Life Extension Magazine®
LE Magazine September 2004
How Humans Died Last Century
|
Most people fail to realize how far medicine has progressed in a remarkably brief period of human history.
For instance, the three leading causes of death in 1900 were pneumonia, tuberculosis, and diarrhea.1 During the first four decades of the twentieth century, bacterial infections ravaged human populations of all ages. Diphtheria, typhoid fever, tuberculosis, scarlet fever, syphilis, staphylococcus, and a host of enteric pathogens were the epidemic killers of the day. By 1946, these diseases were largely brought under control with the advent of antibiotics, improvements in hygiene, and other antimicrobial therapies.
The three leading killers today are heart disease, cancer, and stroke.2 Pneumonia still claims the lives of elderly people with weakened immune systems, but the bacterial infections that caused so many people to perish from pneumonia were eradicated long ago. In less than 65 years, science has turned the death tables upside down to the extent that the major threat now facing health-conscious people is aging and its accompanying diseases.
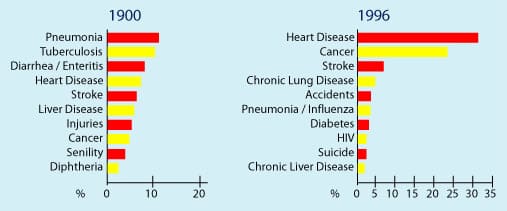 |
| The average life expectancy in 1900 was 47.3 years. In 1993, it was 75.7 years. SOURCE: ODC, National Center for Health Statistics. In 1900, deaths from other causes accounted for almost 30% of total deaths; in 1996, deaths from other causes accounted for about 20% of total deaths. |
Critics of our objectives to radically extend the healthy human life span contend that it is not possible to gain control over aging. Some pessimists even say that cancer may never be cured. We at Life Extension disagree with these cynics who fail to appreciate how far health care has advanced over the past seven decades.
Considering the scientific discoveries that have occurred in recent years, it would appear that both cancer and aging will become controllable diseases long before this century ends. The problem is that most people reading this are likely to die before then.3
We Cannot Forget the Past
To educate more people about how close we may be to eliminating pathological aging as a consequence of human maturation, it is useful to look back just a few generations to see how hopeless the prospects for living a long and healthy life appeared to be then.
At the dawn of the twentieth century, life expectancy at birth was only 47.3 years. In 1900, fewer than 60% of women lived to the age of 50; today, 95% of women can expect to reach at least the age of 50.4
Cancer Used to Be a Death Sentence
We at Life Extension have been highly critical of the medical establishment’s failure to discover more cures for cancer. For many cancers, the mortality rate has not changed much since 1950 and obvious ways to reform today’s broken system have not been implemented.
Despite the many failings we have identified, far more cancer patients are surviving than ever before. Although many of these victims suffer lifelong debilitation from the treatments used to cure their cancer, there has been undeniable progress in many areas of oncology.
In the 1930s, only one of five cancer patients survived five or more years. During the 1940s, the five-year survival rate improved to one of four patients. In the 1960s, one of three survived. Today, 64% of cancer patients survive for five years or more.5
The cancer establishment can brag about these statistics, but we at Life Extension will continue to fight for the lives of cancer victims who are not being effectively treated. One overlooked problem is that despite improved survival rates, cancer deaths continue to mount. While 143 people per 100,000 died of cancer in 1930, today the figure is nearly 180 per 100,000.5 Much of this increase is due to increased life expectancy (cancer incidence increases with age) and smoking-related cancers.
Cancer is the second leading cause of death after heart disease in the US, but it is the major cause of death in women between the ages of 35 and 74. In children under the age of 15, cancer trails only accidents as the leading cause of death.5 If current trends continue, cancer is expected to be the leading cause of death in the US by 2010. It is because of these worrisome trends that we at Life Extension believe that finding a cure for cancer should be a national priority.
Staggeringly High Childhood Mortality
If you visit any old cemetery, you might be surprised to see how large the “babyland” sections are. That is because infants and children used to die at dreadfully high rates.
In the pre-vaccination era, pertussis (whooping cough) was a leading cause of infant death. The number of cases of pertussis dropped by more than 99% between the 1930s and 1970s (though an increase occurred in the US and Europe during the 1980s and 1990s).7
In 1900, 30.4% of all deaths occurred among children less than five years of age. By 1997, that percentage was only 1.4%. These remarkable improvements in saving children’s lives occurred because of improvements in sanitation and hygiene, the discovery of antibiotics, and the implementation of vaccination programs. Scientific advances played a major role in each of these areas.8
|
We at Life Extension would like to see the same commitment aimed at saving the lives of adults. Critics of our ambitious research programs state that it is “natural” for people to succumb to illness as they age. Our rebuttal is that it was perfectly “natural” in 1900 for infants and children to die at a rate 21 times greater than they do today! The reduction in mortality for children and infants is irrefutable proof that the lethal forces of nature can be overcome by scientific innovation.
Life Extension has long advo-cated that a medical research “Manhattan Project” be established to find cures for the diseases of aging and aging itself within the next 17 years. Our organization currently funds almost $5 million of scientific research each year.
Dying from Acute Vitamin Deficiencies
It is hard to imagine that only 65 years ago, thousands of Americans died each year from diseases such as pellagra (niacin deficiency), beriberi (thiamine deficiency), rickets (vitamin D deficiency), and scurvy (vitamin C deficiency).
As many as 3,000 deaths were attributed to pellagra and hundreds of children died from rickets each year as late as 1938. Over 40% of President Franklin D. Roosevelt’s administration was deficient in riboflavin (vitamin B2) and over 20% of preschool children had rickets. These diseases were common and malnutrition was typically a result of not enough food.10
Today, people die of chronic nutrient deficiencies caused by less-than-optimal intake of a wide variety of vitamins, minerals, and amino acids. Thousands of published studies indicate that many of today’s lethal diseases could be prevented if people consumed more than the minimal daily intakes established by the federal government. Yet approximately 40% of the US population takes no dietary supplements, resulting in an epidemic of chronic nutrient deficiency states.11
|
| How Humans Died Last Century | ||||||||||||||||||||||
Challenging the Pessimists Historians later this century will look back at our generation and wonder why doctors did not take advantage of all the wonderful tools at their disposal to eradicate human suffering and premature death. In 1929, Dr. Alexander Flem-ing’s discovery of penicillin was published in the British Journal of Experimental Pathology. Yet well into the 1940s, humans were still dying of bacterial infections that were curable by penicillin.45 The greatest challenge in battling the bureaucrats and medical establishment is countering their absurd notion that aging will never be controllable. All one has to do, however, is look back 100 years and see how people used to die. In 1900, there was little basis to believe that most lethal bacterial diseases would be eradicated in less than 50 years.
Right now, an abundance of knowledge reveals that it is only a matter of time before aging becomes a horrifying relic of the past. As long as the majority of people believe this will never happen, the resources committed to discovering a cure for aging will be limited. When genomic engineering is perfected, not only will it become possible to stop aging, but we may even be able to reverse it. It is difficult to imagine a nobler objective for humankind than making old people young again. What Must Be Done Today Unfortunately, regulators are seeking greater control over what people are allowed to put into their own bodies, over what companies are allowed to sell, and how individual doctors run their own practices. Those with novel solutions for today’s causes of death first have to contend with the FDA and other bureaucracies before they have any hope of making a new therapy available to the masses.
The Life Extension Foundation was established in 1980 as a nonprofit organization dedicated to funding scientific research and educating the public about staying alive. Regrettably, our efforts increasingly are being hindered by regulations that have caused those with creative medical concepts to fear criminal prosecution. This chilling effect results in attorneys being the first ones called when a significant scientific advance is made. The concern is that the government could initiate actions that would economically destroy years of dedicated work, or even put the inventor in jail. The advice of attorneys is to go “slow” or not pursue the discovery at all. The problem is that we are aging quickly and it will take a Herculean effort to bring together the various scientific disciplines needed to gain control over aging in our lifetimes. Avant-garde scientists are impeded because of antiquated laws that require expensive and time-consuming governmental “approvals” just to test new therapies. These laws must be abolished! For longer life, William Faloon | ||||||||||||||||||||||
| References | ||||||||||||||||||||||
1. Control of infectious diseases. MMWR Morb Mortal Wkly Rep. 1999 Jul 30;48(29):621-9. 2. Available at: http://www.cdc.gov/nchs/pressroom/03facts/mortalitytrends.htm. Accessed April 29, 2004. 3. Available at: www.deathclock.com. Accessed April 29, 2004. 4. Kolata G. Model shows how medical changes let population surge. New York Times. January 7, 1997. 5. American Cancer Society. Annual report to the nation on the status of cancer, 1975- 2001. Available at: http://www.cancer.org/docroot/MED/content/MED_2_1x_Annual_Report.asp. Accessed June 3, 2004. 6. Available at: http://www.msdh.state.ms.us/general/NURSHIST/nh08.htm. Accessed April 29, 2004. 7. Available at: http://www.cdc.gov/nip/diseases/pertussis/faqs.htm http://www.emedicine.com/emerg/byname/pediatrics-pertussis.htm. Accessed April 29, 2004. 8. Available at http://www.cdc.gov/mmwr/preview/mmwrhtml/mm4829a1.htm. Accessed April 29, 2004. 9. Available at: http://www.aihw.gov.au/mortality/data/current_data.html. Accessed April 29, 2004. 10. Available at http://www.cdc.gov/mmwr/preview/mmwrhtml/mm4840a1.htm. Accessed May 15, 2004. 11. Available at: http://www.cnn.com/2001/HEALTH/diet.fitness/04/12/dietary.supplements/?related. Accessed April 29, 2004. 12. Available at: http://www.micklebring.com/bml/chapter19.htm. Accessed April 29, 2004. 13. Kirby M, Jackson G, Betteridge J, Friedili K. Is erectile dysfunction a marker for cardiovascular disease? Int J Clin Pract. 2001 Nov;55(9):614-8. 14. Rajagopalan S, Han Z, Supiano M, Brook R, Pitt B. Prospective randomized evaluation of losartan in endothelial dysfunction of the aged—the PREVAILED Study. JACC. 2001 Feb;37:274A. 15. Heitzer T, Schlinzig T, Krohn K, Meinertz T, Munzel T. Endothelial dysfunction, oxidative stress, and risk of cardiovascular events in patients with coronary artery disease. Circulation. 2001 Nov 27;104(22):2673-8. 16. Celermajer DS, Sorensen KE, Spiegelhalter DJ, Georgakopoulos D, Robinson J, Deanfield JE. Aging is associated with endothelial dysfunction in healthy men years before the age-related decline in women. J Am Coll Cardiol. 1994 Aug;24(2):471-6. 17. Kanani PM, Sinkey CA, Browning RL, Allaman M, Knapp HR, Haynes WG. Role of oxidant stress in endothelial dysfunction produced by experimental hyperhomocyst(e)inemia in humans. Circulation. 1999 Sep 14;100(11):1161-8. 18. Küng CF, Lüscher TF. Different mechanisms of endothelial dysfunction with aging and hypertension in rat aorta. Hypertension. 1995 Feb;25(2):194-00. 19. Taddei S, Galetta F, Virdis A, et al. Physical activity prevents age-related impairment in nitric oxide availability in elderly athletes. Circulation. 2000 Jun 27;101(25):2896-901. 20. Landmesser U, Harrison DG. Oxidant stress as a marker for cardiovascular events. Ox marks the spot. Circulation. 2001 Nov 27;104(22):2638-40. 21. Schram MT, Chaturvedi N, Schalkwisk C, et al. Vascular risk factors and markers of endothelial function as determinants of inflammatory markers in type 1 diabetes: the EURODIAB Prospective Complications Study. Diabetes Care. 2003 July;26(7):2165- 73. 22. Matz RL. Andriantsitohaina R. Age-related endothelial dysfunction: potential implications for pharmacotherapy. Drugs Aging. 2003;20(7):527-50. 23. Pacher P, Mabley JG, Soriano FG, Liaudet L, Szabo C. Activation of poly(ADP-ribose) polymerase contributes to the endothelial dysfunction associated with hypertension and aging. Int J Mol Med. 2002 Jun;9(6):659-64. 24. Shimokawa H. Primary endothelial dysfunction: atherosclerosis. J Mol Cell Cardiol. 1999 Jan;31(1):23-37. 25. Taddei S, Virdis A, Ghiadoni L, et al. Menopause is associated with endothelial dysfunction in women. Hypertension. 1996 Oct;28(4):576-82. 26. Luscher TF, Tanner FC, Tschudi MR, Noll G. Endothelial dysfunction in coronary artery disease. Annu Rev Med. 1993;44:395- 18. 27. Taddei S, Virdis A, Mattei P, et al. Hypertension causes premature aging of endothelial function in humans. Hypertension. 1997 Mar;29(3):736-43. 28. Murakami T, Mizuno S. Intrinsic and extrinsic nitric oxide provides better and worse prognosis respectively for coronary artery disease. Circulation. 2002 Nov 5;106(19 Suppl II). 29. Ross R. Cell biology of atherosclerosis. Annu Rev Physiol. 1995;57:791-04. 30. Ward PA. Cytokines, inflammation, and autoimmune diseases. Hosp Pract. 1995 May 15;30(5):35-41. 31. McCarty MF. Interleukin-6 as a central mediator of cardiovascular risk associated with chronic inflammation, smoking, dia- betes, and visceral obesity: down-regulation with essential fatty acids, ethanol and pen- toxifylline. Med. Hypotheses. 1999 May;52(5):465-77. 32. Brod SA. Unregulated inflammation shortens human functional longevity. Inflamm Res. 2000 Nov;49(11):561-70. 33. Willard LB, Hauss-Wegrzyniak B, Wenk GL. Pathological and biochemical consequences of acute and chronic neuroinflammation within the basal forebrain cholinergic system of rats. Neuroscience. 1999 Jan; 88(1):193- 200. 34. Hogan SP, Mishra A, Brandt EB, et al. A pathological function for eotaxin and eosinophils in eosinophilic gastrointestinal inflammation. Nat. Immunol. 2001 Apr;2(4):353-60. 35. Van der Meide PH, Schellekens H. Cytokines and the immune response. Biotherapy. 1996;8(3-4):243-9. 36. Licinio J, Wong ML. The role of inflammatory mediators in the biology of major depression: central nervous system cytokines modulate the biological substrate of depressive symptoms, regulate stress-responsive systems, and contribute to neurotoxicity and neuroprotection. Mol Psychiatry. 1999 Jul;4(4): 317-27. 37. Brouqui P, Dumler JS, Raoult D. Immunohistologic demonstration of Coxiella burnetii in the valves of patients with Q fever endocarditis. Am J Med. 1994 Nov;97(5):451-8. 38. Devaux B, Scholz D, Hirche A, Klovekorn WP, Schaper J. Upregulation of cell adhe- sion molecules and the presence of low grade inflammation in human chronic heart failure. Eur Heart J. 1997 Mar;18(3):470-9. 39. De Keyser F, Elewaut D, De Vos M, et al. Bowel inflammation and the spondy- loarthropathies. Rheum Dis Clin North Am. 1998 Nov;24(4):785-813. 40. Invitti C. Obesity and low-grade systemic inflammation. Minerva Endocrinol. 2002 Sep;27(3):209-14. 41. Lee H, Liao JJ, Graeler M, Huang MC, Goetzl EJ. Lysophospholipid regulation of mononuclear phagocytes. Biochim Biophys Acta. 2002 May 23;1582(1-3):175-7. 42. Santoro A, Mancini E. Cardiac effects of chronic inflammation in dialysis patients. Nephrol Dial Transplant. 2002;17(Suppl. 8):10-5. 43. Sitzer M, Markus HS, Mendall MA, Liehr R, Knorr U, Steinmetz H. C-reactive protein and carotid intimal medial thickness in a community population. J Cardiovasc Risk. 2002 Apr;9(2):97-103. 44. Lee CK, Klopp RG, Weindruch R, Prolla TA. Gene expression profile of aging and its retardation by caloric restriction. Science. 1999 Aug 27;285(5432):1390-3. 45. Available at: http://www.cdc.gov/mmwr/preview/mmwrhtml/mm4829a1.htm. Accessed April 28, 2004. |