Life Extension Magazine®
One reason I battle the medical establishment is that I was a victim at an early age. Due to ignorance and fraud, my DNA was subjected to significant damage. When I was just 27 years old, I was checked into a hospital because of heartbeat irregularities. The cardiologist insisted that I undergo an angiogram, a procedure that involved threading a catheter into my heart to evaluate my arteries and valves. An X-ray that emits very high amounts of radiation guided the catheter. My diagnosis was mitral valve prolapse, a relatively benign condition that may affect up to 18% of women and 12% of men.1 The proper diagnostic procedure would have been a $300 ultrasound, as opposed to the $7,000 angiogram. My insurance company was defrauded of this money, but I have to live with the fact that my DNA was needlessly exposed to a huge amount of radiation. We at Life Extension long ago warned our members to avoid unnecessary X-rays.2,3 Our rationale has been that even low-level radiation damages DNA in a way that can lead to cancer decades later. Conventional doctors have ridiculed our position that medical X-rays are dangerous. A new report by the National Academy of Sciences shows the medical establishment has underestimated the cancer risks posed by radiation. After you read this article, I hope you will acquire the information and fortitude to say no the next time your doctor tries to perform an unnecessary X-ray. Unsafe at Any DoseDoctors argue that the amount of radiation emitted from medical X-rays is so low that there is no cancer risk. This flies in the face of data showing that any amount of radiation inflicts free radical damage to DNA and adversely affects our genes.4-6 Several years ago, we reported statistics indicating that a significant percentage of today’s cancers are caused by medical radiation.7-11 These radiation-induced cancers are caused by mutations in DNA genes that regulate cellular proliferation. While doctors state that radiation is safe as long as it is kept at a certain level, we argued that even the tiniest particle of radiation inflicts DNA damage. For radiation to be safe, all of the DNA damage must be repaired perfectly. Any damage imperfectly repaired creates mutations, any one of which has the potential to cause cancer. In fact, we pointed to research showing that the lowest possible dose of radiation not only is unsafe, but also does far more damage than previously thought and is indeed mutagenic.12,13
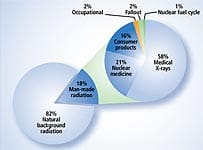
National Academy of Sciences ReportIn June 2005, the National Academy of Sciences released a report stating that even very low doses of radiation can cause cancer. In its report, the National Academy defined low dose as being as low as “near zero.” While the researchers stated that the cancer risk from any given X-ray is very small, their report stated: “Risk would continue at a linear fashion at lower doses without a threshold and that the smallest dose has the potential to cause a small increase in risk to humans.”14 As you can see by the chart on this page, the amount of radiation emitted from a typical medical X-ray is quite low. Typical X-rays, however, are becoming a relic of the past. CT (computerized tomography) scans provide a much better picture of your insides by using much more radiation. A CT scan of your abdomen, for example, exposes you to an amount of radiation equivalent to that of 500 chest X-rays.15 The most worrisome diagnostic procedure is the whole body scan, which experts have estimated is the equivalent of 900 chest X-rays. According to the National Academy of Sciences report, a 45-year-old who planned to undergo 30 annual whole body scans would potentially increase his or her cancer risk to 1 in 50.16 The National Academy of Sciences stated that there is no radiation threshold below which exposure can be viewed as harmless. This finding means that everyone who has had a medical X-ray is at some increased risk for contracting leukemia or a solid cancer. Considering how many X-rays people are exposed to in a lifetime, the risk of contracting cancer from the cumulative effects of many X-rays and CT scans is a serious concern.
My Angiogram: Cause of a Future Heart Attack?John W. Gofman, MD, PhD, Professor Emeritus of Molecular and Cell Biology at the University of California, Berkeley, is one of the world’s most distinguished medical and nuclear scientists. His research shows that no amount of radiation—no matter how small—is safe.18 Dr. Gofman’s data analysis conflicts with the National Academy of Sciences from the standpoint that he believes far more cancers are caused by medical radiation.
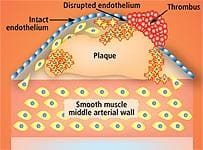
Further, he has come to the conclusion that exposure to radiation from medical procedures is a “highly important (probably principal) cause” of cancer and ischemic heart disease in America.18 How would radiation cause heart disease? According to Dr. Gofman, the same way it causes cancer. Radiation damages DNA—in this case, DNA in the arteries. The radiation-induced changes create a cancer-like phenomenon in the arteries known as atheroma.19,20 Dr. Gofman believes that the interaction between atheromas and lipids blocks arteries and causes blood clots. One of radiation’s most striking effects is causing arterial cells to multiply abnormally. The abnormal growth of cells lining the arteries has the effect of narrowing the arteries. Abnormal growth of smooth muscle tissue inside the artery creates something similar to scar tissue that occludes the arteries and ruins their flexibility. It is not cholesterol that “clogs” arteries—it is abnormal cell growth that narrows arteries. Lipid-laden cells, monocytes, macrophages, cholesterol, fibrin, collagen, elastin, and calcium are all components of plaques, and collect within damaged areas in the inner arterial wall. As early as 1944, scientists showed that radiation could produce plaques and foam cells.21 Since then, additional studies have demonstrated that radiation can produce arterial lesions, sticky platelets, and increased free radicals.22-26 In fact, radiation can create atherosclerosis in its entirety.27 Studies show that people who have undergone radiation of areas containing major blood vessels often develop atherosclerosis in those blood vessels.28,29 So I hope you understand why I am so angry with the medical establishment. At the age of 27, doctors intentionally doused my heart with huge amounts of radiation in a medical procedure that made them a lot of money. This radiation makes it more likely I will develop coronary artery disease. As a result of this medically induced insult, I have to take extraordinary measures to keep all other heart attack risk factors in check.
A Lethal Misconception About AtherosclerosisThe number-one killer in the United States is atherosclerosis, which is the cause of most heart attacks and strokes.30-32 Yet doctors are utterly confused as to how this artery-blocking process occurs. Most cardiologists overlook the specific mechanism that inflicts arterial wall damage and the ensuing progression to occlusive atherosclerotic disease. Most doctors think of an atherosclerotic lesion as a “clog” consisting of fat, cholesterol, and platelets that have accumulated on an arterial wall. So doctors tell patients to eat less fat, take a statin drug (if cholesterol levels are high), and use low-dose (baby) aspirin to prevent platelet aggregation. The problem with these approaches is that while they may postpone a heart attack or stroke, they fail to completely correct the underlying pathology that causes atherosclerotic lesions to form and progress. If we are to be free of the ravages of atherosclerosis in our later years, this lethal misconception must be cleared up. Otherwise, there will be an epidemic of people in their seventies, eighties, and nineties receiving coronary “stents,” undergoing full-blown coronary bypass surgery, or dropping dead from heart attacks, strokes, kidney failure, and other disorders. |
||||||||||||||||||||||||||||||||||||||||||||||||||||
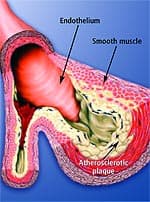
The Fragile Arterial WallA popular medical website describes arteries as “hollow tubes” that are “smooth and elastic, allowing blood to flow freely.”33 This is how healthy arteries look to the naked eye. Under a microscope, however, you see what the illustration on this page shows: that the inner arterial wall comprises a thin layer of cells called the endothelium that acts as a barrier to keep the smooth muscle in the artery from direct contact with the blood. This barrier is important because many blood components are highly toxic to arterial smooth muscle that lies directly beneath the endothelium. When these damaging blood components attack the smooth muscle of the artery, the process of atherosclerosis is initiated. Among the blood components that are damaging to the inner lining of arteries are glucose, homocysteine, low-density lipoprotein (LDL), free radicals, and pro-inflammatory cytokines.34-50 To protect the artery’s elastic smooth muscle against these damaging agents, it is critical to maintain an intact and properly functioning endothelium (or endothelial lining). The endothelium can become dysfunctional at an early age because of poor health habits such as cigarette smoking, bad diet, and nutritional deficiencies.51-57 The resulting disorder is called endothelial dysfunction, in which the cells that line the blood vessels fail to respond normally to increases in blood flow. The most important term that you may ever learn in your life is endothelial dysfunction. It is the pathological condition that causes more Americans to suffer disability and death than any other phenomenon. Atherosclerosis is both initiated and accelerated via the destructive process of endothelial dysfunction. Endothelial dysfunction can also be initiated by hypertension, free radical activity, chronic inflammation, and elevated homocysteine.37-44,58-77 Even those who practice a healthy lifestyle develop endothelial dysfunction if they live long enough.
Protecting Against Endothelial DysfunctionUntil now, aging people could slow the breakdown of the delicate endothelium, but could not adequately prevent it. Folic acid, vitamin C, fish oil, and R-lipoic acid are just a few of the nutrients that help to maintain healthy endothelial function.82-117 It is no coincidence that these same nutrients have been shown to reduce cardiovascular incidence in both animals and humans.118-122 Statin drugs and agents that suppress chronic inflammation also help protect the endothelium. The most significant breakthrough in preventing and treating atherosclerosis may be the discovery of a nutrient combination that maintains healthy endothelial function. What This Means to Aging HumansPeople who lead a healthy lifestyle often develop a false sense of security that they will not suffer from circulatory disorders. One reason for this optimism is that sudden-death heart attack rates have declined dramatically since the mid-1960s.123 Much of this can be attributed to declining tobacco use, improved diets, better control of hypertension, and increased use of supplements and drugs that lower heart attack risk.123,124 What is happening is that people who would have suffered from blocked coronary arteries or a heart attack at the age of 50 are delaying this problem until later in life. Atherosclerosis is usually associated with heart attack risk, but the scientific literature reveals that many disorders of aging are also related to circulatory disorders, including kidney impairment and memory loss.125-127 Sales of sex-enhancing drugs are spiraling mainly because so many men suffer from endothelial dysfunction that causes erectile dysfunction.128-131 In this issue of Life Extension, we introduce the first multi-modal therapy designed to help prevent or partially reverse endothelial dysfunction. Cardiologists may initially reject this approach, as they did 22 years ago when we first recommended the use of low-dose aspirin, folic acid, and coenzyme Q10 to help prevent heart attacks. The science behind this new concept of protecting against endothelial dysfunction is compelling. We predict that in the future, cardiologists will prescribe this novel therapy to a greater extent than they now do aspirin. The Value of InformationWhen a cardiologist told me that I needed an angiogram at the age of 27, there was no one to turn to for guidance. Conventional medicine ruled in that era, and doctors were seldom challenged. If I could have just called an organization like the Life Extension Foundation back then, I would have been told that the ultrasound diagnostic procedure was far safer. As a Life Extension member, you are armed with cutting-edge information that can enable you to make medical choices based on hard science—not on antiquated dogma or financial bias. While some medical X-rays are unavoidable, you should inquire as to whether an ultrasound (sonogram), MRI (magnetic resonance imaging), or MRA (magnetic resonance angiography) might provide alternative imaging. You might also question whether a particular X-ray is necessary, as doctors often prescribe them merely to protect themselves from liability. This may be good for doctors as it confirms their diagnosis, but bad for you as your DNA can sustain irreversible damage. Heart scans, CT scans, whole body scans, PET scans, and virtual colonoscopies all emit tremendous amounts of radiation and should not be used for routine screening. Even MRIs and MRAs emit electromagnetic radiation, but this may not be as dangerous as the ionizing radiation emitted by CT scans. Ultrasounds are completely safe. Most people have never heard the term “endothelial dysfunction,” yet it is the leading cause of disability and death in the Western world. In this month’s issue, you will be the first to learn about specific steps you can take to mitigate this epidemic of arterial occlusive disease. For longer life, William Faloon | |||||
| References |
| 1. Available at: http://www.nursing.wright.edu/ practice/mvp/default.htm. Accessed July 29, 2005. 2. Available at: http://www.lifeextension.com/magazine/ mag2003/feb2003_awsi_01.html. Accessed July 29, 2005. 3. Available at: http://www.lifeextension.com/magazine/ mag2004/mar2004_awsi_death_01.htm. Accessed July 29, 2005. 4. Sutherland BM, Bennett PV, Sidorkina O, Laval J. Clustered DNA damages induced in isolated DNA and in human cells by low doses of ionizing radiation. Proc Natl Acad Sci USA. 2000 Jan 4;97(1):103-8. 5. Boudaiffa B, Cloutier P, Hunting D, Huels MA, Sanche L. Resonant formation of DNA strand breaks by low-energy (3 to 20 eV) electrons. Science. 2000 Mar 3;287(5458):1658-60. 6. Haber D. Roads leading to breast cancer. N Engl J Med. 2000 Nov 23;343(21):1566-8. 7. Available at: http://www.lifeextension.com/magazine/ mag2001/nov2001_report_radiation_01.html. Accessed July 29, 2005. 8. Boice JD, Jr., Monson RR. Breast cancer in women after repeated fluoroscopic examinations of the chest. J Natl Cancer Inst. 1977 Sep;59(3):823-32. 9. Gridley DS, Andres ML, Slater JM. Enhancement of prostate cancer xenograft growth with whole-body radiation and vascular endothelial growth factor. Anticancer Res. 1997 Mar;17(2A):923-8. 10. Available at: http://www.ratical.org/radiation/CNR/RMP/6critiques.html. Accessed July 29, 2005. 11. Available at: http://www.ratical.org/radiation/CNR/RMP/. Accessed July 29, 2005. 12. Available at: http://www.ratical.org/radiation/CNR/RIC/. Accessed July 29, 2005. 13. Available at: http://www.msnbc.msn.com/ id/8389834/. Accessed July 29, 2005. 14. Available at: http://www4.nationalacademies.org/news.nsf/isbn/030909156X?OpenDocument. Accessed July 29, 2005. 15. Available at: http://europa.eu.int/comm/environment/radprot/118/rp-118-en.pdf. Accessed July 29, 2005. 16. Available at: http://www.nap.edu/books/ 030909156X/html/10.html. Accessed July 29, 2005. 17. Available at: http://europa.eu.int/comm/environment/radprot/118/rp-118-en.pdf. Accessed July 29, 2005. 18. Available at: http://www.ratical.org/radiation/CNR/RMP/index.html. Accessed July 29, 2005. 19. Available at: http://cancerweb.ncl.ac.uk/cgi-bin/omd?query=atheromas. Accessed July 29, 2005. 20. Sheehan JF. Foam cell plaques in the intima of irradiated small arteries. Arch Path. 1944;379:297-08. 21. Cheng SW, Ting AC, Wu LL. Ultrasonic analysis of plaque characteristics and intimal-medial thickness in radiation-induced atherosclerotic carotid arteries. Eur J Vasc Endovasc Surg. 2002 Dec;24(6):499-504. 22. Dossing M, Rasmussen S, Fischer-Hansen B, Walbom-Jorgensen S. Radiation-induced lesions of the aorta. Br Med J. 1977 Apr 9;1(6066):973. 23. Tribble DL, Barcellos-Hoff MH, Chu BM, Gong EL. Ionizing radiation accelerates aortic lesion formation in fat-fed mice via SOD-inhibitable processes. Arterioscler Thromb Vasc Biol. 1999 Jun;19(6):1387-92. 24. Notaristefano S, Giombolini C, Santucci S, et al. Radiation-induced ostial stenosis of the coronary artery as a cause of acute coronary syndromes: a novel mechanism of thrombus formation? Ital Heart J. 2003 May;4(5):341-4. 25. Takewa Y, Kawata T, Yoshida Y, Kawachi K, Kitamura S. Radiation-induced coronary ostial stenosis, a case of redo coronary bypass for the restenosis following patch angioplasty. Nippon Kyobu Geka Gakkai Zasshi. 1996 Feb;44(2):220-5. 26. Verheij M, Dewit LG, Boomgaard MN, Brinkman HJ, van Mourik JA. Ionizing radiation enhances platelet adhesion to the extracellular matrix of human endothelial cells by an increase in the release of von Willebrand factor. Radiat Res. 1994 Feb;137(2):202-7. 27. Hicks GL, Jr. Coronary artery operation in radiation-associated atherosclerosis: long-term follow-up. Ann Thorac Surg. 1992 Apr;53(4):670-4. 28. Elkeles A. Metabolic behavior of alpha-ray activity in large human arteries: relationship to atherosclerosis. J Am Geriatr Soc. 1977 Apr;25(4):179-85. 29. Elkeles A. Alpha-ray activity in the large human arteries. Nature. 1969 Feb 15;221(181):662-4. 30. Available at: http://www.nap.edu/nap-cgi/ skimit.cgi?isbn=0309040493&chap=57-76. Accessed July 29, 2005. 31. Available at: http://www.cdc.gov/nchs/about/ major/dvs/mortdata.htm. Accessed July 29, 2005. 32. Available at: http://www.merck.com/mmhe/ sec03/ch033/ch033a.html. Accessed July 29, 2005. 33. Available at: http://my.webmd.com/content/pages/9/1675_57851.htm. Accessed July 29, 2005. 34. MacDonald-Wicks L, Gibson LZ, Godfrey DM, et al. Oxidised LDL in newly diagnosed type 2 diabetes mellitus and impaired glucose tolerance. Asia Pac J Clin Nutr. 2004;13(Suppl):S65. 35. Ceriello A. Impaired glucose tolerance and cardiovascular disease: the possible role of post-prandial hyperglycemia. Am Heart J. 2004 May;147(5):803-7. 36. Bots ML, Launer LJ, Lindemans J, Hofman A, Grobbee DE. Homocysteine, atherosclerosis and prevalent cardiovascular disease in the elderly: The Rotterdam Study. J Intern Med. 1997 Oct;242(4):339-47. 37. Folsom AR, Nieto FJ, McGovern PG, et al. Prospective study of coronary heart disease incidence in relation to fasting total homocysteine, related genetic polymorphisms, and B vitamins: the Atherosclerosis Risk in Communities (ARIC) study. Circulation. 1998 Jul 21;98(3):204-10. 38. Montalescot G, Ankri A, Chadefaux-Vekemans B, et al. Plasma homocysteine and the extent of atherosclerosis in patients with coronary artery disease. Int J Cardiol. 1997 Aug 8;60(3):295-300. 39. Stampfer MJ, Malinow MR, Willett WC, et al. A prospective study of plasma homocyst(e)ine and risk of myocardial infarction in US physicians. JAMA. 1992 Aug 19;268(7):877-81. 40. Verhoef P, Stampfer MJ, Buring JE, et al. Homocysteine metabolism and risk of myocardial infarction: relation with vitamins B6, B12, and folate. Am J Epidemiol. 1996 May 1;143(9):845-59. 41. Robinson K, Mayer EL, Miller DP, et al. Hyperhomocysteinemia and low pyridoxal phosphate. Common and independent reversible risk factors for coronary artery disease. Circulation. 1995 Nov 15;92(10):2825-30. 42. Arnesen E, Refsum H, Bonaa KH, et al. Serum total homocysteine and coronary heart disease. Int J Epidemiol. 1995 Aug;24(4):704-9. 43. Aronow WS, Ahn C. Association between plasma homocysteine and coronary artery disease in older persons. Am J Cardiol. 1997 Nov 1;80(9):1216-8. 44. Berwanger CS, Jeremy JY, Stansby G. Homocysteine and vascular disease. Br J Surg. 1995 Jun;82(6):726-31. 45. Bostom AG, Rosenberg IH, Silbershatz H, et al. Nonfasting plasma total homocysteine levels and stroke incidence in elderly persons: the Framingham Study. Ann Intern Med. 1999 Sep 7;131(5):352-5. 46. Ridker PM, Rifai N, Rose L, Buring JE, Cook NR. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N Engl J Med. 2002 Nov 14;347(20):1557-65. 47. Kanani PM, Sinkey CA, Browning RL, et al. Role of oxidant stress in endothelial dysfunction produced by experimental hyperhomocyst(e)inemia in humans. Circulation. 1999 Sep 14;100(11):1161-8. 48. Van der Meide PH, Schellekens H. Cytokines and the immune response. Biotherapy. 1996;8(3-4):243-9. 49. No author. Role of inflammatory and hemostatic mediators in preclinical endothelial dysfunction: Relevance to high-risk patients with hypertension. JACC. 2002 Mar 6;39(5):209A. 50. Tentolouris C, Tousoulis D, Antoniades C, et al. Endothelial function and proinflammatory cytokines in patients with ischemic heart disease and dilated cardiomyopathy. Int J Cardiol. 2004 Apr;94(2-3):301-5. 51. Ikonomidis I, Lekakis J, Vamvakou G, Andreotti F, Nihoyannopoulos P. Cigarette smoking is associated with increased circulating proinflammatory and procoagulant markers in patients with chronic coronary artery disease: effects of aspirin treatment. Am Heart J. 2005 May;149(5):832-9. 52. Esen AM, Barutcu I, Acar M, et al. Effect of smoking on endothelial function and wall thickness of brachial artery. Circ J. 2004 Dec;68(12):1123-6. 53. Ambrose JA, Barua RS. The pathophysiology of cigarette smoking and cardiovascular disease: an update. J Am Coll Cardiol. 2004 May 19;43(10):1731-7. 54. Poreba R, Skoczynska A, Derkacz A. Effect of tobacco smoking on endothelial function in patients with coronary arteriosclerosis. Pol Arch Med Wewn. 2004 Jan;111(1):27-36. 55. Puranik R, Celermajer DS. Smoking and endothelial function. Prog Cardiovasc Dis. 2003 May;45(6):443-58. 56. Vlassara H, Cai W, Crandall J, et al. Inflammatory mediators are induced by dietary glycotoxins, a major risk factor for diabetic angiopathy. Proc Natl Acad Sci USA. 2002 Nov 26;99(24):15596-601. 57. Cuevas AM, Germain AM. Diet and endothelial function. Biol Res. 2004;37(2):225-30. 58. Chang HJ, Chung J, Choi SY, et al. Endothelial dysfunction in patients with exaggerated blood pressure response during treadmill test. Clin Cardiol. 2004 Jul;27(7):421-5. 59. Tu L, Wei W, Liu X, Deng Y, Yu S. Endothelial function and carotid artery wall thickening in patients with early essential hypertension. J Tongji Med Univ. 1999;19(4):288-90, 303. 60. Rodriguez-Porcel M, Lerman LO, Herrmann J, et al. Hypercholesterolemia and hypertension have synergistic deleterious effects on coronary endothelial function. Arterioscler Thromb Vasc Biol. 2003 May 1;23(5):885-91. 61. Higashi Y, Yoshizumi M. Exercise and endothelial function: role of endothelium-derived nitric oxide and oxidative stress in healthy subjects and hypertensive patients. Pharmacol Ther. 2004 Apr;102(1):87-96. 62. Fan J, Watanabe T. Inflammatory reactions in the pathogenesis of atherosclerosis. J Atheroscler Thromb. 2003;10(2):63-71. 63. Li JJ, Chen JL. Inflammation may be a bridge connecting hypertension and atherosclerosis. Med Hypotheses. 2005;64(5):925-9. 64. Johnson LK, Longenecker JP, Fajardo LF. Differential radiation response of cultured endothelial cells and smooth myocytes. Anal Quant Cytol. 1982 Sep;4(3):188-98. 65. Maresca G, Di Blasio A, Marchioli R, Di Minno G. Measuring plasma fibrinogen to predict stroke and myocardial infarction: an update. Arterioscler Thromb Vasc Biol. 1999 Jun;19(6):1368-77. 66. Acevedo M, Foody JM, Pearce GL, Sprecher DL. Fibrinogen: associations with cardiovascular events in an outpatient clinic. Am Heart J. 2002 Feb;143(2):277-82. 67. Ridker PM, Stampfer MJ, Rifai N. Novel risk factors for systemic atherosclerosis: a comparison of C-reactive protein, fibrinogen, homocysteine, lipoprotein(a), and standard cholesterol screening as predictors of peripheral arterial disease. JAMA. 2001 May 16;285(19):2481-5. 68. Lindahl B, Toss H, Siegbahn A, Venge P, Wallentin L. Markers of myocardial damage and inflammation in relation to long-term mortality in unstable coronary artery disease. FRISC Study Group. Fragmin during Instability in Coronary Artery Disease. N Engl J Med. 2000 Oct 19;343(16):1139-47. 69. Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000 Mar 23;342(12):836-43. 70. Kuller LH, Tracy RP, Shaten J, Meilahn EN. Relation of C-reactive protein and coronary heart disease in the MRFIT nested case-control study. Multiple Risk Factor Intervention Trial. Am J Epidemiol. 1996 Sep 15;144(6):537-47. 71. Mendall MA, Strachan DP, Butland BK, et al. C-reactive protein: relation to total mortality, cardiovascular mortality and cardiovascular risk factors in men. Eur Heart J. 2000 Oct;21(19):1584-90. 72. Pasceri V, Willerson JT, Yeh ET. Direct proinflammatory effect of C-reactive protein on human endothelial cells. Circulation. 2000 Oct 31;102(18):2165-8. 73. Ridker PM, Buring JE, Shih J, Matias M, Hennekens CH. Prospective study of C-reactive protein and the risk of future cardiovascular events among apparently healthy women. Circulation. 1998 Aug 25;98(8):731-3. 74. Auer J, Berent R, Lassnig E, Eber B. C-reactive protein and coronary artery disease. Jpn Heart J. 2002 Nov;43(6):607-19. 75. Maxwell SR. Coronary artery disease—free radical damage, antioxidant protection and the role of homocysteine. Basic Res Cardiol. 2000;95 Suppl 1:I65-71. 76. Johansen JS, Harris AK, Rychly DJ, Ergul A. Oxidative stress and the use of antioxidants in diabetes: linking basic science to clinical practice. Cardiovasc Diabetol. 2005 Apr 29;4(1):5. 77. Schulz E, Anter E, Keaney JF Jr. Oxidative stress, antioxidants, and endothelial function. Curr Med Chem. 2004 May;11(9):1093-104. 78. Basta G, Lazzerini G, Massaro M, et al. Advanced glycation end products activate endothelium through signal-transduction receptor RAGE: a mechanism for amplification of inflammatory responses. Circulation. 2002 Feb 19;105(7):816-22. 79. Baynes JW. From life to death—the struggle between chemistry and biology during aging: the Maillard reaction as an amplifier of genomic damage. Biogerontology. 2000;1(3):235-46. 80. Lebovitz HE. Effect of the postprandial state on nontraditional risk factors. Am J Cardiol. 2001 Sep 20;88(6A):20H-5H. 81. Sasso FC, Carbonara O, Nasti R, et al. Glucose metabolism and coronary heart disease in patients with normal glucose tolerance. JAMA. 2004 Apr 21;291(15):1857-63. 82. Woo KS, Chook P, Chan LL, et al. Long-term improvement in homocysteine levels and arterial endothelial function after 1-year folic acid supplementation. Am J Med. 2002 May;112(7):535-9. 83. Doshi S, McDowell I, Moat S, Lewis M, Goodfellow J. Folate improves endothelial function in patients with coronary heart disease. Clin Chem Lab Med. 2003 Nov;41(11):1505-12. 84. Doshi SN, McDowell IF, Moat SJ, et al. Folic acid improves endothelial function in coronary artery disease via mechanisms largely independent of homocysteine lowering. Circulation. 2002 Jan 1;105(1):22-6. 85. Paradisi G, Cucinelli F, Mele MC, et al. Endothelial function in post-menopausal women: effect of folic acid supplementation. Hum Reprod. 2004 Apr;19(4):1031-5. 86. Pena AS, Wiltshire E, Gent R, Hirte C, Couper J. Folic acid improves endothelial function in children and adolescents with type 1 diabetes. J Pediatr. 2004 Apr;144(4):500-4. 87. Moat SJ, Lang D, McDowell IF, et al. Folate, homocysteine, endothelial function and cardiovascular disease. J Nutr Biochem. 2004 Feb;15(2):64-79. 88. Hernandez-Diaz S, Martinez-Losa E, Fernandez-Jarne E, Serrano-Martinez M, Martinez-Gonzalez MA. Dietary folate and the risk of nonfatal myocardial infarction. Epidemiology. 2002 Nov;13(6):700-6. 89. Czeizel E, Kalina A. Public health control of hyperhomocysteinemia and its consequences. Orv Hetil. 2003 Oct 5;144(40):1981-9. 90. Gokce N, Keaney JF, Jr., Frei B, et al. Long-term ascorbic acid administration reverses endothelial vasomotor dysfunction in patients with coronary artery disease. Circulation. 1999 Jun 29;99(25):3234-40. 91. Jeserich M, Schindler T, Olschewski M, et al. Vitamin C improves endothelial function of epicardial coronary arteries in patients with hypercholesterolaemia or essential hypertension—assessed by cold pressor testing. Eur Heart J. 1999 Nov;20(22):1676-80. 92. Deng YB, Xiang HJ, Chang Q, Li CL. Evaluation by high-resolution ultrasonography of endothelial function in brachial artery after Kawasaki disease and the effects of intravenous administration of vitamin C. Circ J. 2002 Oct;66(10):908-12. 93. Ling L, Zhao SP, Gao M, et al. Vitamin C preserves endothelial function in patients with coronary heart disease after a high-fat meal. Clin Cardiol. 2002 May;25(5):219-24. 94. Singh N, Graves J, Taylor PD, MacAllister RJ, Singer DR. Effects of a ‘healthy’ diet and of acute and long-term vitamin C on vascular function in healthy older subjects. Cardiovasc Res. 2002 Oct;56(1):118-25. 95. Mostafa S el-D, Garner DD, Garrett L, et al. Beneficial effects of vitamin C on risk factors of cardiovascular diseases. J Egypt Public Health Assoc. 1989;64(1-2):123-33. 96. Chin JP, Dart AM. HBPRCA Astra Award. Therapeutic restoration of endothelial function in hypercholesterolaemic subjects: effect of fish oils. Clin Exp Pharmacol Physiol. 1994 Oct;21(10):749-55. 97. Goodfellow J, Bellamy MF, Ramsey MW, Jones CJ, Lewis MJ. Dietary supplementation with marine omega-3 fatty acids improve systemic large artery endothelial function in subjects with hypercholesterolemia. J Am Coll Cardiol. 2000 Feb;35(2):265-70. 98. Flaten H, Hostmark AT, Kierulf P, et al. Fish-oil concentrate: effects on variables related to cardiovascular disease. Am J Clin Nutr. 1990 Aug;52(2):300-6. 99. von Schacky C, Angerer P, Kothny W, Theisen K, Mudra H. The effect of dietary omega-3 fatty acids on coronary atherosclerosis. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 1999 Apr 6;130(7):554-62. 100. Daviglus ML, Stamler J, Orencia AJ, et al. Fish consumption and the 30-year risk of fatal myocardial infarction. N Engl J Med. 1997 Apr 10;336(15):1046-53. 101. Kremer JM. n-3 fatty acid supplements in rheumatoid arthritis. Am J Clin Nutr. 2000 Jan;71(1 Suppl):349S-51S. 102. James MJ, Gibson RA, Cleland LG. Dietary polyunsaturated fatty acids and inflammatory mediator production. Am J Clin Nutr. 2000 Jan;71(1 Suppl):343S-8S. 103. De Caterina R, Massaro M. Effects of diet and of dietary components on endothelial leukocyte adhesion molecules. Curr Atheroscler Rep. 1999 Nov;1(3):188-95. 104. Yaqoob P, Calder PC. N-3 polyunsaturated fatty acids and inflammation in the arterial wall. Eur J Med Res. 2003 Aug 20;8(8):337-54. 105. German JB, Lokesh B, Kinsella JE. The effect of dietary fish oils on eicosanoid biosynthesis in peritoneal macrophages is influenced by both dietary N-6 polyunsaturated fats and total dietary fat. Prostaglandins Leukot Essent Fatty Acids. 1988 Oct;34(1):37-45. 106. De Caterina R, Spiecker M, Solaini G, et al. The inhibition of endothelial activation by unsaturated fatty acids. Lipids. 1999;34 SupplS191-4. 107. Khalfoun B, Thibault F, Watier H, Bardos P, Lebranchu Y. Docosahexaenoic and eicosapentaenoic acids inhibit in vitro human endothelial cell production of interleukin-6. Adv Exp Med Biol. 1997;400B:589-97. 108. Das UN. Beneficial effect(s) of n-3 fatty acids in cardiovascular diseases: but, why and how? Prostaglandins Leukot Essent Fatty Acids. 2000 Dec;63(6):351-62. 109. Smith AR, Hagen TM. Vascular endothelial dysfunction in aging: loss of Akt-dependent endothelial nitric oxide synthase phosphorylation and partial restoration by (R)-alpha-lipoic acid. Biochem Soc Trans. 2003 Dec;31(Pt 6):1447-9. 110. Jones W, Li X, Qu ZC, et al. Uptake, recycling, and antioxidant actions of alpha-lipoic acid in endothelial cells. Free Radic Biol Med. 2002 Jul 1;33(1):83-93. 111. Zhang WJ, Frei B. Alpha-lipoic acid inhibits TNF-alpha-induced NF-kappaB activation and adhesion molecule expression in human aortic endothelial cells. FASEB J. 2001 Nov;15(13):2423-32. 112. Morcos M, Borcea V, Isermann B, et al. Effect of alpha-lipoic acid on the progression of endothelial cell damage and albuminuria in patients with diabetes mellitus: an exploratory study. Diabetes Res Clin Pract. 2001 Jun;52(3):175-83. 113. Kunt T, Forst T, Wilhelm A, et al. Alpha-lipoic acid reduces expression of vascular cell adhesion molecule-1 and endothelial adhesion of human monocytes after stimulation with advanced glycation end products. Clin Sci (Lond). 1999 Jan;96(1):75-82. 114. Gao TL and Huang YZ. Effects of lipoic acid on reperfusion induced arrhythmias and myocardiac action potential alterations induced by free radical generating system. Sheng Li Xue Bao. 1991 Apr;43(2):149-55. 115. Coombes JS, Powers SK, Hamilton KL, et al. Improved cardiac performance after ischemia in aged rats supplemented with vitamin E and alpha-lipoic acid. Am J Physiol Regul Integr Comp Physiol. 2000 Dec;279(6):R2149-55. 116. Vodoevich VP. Effect of lipoic acid, biotin and pyridoxine on blood content of saturated and unsaturated fatty acids in ischemic heart disease and hypertension. Vopr Pitan. 1983 Sep;(5):14-16. 117. Shih JC. Atherosclerosis in Japanese quail and the effect of lipoic acid. Fed Proc. 1983 May 15;42(8):2494-7. 118. Moat SJ, Lang D, McDowell IF, et al. Folate, homocysteine, endothelial function and cardiovascular disease. J Nutr Biochem. 2004 Feb;15(2):64-79. 119. Ness AR, Powles JW, Khaw KT. Vitamin C and cardiovascular disease: a systematic review. J Cardiovasc Risk. 1996 Dec;3(6):513-21. 120. Wilkinson IB, Megson IL, MacCallum H, et al. Oral vitamin C reduces arterial stiffness and platelet aggregation in humans. J Cardiovasc Pharmacol. 1999 Nov;34(5):690-3. 121. FTC reaches record price-fixing settlement to settle charges of price-fixing in generic drug market [press release]. Washington, DC: Federal Trade Commission; November 29, 2000. 122.Yam D, Bott-Kanner G, Genin I, Shinitzky M, Klainman E. The effect of omega-3 fatty acids on risk factors for cardiovascular diseases. Harefuah. 2001 Dec;140(12):1156-8, 1230. 123. Available at: http://www.cdc.gov/ cvh/hp2010/objectives.htm. Accessed July 29, 2005. 124. Esposito K, Marfella R, Ciotola M, et al. Effect of a mediterranean-style diet on endothelial dysfunction and markers of vascular inflammation in the metabolic syndrome: a randomized trial. JAMA. 2004 Sep 22;292(12):1440-6. 125. Chade AR, Lerman A, Lerman LO. Kidney in early atherosclerosis. Hypertension. 2005 Jun;45(6):1042-49. 126. Available at: http://www.mayoclinic.org/ news2004-mchi/2334.html. Accessed July 29, 2005. 127. Available at: http://www.wholehealthmd.com/ print/view/1,1560,RA_489_learn,00.html. Accessed July 29, 2005. 128. Maas R, Schwedhelm E, Albsmeier J, Boger RH. The pathophysiology of erectile dysfunction related to endothelial dysfunction and mediators of vascular function. Vasc Med. 2002 Aug;7(3):213-25. 129. Feldman HA, Johannes CB, Derby CA, et al. Erectile dysfunction and coronary risk factors: prospective results from the Massachusetts male aging study. Prev Med. 2000 Apr;30(4):328-38. 130. Fung MM, Bettencourt R, Barrett-Connor E. Heart disease risk factors predict erectile dysfunction 25 years later: the Rancho Bernardo Study. J Am Coll Cardiol. 2004 Apr 21;43(8):1405-11. 131. Bivalacqua TJ, Usta MF, Champion HC, Kadowitz PJ, Hellstrom WJ. Endothelial dysfunction in erectile dysfunction: role of the endothelium in erectile physiology and disease. J Androl. 2003 Nov;24(6 Suppl):S17-S37. |