Life Extension Magazine®
Two recent studies of progesterone supplementation validate what the Life Extension Foundation has contended for the past 12 years: restoring the body’s supply of natural progesterone confers multiple health benefits, including balancing blood sugar levels, promoting normal sleep, reducing anxiety, and stimulating new bone growth.1,2 Controlled studies and most observational studies published in the last five years suggest that the addition of progestins (synthetic progesterone) to hormone replacement therapy, particularly in a continuous combined regimen, increases the risk of breast cancer compared to estrogen alone.1 While the results of clinical trials may accurately assess the risks associated with synthetic progestin compounds and estrogen/ progestin combinations, the data do not reflect what might have been the result had natural progesterone been used instead of synthetic progesterone.2 Recent studies suggest that the addition of natural progesterone in a cyclic manner does not increase breast cancer risk. These findings are consistent with in-vivo data suggesting that progesterone does not have a detrimental effect on breast tissue.1
Nature has given progesterone to men and women alike to balance and offset the powerful effects of estrogen. Some of the most common concerns of aging women are weight gain, insomnia, anxiety, depression, and migraine. For other women, even more debilitating conditions such as cancer, uterine fibroids, ovarian cysts, and osteoporosis now play a predominant role in their lives. As men age, complaints of weight gain, loss of libido, and prostate enlargement top their list of health concerns. Many physicians and scientists are becoming more aware of a common link between these symptoms and conditions. That common link is often an imbalance between two sex hormones, progesterone and estrogen. In menstruating women, progesterone is one of two primary sex hormones (the other being estrogen) produced each month by the ovaries. During the first 14 days of the menstrual cycle, the ovaries secrete increasing amounts of estrogens. This two-week period is named the follicular phase. Halfway through a woman’s cycle, around day 14, one of her two ovaries will ovulate and release an egg. After ovulation, the ruptured follicle from which the egg has been released is transformed into the corpus luteum and begins producing progesterone. The portion of the menstrual cycle that follows ovulation, called the luteal phase, is orchestrated by progesterone. As its name implies, progesterone prepares (promotes) the womb for pregnancy (gestation). If the egg fails to be fertilized and no pregnancy occurs, the production of both progesterone and estrogen will rapidly decline, resulting in a period (menses). If pregnancy does occur, the placenta begins to secrete progesterone (the corpus luteum continues to produce progesterone as well). In fact, by the fifth month of pregnancy, the placenta itself secretes sufficient progesterone such that the corpus luteum is no longer essential to maintain pregnancy. These high levels of progesterone act as natural birth control agents, shutting down ovulation for the duration of the pregnancy. Plasma concentrations of progesterone in women vary throughout the menstrual cycle. During the follicular phase, plasma concentrations of progesterone are generally below 2 nanograms per milliliter (ng/mL). Throughout the luteal phase, which prepares the body for pregnancy, progesterone levels can rise to 28 ng/mL. Dramatic increases in progesterone occur throughout pregnancy: plasma levels may reach 40 ng/mL in the first trimester, and climb to 100-200 ng/mL near the delivery date.3 Progesterone is a key precursor to other steroid hormones, including cortisol, testosterone, and the estrogens (estriol, estradiol, and estrone). When progesterone circulates in the blood, 90% is bound to a protein or albumin fraction. Only a small percentage (3%) circulates unbound.4 While a woman’s estrogen may eventually drop 40-60% below her baseline level by menopause, her progesterone level can drop even more dramatically. Although the adrenal glands still produce some progesterone, the decline in progesterone upsets the body’s natural hormone balance. Following menopause, a woman’s progesterone level drops to nearly zero. Actions of ProgesteroneProgesterone plays a key role in the tasks necessary for reproduction. Beyond preparation for pregnancy, progesterone has a multitude of effects throughout the body, many of which may be attributable to its ability to oppose the action of estrogen. Multiple physical and psychological problems at midlife are often caused by an imbalance between progesterone and estrogen. The term “estrogen dominance” describes the condition of lacking sufficient progesterone to counteract the effects of estrogen. A common misconception is that estrogen dominance results only from extremely high levels of estrogen. To the contrary, this condition also may be caused by normal levels of estrogen and relatively low levels of progesterone, or by low levels of estrogen and extremely low levels of progesterone. Estrogen levels may be elevated by a number of external influences. Xenoestrogens (foreign estrogens) are among a group of chemicals known to alter hormone levels. Environmental pesticides, including those found on commercially grown fruits and vegetables, are perhaps the primary source of xenoestrogens. Cosmetics, shampoo, and plastics also may contribute to the accumulation of these foreign estrogens. Progesterone’s many functions in the body include:
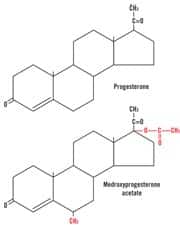
Natural vs. Synthetic ProgesteroneWhen discussing progesterone, it is important to understand the difference between natural progesterone and the synthetic progesterone analogs called progestins. Progestogens is an umbrella term for both natural progesterone and the synthetic progestins, because they all have progestational effects in the uterus. Natural progesterone is synthesized in the laboratory from either soybeans or the Mexican wild yam (Dioscorea villosa). The process was discovered in the 1930s by Pennsylvania State University professor Russell Marker, who transformed diosgenin from wild yams into natural progesterone. Natural progesterone refers to bioidentical hormone products that have a molecular structure identical to the hormones our bodies manufacture naturally. The most effective form of bioidentical progesterone is called micronized progesterone USP. The process of micronization allows for steady and even absorption of the medication. Micronized progesterone is available only through a doctor’s prescription. An alternative is natural progesterone creams sold over the counter worldwide. Both the micronized progesterone and commercially available progesterone creams contain bioidentical progesterone.
Unlike progesterone, synthetic progestins are not molecularly identical to the hormones found naturally in the body. Synthetic progestins were first developed for use as contraceptive agents. Because the half-life of natural progesterone is very short, researchers sought an agent that would produce longer-lasting, more potent effects than natural progesterone. Birth control pills usually contain a synthetic progestin and a synthetic estrogen. Synthetic progestins are very potent, with just a small dose preventing ovulation and thus functioning as birth control. A slight change in the chemical structure of progesterone has allowed pharmaceutical companies to create patentable and profitable birth control products.
One of the most common progestins, medroxyprogesterone acetate (Provera®), has been linked to blood clots, fluid retention, acne, rashes, weight gain, and depression. Progestins are also able to bind to glucocorticoid, androgen, and mineralocorticoid receptors, which explains the wide range of side effects many women experience while taking progestins.17,18 The vast majority of research studies have been conducted using progestins rather than natural progesterone, which explains the disparity and negativity of the results. The FDA has also approved a drug called Prometrium®, an oral pill containing 200 mg of natural progesterone taken daily. Because orally administered progesterone is metabolized by the liver, it may be contraindicated in patients with certain liver conditions. Natural progesterone cream may be more efficiently used, since its highly lipophilic (fat-soluble) molecules of low molecular weight allow it be well absorbed through the skin. Another advantage of topical natural progesterone cream is that individualized dosing can be easily facilitated by varying the amount of cream applied. | |||||||
Progesterone and the BrainThe brain is highly responsive to progesterone. In fact, progesterone concentrations in the brain have been shown to be 20 times higher than in the blood.2 Insomnia, anxiety, and migraine are just a few of the conditions linked to an imbalance of progesterone and estrogen.19-22 In the brain as elsewhere in the body, progesterone counterbalances the effects of estrogen. Whereas estrogen has an excitatory effect on the brain, progesterone’s effect is calming.9 Clinical and anecdotal experience indicates that women with estrogen dominance sleep restlessly, whereas progesterone replenishment enhances sleep. It remains unexplained why anxiety disorders are more prevalent in women than in men, and how female hormone-related events (such as menstrual cycle and postpartum) influence the course of anxiety disorders. However, it would appear logical that female hormones and their derivatives play a major role in these observations.23 Several studies have shown progesterone to have anxiolytic (anti-anxiety) effects by acting on gamma-aminobutyric acid (GABA) receptors in the brain.20-22 GABA is an inhibitory neurotransmitter that aids in relaxation and sleep. In the brain, GABA helps balance excitation with inhibition. Furthermore, withdrawal from endogenous progesterone supplementation after chronic administration increases anxiety via declining levels of its potent GABA-modulatory metabolites.24
A variety of evidence suggests a link between migraine and the female sex hormones. According to the American Migraine Study, 17.6% of females versus only 6% of males in the US currently suffer from severe migraine.25 Many women with migraine, especially those with a history of menstrual migraine, experience an exacerbation of the condition as they approach menopause. During this time, the orderly pattern of estrogen and progesterone secretion is lost.26 In 1953, two English physicians, Drs. Katharina Dalton and Raymond Greene, published the first medical report on premenstrual syndrome (PMS). Dr. Dalton observed that progesterone injections relieved her own menstrual migraine headaches. Dr. Dalton then injected progesterone into other women and found that their PMS was cured.11 Hormone replacement with estrogen exacerbates migraine, and oral contraceptives can change the character and frequency of migraine.27 Higher levels of estradiol and the estradiol:progesterone ratio are directly correlated to increased headache activity.19 Migraine syndromes, particularly in women, are associated with deficits in brain and serum ionized magnesium levels.28 Scientists believe that magnesium’s ability to relieve premenstrual distress may be due to the mineral’s ability to relax vascular smooth muscle.29 Researchers have demonstrated that with each increase in estrogen, a decrease in ionized magnesium occurs. However, as progesterone levels rise, ionized magnesium levels increase as well.30 In one study, physicians set out to test the hypothesis that migraine is a specific consequence of an imbalance between neurohormonal and metabolic integrity. Restoration of progesterone along with several other steroid hormones led to a complete resolution of migraine.8 Progesterone and OsteoporosisBone is a very metabolically active tissue, and bone remodeling continues throughout life. The remodeling process is an active coupling of bone formation and bone resorption. Bone loss occurs when the cellular events of bone resorption are quantitatively greater than the events of bone formation. Conventional medicine widely attributes osteoporosis to a decline in estrogen. Before the onset of menopause, however, luteal levels of progesterone decline, whereas levels of estrogen, lutenizing hormone, follicle stimulating hormone, and other reproductive hormones remain intact. In addition, we know that bone loss begins in women well before menopause. In fact, a woman attains her peak bone density at approximately 30 years of age, after which she begins to lose bone at a rate of about 1-1.5% per year.9 Countless women who use estrogen therapy and consume the proper nutrients still have disappointing bone density test results. These facts raise an interesting question: is the decline in estrogen responsible for bone loss, or is progesterone involved, possibly even more so than estrogen? The two types of bone-regulating cells are osteoclasts and osteoblasts. Osteoclasts function to dissolve older bone, leaving tiny unfilled spaces behind. Osteoblasts are then able to move into these spaces to produce new bone. Like all living cells, osteoblasts and osteoclasts require hormonal guidance to function properly. Estrogen is able to help slow bone loss by curbing the activity of bone-dissolving osteoclasts.31,32 On the other hand, osteoblasts depend primarily on progesterone and testosterone to facilitate the building of new bone. In the absence of these hormones, osteoblasts and osteoclasts cease to function properly, and rapid deterioration of bone occurs. Natural progesterone stimulates the new bone formation required to prevent and reverse osteoporosis.7 Progesterone also appears to increase levels of insulin-like growth factor 1, which promotes bone formation.33 It remains uncertain whether progesterone or estrogen plays a more prominent role in bone remodeling. An optimal balance of both hormones appears to be most efficient in preventing and treating osteoporosis. Progesterone and CancerDisturbances in gonadal hormones have been associated with an increased risk of both breast and endometrial cancers. As previously stated, most controlled studies and observational studies in the past five years suggest that the addition of synthetic progestins to synthetic estrogen in hormone replacement therapy, particularly in a continuous combined regimen, increases breast cancer risk compared to synthetic estrogen alone.1 By contrast, recent studies suggest that the addition of natural progesterone does not affect breast cancer risk.1,2 In fact, a large base of evidence suggests that progesterone is a protective agent against, as well as a potential adjunctive treatment for, breast and endometrial cancers.34-44 Whereas estrogen is pro-proliferative, causing the cells in the breast and uterus to multiply, progesterone does not promote proliferation of these tissues. One of the most significant studies of the relationship between low levels of natural progesterone and increased breast cancer risk was published in the American Journal of Epidemiology in 1981. The study followed 1,083 women with a history of difficulty becoming pregnant for periods ranging from 13 to 33 years. The researchers found that infertile women who demonstrated a progesterone deficiency had a premenopausal breast cancer risk that was 540% greater than that of women whose infertility was due to non-hormonal causes. Furthermore, the women with a progesterone deficiency had a 1,000% greater chance of death from all types of cancer.34
In a study conducted at the National Taiwan University Hospital, researchers showed that transdermal estradiol increased breast cell proliferation by 230%, while transdermal progesterone decreased cell proliferation by over 400%.6 A combination of estradiol and progesterone cream was able to maintain the normal proliferation rate. This provides direct evidence that estradiol stimulates hyperproliferation of breast tissue cells and progesterone decreases hyperproliferation. Serum progesterone levels at the time of breast cancer surgery influence survival rates, according to a 1996 study published in the British Journal of Cancer. Women who had progesterone levels of 4 ng/mL or greater at the time of breast cancer surgery had significantly better survival rates at 18 years than those with lower serum levels of progesterone at the time of surgery. In women with higher progesterone levels at the time of surgery, approximately 65% were alive 18 years later, whereas only 35% of the women with low progesterone levels survived 18 years.45 Conventional estrogen replacement therapy with synthetic estrogens increases the incidence of endometrial (uterine lining) abnormalities, including cancer.37 However, co-administration of progesterone counteracts this effect.36-44 Women are also becoming increasingly aware of other serious health conditions that may result from an imbalance of their gonadal hormones. Some scientists believe that conditions such as ovarian cysts, uterine fibroids, fibrocystic breast disease, and cervical erosions may stem from an imbalance between progesterone and estrogen.12 Progesterone After a Hysterectomy?Hysterectomy is the surgical removal of the uterus, which is sometimes performed in conjunction with an oophorectomy or ovariectomy, which is the surgical removal of the ovaries. Doctors often perform a hysterectomy to alleviate patient discomforts associated with conditions such as uterine fibroids, endometriosis, and heavy menses. These conditions, however, are often related to a relative dominance of estrogen.12 Removal of the uterus does not correct the underlying imbalance that may have contributed to these conditions in the first place. Many medical professionals believe that once the uterus has been removed, there is no need to supplement with progesterone. Clinical experience and a review of the scientific literature, however, make it is clear that unopposed estrogen therapy can lead to many undesirable health conditions. When women are young, they have optimal levels of all the steroid hormones, not just estrogen. Replacing only estrogen after a complete hysterectomy is a sure-fire way to increase existing estrogen dominance. Whether a woman has a uterus or not, research suggests that estrogen replacement therapy should not be given without natural progesterone.
Progesterone and MenTypically thought of as a female hormone, progesterone can also be an invaluable tool in hormone modulation in men. Progesterone is manufactured in men by the adrenal glands and testes. Just as estrogen dominance can severely affect the quality of life for women, excess estrogen can be equally detrimental to men. Elevated estrogen in men has been linked to gynecomastia (breast enlargement in men), decreased sexual function, weight gain, and prostate enlargement.46-48 Benign prostatic hyperplasia seems to be related to long-term exposure of the prostate gland to the strong androgen dihydrotestosterone and possibly to estrogens. In fact, the late Dr. John R. Lee, considered a pioneer in natural progesterone therapy, believed that excessive exposure to estrogen was a primary cause of prostate enlargement and prostate cancer. In addition to counterbalancing the negative aspects of estrogen, progesterone may also inhibit 5-alpha-reductase, the enzyme that converts testosterone to dihydrotestosterone.49 ConclusionIn the past century, progesterone and hormone restoration has become increasing popular with men and women alike. In the early 1900s, the average life expectancy was only 49 years.50 Men and women simply did not live long enough to experience the detrimental effects of an imbalance of progesterone and estrogen. Since then, advances in health and medicine have significantly extended the human life span. Today, it is up to each individual to ensure that his or her quality of life is also extended. The risks and side effects of synthetic progestins like medroxyprogesterone acetate have clouded the true health benefits of progesterone supplementation and restoration. Due to monetary interest, research has focused almost exclusively on synthetic derivatives rather than the natural hormones found within the human body. Natural progesterone is associated with few side effects, and is less expensive than its synthetic counterpart.51 Assessing levels of progesterone and other hormones through regular blood testing is essential to attaining optimal health. Life Extension suggests using individually modified doses of progesterone in a cyclical manner. Such an approach will mimic a youthful physiology, resulting in much-improved quality of life.
| |||||
| References | |||||
| 1. Campagnoli C, Clavel-Chapelon F, Kaaks R, Peris C, Berrino F. Progestins and progesterone in hormone replacement therapy and the risk of breast cancer. J Steroid Biochem Mol Biol. 2005 Jul;96(2):95-108. 2. Stein DG. The case for progesterone. Ann NY Acad Sci. 2005 Jun;1052:152-69. 3. Yen SSC. Endocrine-Metabolic Alterations in Pregnancy. Philadelphia, PA: WB Saunders Co.; 1991:936-981. 4. Andersen CY. Concentrations of free oestradiol and progesterone in human preovulatory follicular fluid. Hum Reprod. 1991 Mar;6(3):359-64. 5. Clarke CL, Sutherland RL. Progestin regulation of cellular proliferation. Endocr Rev. 1990 May;11(2):266-301. 6. Chang KJ, Lee TT, Linares-Cruz G, Fournier S, de Lignieres B. Influences of percutaneous administration of estradiol and progesterone on human breast epithelial cell cycle in vivo. Fertil Steril. 1995 Apr;63(4):785-91. 7. Heersche JN, Bellows CG, Ishida Y. The decrease in bone mass associated with aging and menopause. J Prosthet Dent. 1998 Jan;79(1):14-6. 8. Dzugan SA, Smith RA. The simultaneous restoration of neurohormonal and metabolic integrity as a very promising method of migraine management. Bull Urg Rec Med. 2003;4(4):622-8. 9. Hotze SF. Hormones, Health, and Happiness. Houston, TX: Forrest Publishing; 2005. 10. Akande EO. Plasma concentration of gonadotrophins, oestrogen and progesterone in hypothyroid women. Br J Obstet Gynaecol. 1975 Jul;82(7):552-6. 11. Dalton, K. The Premenstrual Syndrome and Progesterone Therapy. Chicago, IL: Year Book Medical Publishers; 1977. 12. Lee JR, Zava D, Hopkins V. What Your Doctor May Not Tell You About Breast Cancer. New York, NY: Warner Books; 2002. 13. Huber J. Estrogen substitution therapy in climacteric: should progesterone be omitted in hysterectomized women? Geburtshilfe Frauenheilkd. 1991 Apr;51(4):257-61. 14. Sumino H, Ichikawa S, Itoh H, et al. Hormone replacement therapy decreases insulin resistance and lipid metabolism in Japanese postmenopausal women with impaired and normal glucose tolerance. Horm Res. 2003;60(3):134-42. 15. Kanaya A, Herrington D, Vittinghoff E, et al. Glycemic effects of postmenopausal hormone therapy: the heart and estrogen/progestin replacement study: a randomized, double-blind, placebo-controlled trial. Ann Intern Med. 2003 Jan 7;138(1):1-9. 16. Chmouliovsky L, Habicht F, James RW, Lehmann T, Campana A, Golay A. Beneficial effect of hormone replacement therapy on weight loss in obese menopausal women. Maturitas. 1999 Aug 16;32(3):147-53. 17. Available at: http://www.karger.com/gazette/66/mcewen/art_05.htm. Accessed January 10, 2005. 18. Simoncini T, Mannella P, Fornari L, et al. Differential signal transduction of progesterone and medroxyprogesterone acetate in human endothelial cells. Endocrinology. 2004 Dec; 145(12):5745-56. 19. Beckham JC, Krug LM, Penzien DB, et al. The relationship of ovarian steroids, headache activity and menstrual distress: a pilot study with female migraineurs. Headache. 1992 Jun;32(6):292-7. 20. Smith SS, Waterhouse BD, Chapin JK, Woodward DJ. Progesterone alters GABA and glutamate responsiveness: a possible mechanism for its anxiolytic action. Brain Res. 1987 Jan 6;400(2):353-9. 21. Gulinello M, Smith SS. Anxiogenic effects of neurosteroid exposure: sex differences and altered GABAA receptor pharmacology in adult rats. J Pharmacol Exp Ther. 2003 May;305(2):541-8. 22. Rupprecht R. Neuroactive steroids: mechanisms of action and neuropsychopharmacological properties. Psychoneuroendocrinology. 2003 Feb;28(2):139-68. 23. Le Melledo JM, Baker G. Role of progesterone and other neuroactive steroids in anxiety disorders. Expert Rev Neurother. 2004 Sep;4(5):851-60. 24. Hsu FC, Smith SS. Progesterone withdrawal reduces paired-pulse inhibition in rat hippocampus: dependence on GABA(A) receptor alpha4 subunit upregulation. J Neurophysiol. 2003 Jan;89(1):186-98. 25. Lipton RB, Stewart WF. Migraine in the United States: a review of epidemiology and health care use. Neurology. 1993 Jun;43(6 Suppl 3):S6-10. 26. Fettes I. Migraine in the menopause. Neurology. 1999;53(4 Suppl 1):S29-33. 27. Silberstein SD, Merriam GR. Sex hormones and headache. J Pain Symptom Manage. 1993 Feb;8(2):98-114. 28. Li W, Zheng T, Altura BM, Altura BT. Sex steroid hormones exert biphasic effects on cytosolic magnesium ions in cerebral vascular smooth muscle cells: possible relationships to migraine frequency in premenstrual syndromes and stroke incidence. Brain Res Bull. 2001 Jan 1;54(1):83-9. 29. Tolsa JF, Gao Y, Raj JU. Developmental change in magnesium sulfate-induced relaxation of rabbit pulmonary arteries. J Appl Physiol. 1999 Nov;87(5):1589-94. 30. O’Shaughnessy A, Muneyyirci-Delale O, Nacharaju VL, et al. Circulating divalent cations in asymptomatic ovarian hyperstimulation and in vitro fertilization patients. Gynecol Obstet Invest. 2001;52(4):237-42. 31. Turner RT, Colvard DS, Spelsberg TC. Estrogen inhibition of periosteal bone formation in rat long bones: down-regulation of gene expression for bone matrix proteins. Endocrinology. 1990 Sep;127(3):1346-51. 32. Turner RT, Backup P, Sherman PJ, Hill E, Evans GL, Spelsberg TC. Mechanism of action of estrogen on intramembranous bone formation: regulation of osteoblast differentiation and activity. Endocrinology. 1992 Aug;131(2):883-9. 33. Barengolts EI, Kouznetsova T, Segalene A, et al. Effects of progesterone on serum levels of IGF-1 and on femur IGF-1 mRNA in ovariectomized rats. J Bone Miner Res. 1996 Oct;11(10):1406-12. 34. Cowan LD, Gordis L, Tonascia JA, Jones GS. Breast cancer incidence in women with a history of progesterone deficiency. Am J Epidemiol. 1981 Aug;114(2):209-17. 35. Formby B, Wiley TS. Progesterone inhibits growth and induces apoptosis in breast cancer cells: inverse effects on Bcl-2 and p53. Ann Clin Lab Sci. 1998 Nov-Dec;28(6):360-9. 36. Creasman WT. Hormone replacement therapy after cancers. Curr Opin Oncol. 2005 Sep;17(5):493-9. 37. Medina RA, Meneses AM, Vera JC, et al. Differential regulation of glucose transporter expression by estrogen and progesterone in Ishikawa endometrial cancer cells. J Endocrinol. 2004 Sep;182(3):467-78. 38. De Vivo I, Huggins GS, Hankinson SE, et al. A functional polymorphism in the promoter of the progesterone receptor gene associated with endometrial cancer risk. Proc Natl Acad Sci USA. 2002 Sep 17;99(19):12263-8. 39. La Vecchia C, Brinton LA, McTiernan A. Cancer risk in menopausal women. Best Pract Res Clin Obstet Gynaecol. 2002 Jun;16(3):293-307. 40. Southcott BM. Carcinoma of the endometrium. Drugs. 2001;61(10):1395-405. 41. Mahavni V, Sood AK. Hormone replacement therapy and cancer risk. Curr Opin Oncol. 2001 Sep;13(5):384-9. 42. Beresford SA, Weiss NS, Voigt LF, McKnight B. Risk of endometrial cancer in relation to use of oestrogen combined with cyclic progestagen therapy in postmenopausal women. Lancet. 1997 Feb 15;349(9050):458-61. 43. Ravn SH, Rosenberg J, Bostofte E. Postmenopausal hormone replacement therapy—clinical implications. Eur J Obstet Gynecol Reprod Biol. 1994 Feb;53(2):81-93. 44. Samsioe G. The endometrium: effects of estrogen and estrogen-progestogen replacement therapy. Int J Fertil Menopausal Stud. 1994;39 Suppl 2:84-92. 45. Mohr PE, Wang DY, Gregory WM, Richards MA, Fentiman IS. Serum progesterone and prognosis in operable breast cancer. Br J Cancer. 1996 Jun;73(12):1552-5. 46. Sodi R, Fikri R, Diver M, Ranganath L, Vora J. Testosterone replacement-induced hyperprolactinaemia: case report and review of the literature. Ann Clin Biochem. 2005 Mar;42(Pt 2):153-9. 47. Plourde PV, Reiter EO, Jou HC, et al. Safety and efficacy of anastrozole for the treatment of pubertal gynecomastia: a randomized, double-blind, placebo-controlled trial. J Clin Endocrinol Metab. 2004 Sep;89(9):4428-33. 48. Comhaire F, Mahmoud A. Preventing diseases of the prostate in the elderly using hormones and nutriceuticals. Aging Male. 2004 Jun;7(2):155-69. 49. Tilakaratne A, Soory M. Effects of the anti-androgen finasteride on 5 alpha-reduction of androgens in the presence of progesterone in human gingival fibroblasts: modulatory actions of the alkaline phosphatase inhibitor levamisole. J Periodontal Res. 2000 Aug;35(4):179-85. 50. Available at: www.cdc.gov/nchs/data/nvsr/nvsr53/nvsr53_06.pdf. Accessed October 4, 2005. 51. Apgar BS, Greenberg G. Using progestins in clinical practice. Am Fam Physician. 2000 Oct 15;62(8):1839-50. |