Life Extension Magazine®
Prostate disorders wreak havoc on the majority of aging men. Scientists have identified nutrients and drugs that alleviate symptoms of benign enlargement and reduce prostate cancer risk. A plant extract called beta-sitosterol may be of particular benefit. Published studies indicate that beta-sitosterol consistently improves urinary symptoms related to prostate enlargement. The good news is that beta-sitosterol costs so little that men can increase their consumption without spending extra dollars. For the past several decades, European doctors have routinely prescribed a variety of plant-based drugs to treat benign prostate enlargement and lower urinary tract symptoms. Saw palmetto, pygeum, and nettle root extracts are common plant-based drugs prescribed to millions of men in Europe. Consumers in the U.S. have open access to these same nutrients that are approved as drugs in Europe to combat urinary symptoms of benign prostate enlargement. Beta-sitosterol is a plant fat contained in several European prostate drugs, though it is not routinely used in the United States. Multiple randomized studies have confirmed the efficacy of beta-sitosterol in alleviating the types of prostate discomfort that aging men so frequently encounter. Measuring Symptoms of Prostate EnlargementIn order for scientists around the world to evaluate the efficacy of a particular therapy, certain testing standards have been established. One of the most common standards is the International Prostate Symptom Score (IPSS). The score is stated as a number that can range from 0 to 35, depending on the severity of lower urinary tract symptoms. The International Prostate Symptom Score also includes a scoring of quality of life as it relates to urinary symptoms. A measurement to assess the strength of the urinary stream is called the maximum urinary flow rate (Qmax). The maximum urinary flow is commonly decreased with benign prostate disease such as BPH (benign prostatic hyperplasia). The third test is the amount of residual urine that remains in the bladder after voiding, or post-void residual urine (PVR). This is most easily assessed with pre- and post-void ultrasounds of the bladder.
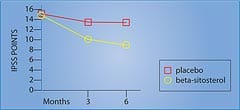
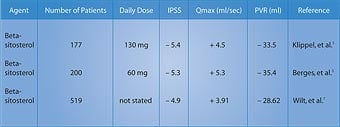
Remarkable Effects of Beta-SitosterolIn a randomized, double-blind, placebo-controlled, multicenter study of 200 men with benign prostate enlargement, half the group received 180 mg of beta-sitosterol daily, while the other half received placebo. After six months, the beta-sitosterol group saw improvement in the International Prostate Symptom Score, the measurement of urine flow (Qmax), and the amount of residual urine remaining in the bladder (PVR).1 The beta-sitosterol group showed a 7.4-point decrease in the International Prostate Symptom Score, compared to a decrease of only 2.1 points in the placebo group. This was a significant 3.5-fold improvement in the men taking beta-sitosterol (Figure 1).1 The measurement of urinary flow increased to an average of 15.2 milliliters (ml) per second from 9.9 ml/second in the men receiving beta- sitosterol. The placebo group only increased to 11.4 ml/second from 10.2 ml/second at baseline. Urinary flow thus improved almost 35% in the group taking beta-sitosterol, compared to only 11% in the placebo group.1 Most remarkably, residual urine in the bladder decreased to 30.4 ml from 65.8 ml in the men using beta-sitosterol ... a reduction of almost 54%! In the placebo group, residual bladder urine declined from 64.8 ml to 54.3 ml ... a reduction of only around 16%.1 In a follow-up study that evaluated durability of response to beta-sitosterol, the beneficial effects for beta-sitosterol were found to be maintained during an additional 18 months of observation.2 Figure 1 shows the significant difference in the International Prostate Symptom Score in men receiving beta-sitosterol compared to placebo. Benefits of Beta-Sitosterol ConfirmedTo confirm these remarkable effects of beta-sitosterol, another study was performed and the results were published in the British Journal of Urology. The study involved 177 patients with benign prostate enlargement. Patients received 130 mg of beta-sitosterol each day and were monitored for more than six months. Measurements of the International Prostate Symptom Score, urinary flow, and residual urine in the bladder after voiding were recorded.3 On average, urinary flow values increased by 4.5 ml/second while residual urine volumes decreased by a substantial 33.5 ml. The International Prostate Symptom Scores showed a statistically significant improvement. These results with beta-sitosterol are comparable to those seen with the commonly prescribed drug Proscar®, used to treat benign prostate enlargement.3
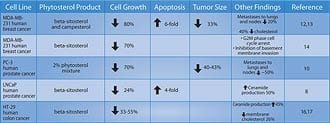
Two years later, a review of all existing studies of beta-sitosterol in the treatment of benign prostate enlargement was conducted. The researchers identified randomized, placebo-controlled, double-blind trials involving a total of 519 men. In three of the trials, beta-sitosterol was used, and in one trial, a glucoside of beta-sitosterol was used. In these studies, beta-sitosterol improved urinary symptom scores and urinary flow rates, and significantly reduced the volume of residual urine in the bladder.4,5 Table 1 summarizes some of the randomized studies of beta-sitosterol in the treatment of BPH. The magnitude of reduction in prostate symptoms and improvement in urinary flow rates is a strong incentive for the use of beta-sitosterol, either alone or in combination with standard pharmacologic interventions such as alpha-adrenergic blockers (Cardura®, Hytrin®, Uroxatral®, Flomax®) or 5-alpha reductase inhibitors (Proscar®, Avodart®). Beta-Sitosterol and Prostate CancerA study using the prostate cancer cell line LNCaP (an androgen dependent tumor) showed that beta-sitosterol decreased cancer cell growth by 24% and induced apoptosis (programmed cell death) four-fold. These findings were correlated with a 50% increase in ceramide production.6 Research suggests that ceramide, an important component of the cell membrane, induces apopotosis.7
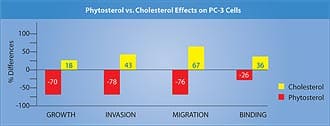
Growth of the human prostate cancer PC-3 cell line (androgen independent) implanted in mice was compared in two groups receiving either a 2% phytosterol mixture or a cholesterol mixture. In the in vitro studies, both beta-sitosterol and campesterol inhibited the growth of PC-3 cells by 70% and 14%, respectively. Cholesterol supplementation, by contrast, increased the growth by 18% when compared with controls.8 Phytosterols inhibited the invasion of PC-3 cells into Matrigel-coated membranes by 78% (a measure of cancer invasiveness), while cholesterol increased it by 43% compared to the cells in the control media.8 Migration of tumor cells through 8-micron pore membranes (a measure of tumor motility) was reduced by 60-93% when the PC-3 cells were in phytosterol media, compared to a 67% increase after cholesterol supplementation.8 Phytosterol supplementation reduced the binding of PC-3 cells to laminin by 15-38% and to fibronectin by 23%, while cholesterol increased binding to type IV collagen (a measure of adhesiveness and ability to form tumor clumps) by 36%.8 The results are presented in Figure 2.
The researchers concluded that phytosterol indirectly (in vivo as a dietary supplement) and directly (in tissue culture media) inhibited the growth and metastasis of PC-3 cells. Beta-sitosterol was more effective than campesterol in offering this protection in most of the parameters studied.8 Results of this study and other cell line trials involving phytosterols in cancer are shown in Table 2 shown on this page. Combining Beta-Sitosterol and ResveratrolDr. Atif B. Awad and his team at the State University of New York at Buffalo have found that beta-sitosterol inhibits prostate and breast cancer cell growth, and induces cell cycle arrest. Yet, since their studies were published in 2000-2001, little research has been done to determine the mechanisms by which this occurs.8-12 In the March 2005 issue of the journal Prostaglandins, Leukotrienes and Essential Fatty Acids, Awad and his team published cutting-edge findings of the possible mechanisms by which resveratrol and beta-sitosterol inhibit the growth of human prostate cancer cells.13 After researchers supplemented prostate cancer cells with resveratrol and beta-sitosterol, they reported that long-term supplementation with either beta-sitosterol or resveratrol elevated basal prostaglandin release, but that beta-sitosterol was more potent in this regard. Of the two phytochemicals, beta-sitosterol was also more effective in inducing apoptosis and inhibiting the growth at concentrations tested. The scientists concluded that these phytochemicals may induce the inhibition of tumor growth by stimulating apoptosis and arresting cells at different locations in the cell cycle, and that the mechanism may involve alterations in reactive oxygen species and prostaglandin production.13
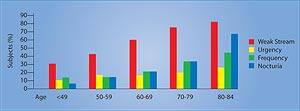
ConclusionAs men grow older, cells in their prostate glands often overgrow, causing a swelling that obstructs the bladder opening, resulting in slowness in urination and bladder emptying. This non-malignant enlargement of the prostate gland causes pressure on the urethra, acting like a clamp. The result is a weak urinary stream, hesitancy, and other uncomfortable urinary symptoms such as increased nighttime frequency and urgency. See Figure 3. While a man aged 31 to 40 has only an 8% chance of having benign prostate enlargement, the risk increases to 40-50% in men aged 51 to 60 and to over 80% in men older than 80.14 For millions of men in America, benign prostate enlargement will severely downgrade their quality of life. Yet across the ocean are drugs that have been shown in carefully controlled studies to alleviate much of the discomfort associated with prostate gland overgrowth. Europeans use beta-sitosterol by itself or in combination with saw palmetto to alleviate urinary symptoms of benign prostate enlargement. As the word gets out about the documented benefits of beta-sitosterol, American consumers can expect to see more prostate support products that contain this low-cost plant sterol. | |||||||||||
| References | |||||||||||
| 1. Berges RR, Kassen A, Senge T. Treatment of symptomatic benign prostatic hyperplasia with beta-sitosterol: an 18-month follow-up. BJU Int. 2000 May;85(7):842-6. 2. Berges RR, Windeler J, Trampisch HJ, Senge T. Randomised, placebo-controlled, double-blind clinical trial of beta-sitosterol in patients with benign prostatic hyperplasia. Beta-sitosterol Study Group. Lancet. 1995 Jun 17;345(8964):1529-32. 3. Klippel KF, Hiltl DM, Schipp B. A multicentric, placebo-controlled, double-blind clinical trial of beta-sitosterol (phytosterol) for the treatment of benign prostatic hyperplasia. German BPH-Phyto Study group. Br J Urol. 1997 Sep;80(3):427-32. 4. Wilt TJ, MacDonald R, Ishani A. beta-sitosterol for the treatment of benign prostatic hyperplasia: a systematic review. BJU Int. 1999 Jun;83(9):976-83. 5. Wilt T, Ishani A, MacDonald R, Stark G, Mulrow C, Lau J. Beta-sitosterols for benign prostatic hyperplasia. Cochrane Database Syst Rev. 2000;(2):CD001043. 6. von Holtz RL, Fink CS, Awad AB. Beta-Sitosterol activates the sphingomyelin cycle and induces apoptosis in LNCaP human prostate cancer cells. Nutr Cancer. 1998;32(1):8-12. 7. Duan RD. Anticancer compounds and sphingolipid metabolism in the colon. In Vivo. 2005 Jan-Feb;19(1):293-300. 8. Awad AB, Fink CS, Williams H, Kim U. In vitro and in vivo (SCID mice) effects of phytosterols on the growth and dissemination of human prostate cancer PC-3 cells. Eur J Cancer Prev. 2001 Dec;10(6):507-13. 9. Awad AB, Gan Y, Fink CS. Effect of beta-sitosterol, a plant sterol, on growth, protein phosphatase 2A, and phospholipase D in LNCaP cells. Nutr Cancer. 2000;36(1):74-8. 10. Awad AB, Downie A, Fink CS, Kim U. Dietary phytosterol inhibits the growth and metastasis of MDA-MB-231 human breast cancer cells grown in SCID mice. Anticancer Res. 2000 Mar;20(2A):821-4. 11. Awad AB, Downie AC, Fink CS. Inhibition of growth and stimulation of apoptosis by beta-sitosterol treatment of MDA-MB-231 human breast cancer cells in culture. Int J Mol Med. 2000 May;5(5):541-5. 12. Awad AB, Williams H, Fink CS. Phytosterols reduce in vitro metastatic ability of MDA-MB-231 human breast cancer cells. Nutr Cancer. 2001;40(2):157-64. 13. Awad AB, Burr AT, Fink CS. Effect of resveratrol and beta-sitosterol in combination on reactive oxygen species and prostaglandin release by PC-3 cells. Prostaglandins Leukot Essent Fatty Acids. 2005 Mar;72(3):219-26. 14. Glynn RJ, Campion EW, Bouchard GR, Silbert JE. The development of benign prostatic hyperplasia among volunteers in the Normative Aging Study. Am J Epidemiol. 1985 Jan;121(1):78-90. |