Ounpuu S, Anand S. Global burden of cardiovascular diseases: part I: general considerations, the epidemiologic transition, risk factors, and impact of urbanization. Circulation. 2001 Nov 27;104(22):2746-53. 4. Chang HJ, Chung J, Choi SY, et al. Endothelial dysfunction in patients with exaggerated blood pressure response during treadmill test. Clin Cardiol. 2004 Jul;27(7):421-25. 5. Higashi Y, Yoshizumi M. Exercise and endothelial function: role of endothelium-derived nitric oxide and oxidative stress in healthy subjects and hypertensive patients. Pharmacol Ther. 2004 Apr;102(1):87-96. 6. Rodriguez-Porcel M, Lerman LO, Herrmann J, et al. Hypercholesterolemia and hypertension have synergistic deleterious effects on coronary endothelial function. Arterioscler Thromb Vasc Biol. 2003 May 1;23(5):885-91. 7. Tu L, Wei W, Liu X, Deng Y, Yu S. Endothelial function and carotid artery wall thickening in patients with early essential hypertension. J Tongji Med Univ. 1999;19(4):288-90, 303. 8. Sutton-Tyrrell K, Bostom A, Selhub J, Zeigler-Johnson C. High homocysteine levels are independently related to isolated systolic hypertension in older adults. Circulation. 1997 Sep 16;96(6):1745-9. 9. Puddu P, Puddu GM, Zaca F, Muscari A. Endothelial dysfunction in hypertension. Acta Cardiol. 2000 Aug;55(4):221-32. 10. Bolad I, Delafontaine P. Endothelial dysfunction: its role in hypertensive coronary disease. Curr Opin Cardiol. 2005 Jul;20(4):270-4. 11. Maggi FM, Raselli S, Grigore L, et al. Lipoprotein remnants and endothelial dysfunction in the postprandial phase. J Clin Endocrinol Metab. 2004 Jun;89(6):2946-50. 12. Laaksonen R, Janatuinen T, Vesalainen R, et al. High oxidized LDL and elevated plasma homocysteine contribute to the early reduction of myocardial flow reserve in healthy adults. Eur J Clin Invest. 2002 Nov;32(11):795-802. 13. Dardik R, Varon D, Tamarin I, et al. Homocysteine and oxidized low density lipoprotein enhanced platelet adhesion to endothelial cells under flow conditions: distinct mechanisms of thrombogenic modulation. Thromb Haemost. 2000 Feb;83(2):338-44. 14. Voutilainen S, Morrow JD, Roberts LJ, et al. Enhanced in vivo lipid peroxidation at elevated plasma total homocysteine levels. Arterioscler Thromb Vasc Biol. 1999 May;19(5):1263-6. 15. De Caterina R, Lenzi S. The role of LDL in the origin and progression of atherosclerosis: pathobiological concepts on the origin and development of atherosclerotic lesions and the role of the endothelium. G Ital Cardiol. 1998 Feb;28(2):158-67. 16. Drexel H, Amann FW, Beran J, et al. Plasma triglycerides and three lipoprotein cholesterol fractions are independent predictors of the extent of coronary atherosclerosis. Circulation. 1994 Nov;90(5):2230-5. 17. Sharrett AR, Patsch W, Sorlie PD, et al. Associations of lipoprotein cholesterols, apolipoproteins A-I and B, and triglycerides with carotid atherosclerosis and coronary heart disease. The Atherosclerosis Risk in Communities (ARIC) Study. Arterioscler Thromb. 1994 Jul;14(7):1098-104. 18. Vasques E, Almeida AL, Noya V, et al. Impairment of endothelium-dependent aorta relaxation by phospholipid components of oxidized low-density lipoprotein. Endothelium. 2006 Jan;13(1):1-8. 19. Badimon L, Martinez-Gonzalez J, Llorente-Cortes V, Rodriguez C, Padro T. Cell biology and lipoproteins in atherosclerosis. Curr Mol Med. 2006 Aug;6(5):439-56. 20. Vakkilainen J, Makimattila S, Seppala-Lindroos A, et al. Endothelial dysfunction in men with small LDL particles. Circulation. 2000 Aug 15;102(7):716-21. 21. Calabresi L, Gomaraschi M, Franceschini G. Endothelial protection by high-density lipoproteins: from bench to bedside. Arterioscler Thromb Vasc Biol. 2003 Oct 1;23(10):1724-31. 22. Spieker LE, Sudano I, Hurlimann D, et al. High-density lipoprotein restores endothelial function in hypercholesterolemic men. Circulation. 2002 Mar 26;105(12):1399-402. 23. Toikka JO, Ahotupa M, Viikari JS, et al. Constantly low HDL-cholesterol concentration relates to endothelial dysfunction and increased in vivo LDL-oxidation in healthy young men. Atherosclerosis. 1999 Nov 1;147(1):133-8. 24. Ikonomidis I, Lekakis J, Vamvakou G, Andreotti F, Nihoyannopoulos P. Cigarette smoking is associated with increased circulating proinflammatory and procoagulant markers in patients with chronic coronary artery disease: effects of aspirin treatment. Am Heart J. 2005 May;149(5):832-9. 25. Esen AM, Barutcu I, Acar M, et al. Effect of smoking on endothelial function and wall thickness of brachial artery. Circ J. 2004 Dec;68(12):1123-6. 26. Wanner A, Campos MA, Mendes E. Airway blood flow reactivity in smokers. Pulm Pharmacol Ther. 2007;20(2):126-9. 27. Ambrose JA, Barua RS. The pathophysiology of cigarette smoking and cardiovascular disease: an update. J Am Coll Cardiol. 2004 May 19;43(10):1731-7. 28. Poreba R, Skoczynska A, Derkacz A. Effect of tobacco smoking on endothelial function in patients with coronary arteriosclerosis. Pol Arch Med Wewn. 2004 Jan;111(1):27-36. 29. Puranik R, Celermajer DS. Smoking and endothelial function. Prog Cardiovasc Dis. 2003 May;45(6):443-58. 30. Newby DE, McLeod AL, Uren NG, et al. Impaired coronary tissue plasminogen activator release is associated with coronary atherosclerosis and cigarette smoking: direct link between endothelial dysfunction and atherothrombosis. Circulation. 2001 Apr 17;103(15):1936-41. 31. Papamichael CM, Aznaouridis KA, Stamatelopoulos KS, et al. Endothelial dysfunction and type of cigarette smoked: the impact of ‘light’ versus regular cigarette smoking. Vasc Med. 2004 May;9(2):103-5. 32. O’Callaghan P, Meleady R, Fitzgerald T, Graham I. Smoking and plasma homocysteine. Eur Heart J. 2002 Oct;23(20):1580-6. 33. Targher G, Bertolini L, Zoppini G, Zenari L, Falezza G. Increased plasma markers of inflammation and endothelial dysfunction and their association with microvascular complications in Type 1 diabetic patients without clinically manifest macroangiopathy. Diabet Med. 2005 Aug;22(8):999-1004. 34. Jarvisalo MJ, Raitakari M, Toikka JO, et al. Endothelial dysfunction and increased arterial intima-media thickness in children with type 1 diabetes. Circulation. 2004 Apr 13;109(14):1750-5. 35. Vlassara H, Cai W, Crandall J, et al. Inflammatory mediators are induced by dietary glycotoxins, a major risk factor for diabetic angiopathy. Proc Natl Acad Sci USA. 2002 Nov 26;99(24):15596-601. 36. Najemnik C, Sinzinger H, Kritz H. Endothelial dysfunction, atherosclerosis and diabetes. Acta Med Austriaca. 1999;26(5):148-53. 37. Hink U, Tsilimingas N, Wendt M, Munzel T. Mechanisms underlying endothelial dysfunction in diabetes mellitus: therapeutic implications. Treat Endocrinol. 2003;2(5):293-304. 38. Panus C, Mota M, Vladu D, Vanghelie L, Raducanu CL. The endothelial dysfunction in diabetes mellitus. Rom J Intern Med. 2003;41(1):27-33. 39. Bakker SJ, IJzerman RG, Teerlink T, et al. Cytosolic triglycerides and oxidative stress in central obesity: the missing link between excessive atherosclerosis, endothelial dysfunction, and beta-cell failure? Atherosclerosis. 2000 Jan;148(1):17-21. 40. Yu YR, Li HL, Yu HL, Wang C, Pu S. The relationship between insulin resistance and endothelium-dependent vasodilatation in obese subjects. Zhonghua Yi Xue Za Zhi. 2003 Sep 10;83(17):1467-70. 41. Blann AD, Bushell D, Davies A, et al. von Willebrand factor, the endothelium and obesity. Int J Obes Relat Metab Disord. 1993 Dec;17(12):723-5. 42. Lteif AA, Han K, Mather KJ. Obesity, insulin resistance, and the metabolic syndrome: determinants of endothelial dysfunction in whites and blacks. Circulation. 2005 Jul 5;112(1):32-8. 43. Edwards DG, Schofield RS, Lennon SL, et al. Effect of exercise training on endothelial function in men with coronary artery disease. Am J Cardiol. 2004 Mar 1;93(5):617-20. 44. Mitu F, Mitu M. Physical exercise and vascular endothelium. Rev Med Chir Soc Med Nat Iasi. 2003 Jul;107(3):487-93. 45. Gokce N, Vita JA, Bader DS, et al. Effect of exercise on upper and lower extremity endothelial function in patients with coronary artery disease. Am J Cardiol. 2002 Jul 15;90(2):124-7. 46. Chakraphan D, Sridulyakul P, Thipakorn B, et al. Attenuation of endothelial dysfunction by exercise training in STZ-induced diabetic rats. Clin Hemorheol Microcirc. 2005;32(3):217-26. 47. Harvey PJ, Picton PE, Su WS, et al. Exercise as an alternative to oral estrogen for amelioration of endothelial dysfunction in postmenopausal women. Am Heart J. 2005 Feb;149(2):291-7. 48. Suvorava T, Lauer N, Kojda G. Physical inactivity causes endothelial dysfunction in healthy young mice. J Am Coll Cardiol. 2004 Sep 15;44(6):1320-7. 49. Superko HR. Exercise and lipoprotein metabolism. J Cardiovasc Risk. 1995 Aug;2(4):310-5. 50. Ceriello A. Impaired glucose tolerance and cardiovascular disease: the possible role of post-prandial hyperglycemia. Am Heart J. 2004 May;147(5):803-7. 51. Thomas GN, Chook P, Qiao M, et al. Deleterious impact of “high normal” glucose levels and other metabolic syndrome components on arterial endothelial function and intima-media thickness in apparently healthy Chinese subjects: the CATHAY study. Arterioscler Thromb Vasc Biol. 2004 Apr;24(4):739-43. 52. Stochmal E, Szurkowska M, Czarnecka D, et al. Association of coronary atherosclerosis with insulin resistance in patients with impaired glucose tolerance. Acta Cardiol. 2005 Jun;60(3):325-31. 53. Muis MJ, Bots ML, Bilo HJ, et al. High cumulative insulin exposure: a risk factor of atherosclerosis in type 1 diabetes? Atherosclerosis. 2005 Jul;181(1):185-92. 54. Yki-Jarvinen H. Nonglycemic effects of insulin. Clin Cornerstone. 2003;Suppl 4S6-12. 55. Howes PS, Zacharski LR, Sullivan J, Chow B. Role of stored iron in atherosclerosis. J Vasc Nurs. 2000 Dec;18(4):109-14. 56. de VB, Marx JJ. Iron, atherosclerosis, and ischemic heart disease. Arch Intern Med. 1999 Jul 26;159(14):1542-8. 57. Chau LY. Iron and atherosclerosis. Proc Natl Sci Counc Repub China B. 2000 Oct;24(4):151-5. 58. Hoogeveen EK, Kostense PJ, Beks PJ, et al. Hyperhomocysteinemia is associated with an increased risk of cardiovascular disease, especially in non-insulin-dependent diabetes mellitus: a population-based study. Arterioscler Thromb Vasc Biol. 1998 Jan;18(1):133-8. 59. Sainani GS, Sainani R. Homocysteine and its role in the pathogenesis of atherosclerotic vascular disease. J Assoc Physicians India. 2002 May;50 Suppl16-23. 60. Zeng XK, Guan YF, Remick DG, Wang X. Signal pathways underlying homocysteine-induced production of MCP-1 and IL-8 in cultured human whole blood. Acta Pharmacol Sin. 2005 Jan;26(1):85-91. 61. Zeng XK, Remick DG, Wang X. Homocysteine induces production of monocyte chemoattractant protein-1 and interleukin-8 in cultured human whole blood. Acta Pharmacol Sin. 2004 Nov;25(11):1419-25. 62. Hassan A, Hunt BJ, O’Sullivan M, et al. Homocysteine is a risk factor for cerebral small vessel disease, acting via endothelial dysfunction. Brain. 2004 Jan;127(Pt 1):212-9. 63. Devlin AM, Arning E, Bottiglieri T, et al. Effect of Mthfr genotype on diet-induced hyperhomocysteinemia and vascular function in mice. Blood. 2004 Apr 1;103(7):2624-9. 64. Ungvari Z, Csiszar A, Edwards JG, et al. Increased superoxide production in coronary arteries in hyperhomocysteinemia: role of tumor necrosis factor-alpha, NAD(P)H oxidase, and inducible nitric oxide synthase. Arterioscler Thromb Vasc Biol. 2003 Mar 1;23(3):418-24. 65. Loscalzo J. Oxidant stress: a key determinant of atherothrombosis. Biochem Soc Trans. 2003 Oct;31(Pt 5):1059-61. 66. Symons JD, Mullick AE, Ensunsa JL, Ma AA, Rutledge JC. Hyperhomocysteinemia evoked by folate depletion: effects on coronary and carotid arterial function. Arterioscler Thromb Vasc Biol. 2002 May 1;22(5):772-80. 67. Eberhardt RT, Forgione MA, Cap A, et al. Endothelial dysfunction in a murine model of mild hyperhomocyst(e)inemia. J Clin Invest. 2000 Aug;106(4):483-91. 68. Folsom AR, Nieto FJ, McGovern PG, et al. Prospective study of coronary heart disease incidence in relation to fasting total homocysteine, related genetic polymorphisms, and B vitamins: the Atherosclerosis Risk in Communities (ARIC) study. Circulation. 1998 Jul 21;98(3):204-10. 69. Woo KS, Chook P, Lolin YI, et al. Hyperhomocyst(e)inemia is a risk factor for arterial endothelial dysfunction in humans. Circulation. 1997 Oct 21;96(8):2542-4. 70. Bots ML, Launer LJ, Lindemans J, Hofman A, Grobbee DE. Homocysteine, atherosclerosis and prevalent cardiovascular disease in the elderly: The Rotterdam Study. J Intern Med. 1997 Oct;242(4):339-47. 71. Montalescot G, Ankri A, Chadefaux-Vekemans B, et al. Plasma homocysteine and the extent of atherosclerosis in patients with coronary artery disease. Int J Cardiol. 1997 Aug 8;60(3):295-300. 72. Lentz SR, Sobey CG, Piegors DJ, et al. Vascular dysfunction in monkeys with diet-induced hyperhomocyst(e)inemia. J Clin Invest. 1996 Jul 1;98(1):24-9. 73. Verhoef P, Stampfer MJ, Buring JE, et al. Homocysteine metabolism and risk of myocardial infarction: relation with vitamins B6, B12, and folate. Am J Epidemiol. 1996 May 1;143(9):845-59. 74. Arnesen E, Refsum H, Bonaa KH, et al. Serum total homocysteine and coronary heart disease. Int J Epidemiol. 1995 Aug;24(4):704-9. 75. Berwanger CS, Jeremy JY, Stansby G. Homocysteine and vascular disease. Br J Surg. 1995 Jun;82(6):726-31. 76. Tsai JC, Perrella MA, Yoshizumi M, et al. Promotion of vascular smooth muscle cell growth by homocysteine: a link to atherosclerosis. Proc Natl Acad Sci USA. 1994 Jul 5;91(14):6369-73. 77. Fryer RH, Wilson BD, Gubler DB, Fitzgerald LA, Rodgers GM. Homocysteine, a risk factor for premature vascular disease and thrombosis, induces tissue factor activity in endothelial cells. Arterioscler Thromb. 1993 Sep;13(9):1327-33. 78. Harker LA, Harlan JM, Ross R. Effect of sulfinpyrazone on homocysteine-induced endothelial injury and arteriosclerosis in baboons. Circ Res. 1983 Dec;53(6):731-9. 79. Wall RT, Rubenstein MD, Cooper SL. Studies on the cellular basis of atherosclerosis: the effects of atherosclerosis risk factors on platelets and the vascular endothelium. Diabetes. 1981;30(Suppl 2):39-43. 80. Rasouli ML, Nasir K, Blumenthal RS, et al. Plasma homocysteine predicts progression of atherosclerosis. Atherosclerosis. 2005 Jul;181(1):159-65. 81. Anon. Homocysteine and risk of ischemic heart disease and stroke: a meta-analysis. JAMA. 2002 Oct 23;288(16):2015-22. 82. Refsum H, Ueland PM, Nygard O, Vollset SE. Homocysteine and cardiovascular disease. Annu Rev Med. 1998;49:31-62. 83. Kunz J. Initial lesions of vascular aging disease (arteriosclerosis). Gerontology. 2000 Nov;46(6):295-9. 84. Nappo F, De RN, Marfella R, et al. Impairment of endothelial functions by acute hyperhomocysteinemia and reversal by antioxidant vitamins. JAMA. 1999 Jun 9;281(22):2113-8. 85. Drouet L, Bal dit SC. Is fibrinogen a predictor or a marker of the risk of cardiovascular events? Therapie. 2005 Mar;60(2):125-36. 86. Coppola G, Rizzo M, Abrignani MG, et al. Fibrinogen as a predictor of mortality after acute myocardial infarction: a forty-two-month follow-up study. Ital Heart J. 2005 Apr;6(4):315-22. 87. Danesh J, Lewington S, Thompson SG, et al. Plasma fibrinogen level and the risk of major cardiovascular diseases and nonvascular mortality: an individual participant meta-analysis. JAMA. 2005 Oct 12;294(14):1799-809. 88. Acevedo M, Foody JM, Pearce GL, Sprecher DL. Fibrinogen: associations with cardiovascular events in an outpatient clinic. Am Heart J. 2002 Feb;143(2):277-82. 89. Bots ML, Elwood PC, Salonen JT, et al. Level of fibrinogen and risk of fatal and non-fatal stroke. EUROSTROKE: a collaborative study among research centres in Europe. J Epidemiol Community Health. 2002 Feb;56 Suppl 1:i14-8. 90. de Maat MP. Effects of diet, drugs, and genes on plasma fibrinogen levels. Ann NY Acad Sci. 2001;936:509-21. 91. Maresca G, Di BA, Marchioli R, Di MG. Measuring plasma fibrinogen to predict stroke and myocardial infarction: an update. Arterioscler Thromb Vasc Biol. 1999 Jun;19(6):1368-77. 92. Ma J, Hennekens CH, Ridker PM, Stampfer MJ. A prospective study of fibrinogen and risk of myocardial infarction in the Physicians’ Health Study. J Am Coll Cardiol. 1999 Apr;33(5):1347-52. 93. Behar S. Lowering fibrinogen levels: clinical update. BIP Study Group. Bezafibrate Infarction Prevention. Blood Coagul Fibrinolysis. 1999 Feb;10 Suppl 1S41-3. 94. Thompson SG, Kienast J, Pyke SD, Haverkate F, van de Loo JC. Hemostatic factors and the risk of myocardial infarction or sudden death in patients with angina pectoris. European Concerted Action on Thrombosis and Disabilities Angina Pectoris Study Group. N Engl J Med. 1995 Mar 9;332(10):635-41. 95. Wilhelmsen L, Svardsudd K, Korsan-Bengtsen K, et al. Fibrinogen as a risk factor for stroke and myocardial infarction. N Engl J Med. 1984 Aug 23;311(8):501-5. 96. Levenson J, Giral P, Megnien JL, et al. Fibrinogen and its relations to subclinical extracoronary and coronary atherosclerosis in hypercholesterolemic men. Arterioscler Thromb Vasc Biol. 1997 Jan;17(1):45-50. 97. Koenig W. Fibrin(ogen) in cardiovascular disease: an update. Thromb Haemost. 2003 Apr;89(4):601-9. 98. Palmieri V, Celentano A, Roman MJ, et al. Relation of fibrinogen to cardiovascular events is independent of preclinical cardiovascular disease: the Strong Heart Study. Am Heart J. 2003 Mar;145(3):467-74. 99. Channer KS, Jones TH. Cardiovascular effects of testosterone: implications of the “male menopause”? Heart. 2003 Feb;89(2):121-2. 100. English KM, Mandour O, Steeds RP, et al. Men with coronary artery disease have lower levels of androgens than men with normal coronary angiograms. Eur Heart J. 2000 Jun;21(11):890-4. 101. Malkin CJ, Pugh PJ, Jones RD, Jones TH, Channer KS. Testosterone as a protective factor against atherosclerosis—immunomodulation and influence upon plaque development and stability. J Endocrinol. 2003 Sep;178(3):373-80. 102. Jones RD, Nettleship JE, Kapoor D, Jones HT, Channer KS. Testosterone and atherosclerosis in aging men: purported association and clinical implications. Am J Cardiovasc Drugs. 2005;5(3):141-54. 103. Lindahl B, Toss H, Siegbahn A, Venge P, Wallentin L. Markers of myocardial damage and inflammation in relation to long-term mortality in unstable coronary artery disease. FRISC Study Group. Fragmin during Instability in Coronary Artery Disease. N Engl J Med. 2000 Oct 19;343(16):1139-47. 104. Auer J, Berent R, Lassnig E, Eber B. C-reactive protein and coronary artery disease. Jpn Heart J. 2002 Nov;43(6):607-19. 105. Ridker PM, Rifai N, Rose L, Buring JE, Cook NR. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N Engl J Med. 2002 Nov 14;347(20):1557-65. 106. Wang TJ, Larson MG, Levy D, et al. C-reactive protein is associated with subclinical epicardial coronary calcification in men and women: the Framingham Heart Study. Circulation. 2002 Sep 3;106(10):1189-91. 107. Bermudez EA, Ridker PM. C-reactive protein, statins, and the primary prevention of atherosclerotic cardiovascular disease. Prev Cardiol. 2002;5(1):42-6. 108. Virmani R, Burke AP, Kolodgie FD, Farb A. Vulnerable plaque: the pathology of unstable coronary lesions. J Interv Cardiol. 2002 Dec;15(6):439-46. 109. Rifai N, Ridker PM. Inflammatory markers and coronary heart disease. Curr Opin Lipidol. 2002 Aug;13(4):383-9. 110. Zairis MN, Papadaki OA, Manousakis SJ, et al. C-reactive protein and multiple complex coronary artery plaques in patients with primary unstable angina. Atherosclerosis. 2002 Oct;164(2):355-9. 111. Ridker PM. High-sensitivity C-reactive protein: potential adjunct for global risk assessment in the primary prevention of cardiovascular disease. Circulation. 2001 Apr 3;103(13):1813-8. 112. Di Napoli M, Papa F, Bocola V. Prognostic influence of increased C-reactive protein and fibrinogen levels in ischemic stroke. Stroke. 2001 Jan;32(1):133-8. 113. Ridker PM, Stampfer MJ, Rifai N. Novel risk factors for systemic atherosclerosis: a comparison of C-reactive protein, fibrinogen, homocysteine, lipoprotein(a), and standard cholesterol screening as predictors of peripheral arterial disease. JAMA. 2001 May 16;285(19):2481-5. 114. Rifai N. C-reactive protein and coronary heart disease: diagnostic and therapeutic implications for primary prevention. Cardiovasc Toxicol. 2001;1(2):153-7. 115. Higuchi M, Castelli JB, Aiello VD, et al. Great amount of C.pneumoniae in ruptured plaque vessel segments at autopsy. A comparative study with stable plaques. Arq Bras Cardiol. 2000 Feb;74(2):149-51. 116. Pasceri V, Willerson JT, Yeh ET. Direct proinflammatory effect of C-reactive protein on human endothelial cells. Circulation. 2000 Oct 31;102(18):2165-8. 117. Mendall MA, Strachan DP, Butland BK, et al. C-reactive protein: relation to total mortality, cardiovascular mortality and cardiovascular risk factors in men. Eur Heart J. 2000 Oct;21(19):1584-90. 118. Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000 Mar 23;342(12):836-43. 119. Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH. Plasma concentration of C-reactive protein and risk of developing peripheral vascular disease. Circulation. 1998 Feb 10;97(5):425-8. 120. Ridker PM, Buring JE, Shih J, Matias M, Hennekens CH. Prospective study of C-reactive protein and the risk of future cardiovascular events among apparently healthy women. Circulation. 1998 Aug 25;98(8):731-3. 121. Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med. 1997 Apr 3;336(14):973-9. 122. Albert CM, Ma J, Rifai N, Stampfer MJ, Ridker PM. Prospective study of C-reactive protein, homocysteine, and plasma lipid levels as predictors of sudden cardiac death. Circulation. 2002 Jun 4;105(22):2595-9. 123. Xie LQ and Wang X. C-reactive protein and atherosclerosis. Sheng Li Ke Xue Jin Zhan. 2004 Apr;35(2):113-8. 124. Verma S. C-reactive protein incites atherosclerosis. Can J Cardiol. 2004 Aug;20 Suppl B29B-31B. 125. Chambless LE, Folsom AR, Sharrett AR, et al. Coronary heart disease risk prediction in the Atherosclerosis Risk in Communities (ARIC) study. J Clin Epidemiol. 2003 Sep;56(9):880-90. 126. Schurgers LJ, Dissel PE, Spronk HM, et al. Role of vitamin K and vitamin K-dependent proteins in vascular calcification. Z Kardiol. 2001;90 Suppl 357-63. 127. Geleijnse JM, Vermeer C, Grobbee DE et al. Dietary intake of menaquinone is associated with a reduced risk of coronary heart disease: the Rotterdam Study. J Nutr. 2004 Nov;134(11):3100-3105. 128. Kawashima H, Nakajima Y, Matubara Y, et al. Effects of vitamin K2 (menatetrenone) on atherosclerosis and blood coagulation in hypercholesterolemic rabbits. Jpn J Pharmacol. 1997 Oct;75(2):135-43. 129. Shearer MJ, Bach A, Kohlmeier M. Chemistry, nutritional sources, tissue distribution and metabolism of vitamin K with special reference to bone health. J Nutr. 1996 Apr;126(4 Suppl):1181S-6S. 130. Dandona P. Endothelium, inflammation, and diabetes. Curr Diab Rep. 2002 Aug;2(4):311-5. 131. Bazzano LA, Reynolds K, Holder KN, He J. Effect of folic acid supplementation on risk of cardiovascular diseases: a meta-analysis of randomized controlled trials. JAMA. 2006 Dec 13;296(22):2720-6. 132. Robinson K, Mayer EL, Miller DP, et al. Hyperhomocysteinemia and low pyridoxal phosphate. Common and independent reversible risk factors for coronary artery disease. Circulation. 1995 Nov 15;92(10):2825-30. 133. Toole JF, Malinow MR, Chambless LE, et al. Lowering homocysteine in patients with ischemic stroke to prevent recurrent stroke, myocardial infarction, and death: the Vitamin Intervention for Stroke Prevention (VISP) randomized controlled trial. JAMA. 2004 Feb 4;291(5):565-75. 134. Baker F, Picton D, Blackwood S, et al. Blinded comparison of folic acid and placebo in patients with ischaemic heart disease: an outcome trial. Circulation 2002;106:(suppl II):2-741S. 135. Schnyder G, Roffi M, Flammer Y, Pin R, Hess OM. Effect of homocysteine-lowering therapy with folic acid, vitamin B12, and vitamin B6 on clinical outcome after percutaneous coronary intervention: the Swiss Heart study: a randomized controlled trial. JAMA. 2002 Aug 28;288(8):973-9. 136. Righetti M, Ferrario GM, Milani S, et al. Effects of folic acid treatment on homocysteine levels and vascular disease in hemodialysis patients. Med Sci Monit. 2003 Apr;9(4):I19-24. 137. Lange H, Suryapranata H, De LG, et al. Folate therapy and in-stent restenosis after coronary stenting. N Engl J Med. 2004 Jun 24;350(26):2673-81. 138. Wrone EM, Hornberger JM, Zehnder JL, et al. Randomized trial of folic acid for prevention of cardiovascular events in end-stage renal disease. J Am Soc Nephrol. 2004 Feb;15(2):420-6. 139. Available at: http://heart.bmj.com/cgi/content/extract/91/9/1213. Accessed January 9, 2007. 140. Bonaa KH, Njolstad I, Ueland PM, et al. Homocysteine lowering and cardiovascular events after acute myocardial infarction. N Engl J Med. 2006 Apr 13;354(15):1578-88. 141. Lonn E, Yusuf S, Arnold MJ, et al. Homocysteine lowering with folic acid and B vitamins in vascular disease. N Engl J Med. 2006 Apr 13;354(15):1567-77. 142. Righetti M, Serbelloni P, Milani S, Ferrario G. Homocysteine-lowering vitamin B treatment decreases cardiovascular events in hemodialysis patients. Blood Purif. 2006;24(4):379-86. 143. Zoungas S, McGrath BP, Branley P, et al. Cardiovascular morbidity and mortality in the Atherosclerosis and Folic Acid Supplementation Trial (ASFAST) in chronic renal failure: a multicenter, randomized, controlled trial. J Am Coll Cardiol. 2006 Mar 21;47(6):1108-16. 144. Ramakrishnan S, Sulochana KN, Lakshmi S, Selvi R, Angayarkanni N. Biochemistry of homocysteine in health and diseases. Indian J Biochem Biophys. 2006 Oct;43(5):275-83. 145. Wang TJ, Gona P, Larson MG, et al. Multiple biomarkers for the prediction of first major cardiovascular events and death. N Engl J Med. 2006 Dec 21;355(25):2631-9. |