Life Extension Magazine®
When a celebrity develops a serious illness, the news media reports not only on the famous person, but also on the disease itself. The media conducts interviews with physicians and discusses what may have caused the celebrity’s ailment. We have long argued that medical ignorance is the leading cause of death in the US. We thus believe that the news media provides a partial public service by revealing intimate details about a celebrity’s disorder, and information about what average people can do to reduce their risk of contracting the same disease. In early September, former President Bill Clinton underwent quadruple coronary artery bypass surgery. This operation was performed after Clinton went to his doctor complaining of chest pains and shortness of breath. An angiogram revealed significant (90%) atherosclerotic occlusion in the major arteries feeding his heart. Immediate bypass surgery was prescribed to prevent the 58-year-old former president from suffering a major heart attack. The news media did a good job of educating the public about coronary artery disease, how it is diagnosed, and what happens during bypass surgery. There was also a lot of reporting on what may have caused the apparently robust former president to develop such a severe case of coronary artery occlusion. Bill Clinton’s penchant for eating artery-clogging fast food was noted, along with his mild hypertension. If these news media reports motivate some Americans to alter their food choices and maintain optimal blood pressure levels, then Clinton’s ordeal will have provided some benefit to the public. Regrettably, the news media spent so much time focusing on Clinton’s cholesterol level that people could have been misled into believing that keeping cholesterol low is all it takes prevent coronary artery occlusion. While cholesterol (especially the more dangerous low-density lipoprotein, or LDL) facilitates arterial blockage, it represents only part of the reason why heart attacks continue to strike Americans at epidemic levels. Misconceptions About Atherosclerosis Most doctors think of an ath-erosclerotic lesion as a “clog” consisting of fat, cholesterol, and platelets that have accumulated on an inner arterial wall. As a result, they tell their patients to eat less fat, take a statin drug (if cholesterol levels are high), and use a baby aspirin to prevent arterial platelet aggregation. The problem with these approaches is that while they may postpone a heart attack or stroke, they fail to correct the underlying pathologies that cause atherosclerotic lesions to form and progress. If people are to live long lives free of the ravages of atherosclerosis, these lethal misconceptions must be cleared up. Otherwise, there will be an epidemic of aging people receiving coronary stents, undergoing bypass surgeries, and dropping dead from sudden heart attacks. In reporting on Bill Clinton’s coronary bypass surgery, the news media stated that over 300,000 of these “routine” procedures are performed every year. Considering the miserable adverse consequences these operations can inflict, coronary bypass surgery should be considered only as a last resort rather than as a “routine” procedure.
Why Arteries Clog as We Age For the past 35 years, the standard treatment for coronary ath-erosclerosis has been to bypass the blocked arteries. Recuperation from this procedure can take months, and some patients are afflicted with lifetime impairments such as chronic inflammation, memory loss, and depression.5-15 A review of the scientific literature reveals that atherosclerosis is associated with high blood levels of homocysteine,16-24 fibrinogen,25-28 C-reactive protein,29-36 glucose,37,38 cholesterol,39-43 insulin,44-47 iron,48-51 LDL,39-43 and triglycerides,52-54 along with low levels of HDL55-57 and testosterone.45,58-64 Optimizing blood levels of these substances can dramatically reduce heart attack and stroke risk. Despite thousands of studies validating that atherosclerosis is a multifactorial process, today’s doctors often prescribe a statin drug as the sole therapy to prevent and treat coronary atherosclerosis. Mainstream cardiologists fail to appreciate that coronary atherosclerosis is a sign of systemic arterial dysfunction requiring aggressive therapy to correct it. Conventional medicine’s failure became self-evident when the news media interviewed cardiologists about Bill Clinton’s diseased arteries. The doctors focused on his elevated cholesterol as the cause of his problem. Life Extension members, on the other hand, have grown inpatient with doctors who fail to translate research findings into improved therapies. More than ever before, health-conscious people are taking responsibility for the health of their arteries by correcting as many of the known risk factors as possible.
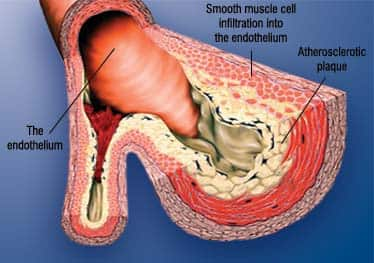
Anatomy of the Artery The outer layer of the artery comprises mostly connective tissue and provides structural containment for the two layers beneath. The middle area comprises elastic smooth muscle that provides the contractile strength to make possible the artery’s expansion and contraction with each heartbeat. The inner layer, known as the endothelium, comprises a thin area of endothelial cells whose integrity is crucial if atherosclerosis is to be prevented. Poor health habits and normal aging result in endothelial dysfunction, a process in which the endothelium boundary is broken, arterial flexibility is diminished, abnormal platelet aggregation occurs, and atherosclerotic lesions form in response to arterial wall (endothelium) injuries. Folic acid,65-71 vitamin C,72-76 fish oil,77-79 and lipoic acid80-84 are just a few of the nutrients that help maintain healthy endothelial function. It is no coincidence that these same nutrients have been shown to reduce cardiovascular incidence in both animals and people.85-94 Agents that suppress chronic inflammation also help protect the endothelium.95-133 | |||||||
| How Atherosclerosis Develops Atherosclerosis begins with changes in endothelial cell function that cause white blood cells moving through the blood to stick to the endothelium (inner arterial wall) instead of flowing by normally. The endothelium then becomes weakened. This allows blood cells and toxic substances circulating in the blood to pass through the endothelium and enter the artery’s sub-endothelial compartment. Lipid or fat-cell-like substances such as LDL and triglycerides in the blood then accumulate in this area. The lipids that accumulate in the broken endothelium become oxidized, causing the smooth muscle cells to try to “repair” the damaged endothelium. The result of this repair process is smooth muscle cell infiltration into the endothelium causing the formation of the initial atherosclerotic lesion. Depending on an individual’s risk factors—such as poor diet, lack of exercise, smoking, high blood pressure, and the aging process itself—fat accumulation continues and the atherosclerotic process accelerates. Immune cells called macro-phages then invade the damaged arterial area to digest the fat. But smooth muscle cells that have migrated to the area have already changed their nature to scavenge fat. These fat-laden white blood cells and smooth muscle cells are called “foam cells,” and provoke a chronic inflammatory attack by various immune components. Smooth muscle cells try to curtail the injury to the endothelium by producing collagen, which forms a cap over the injury site. Calcium then accumulates over the injury site to form a material resembling bone. This is why atherosclerosis used to be referred to as “hardening of the arteries.” This complex array of foam cells, calcification, and lipid accumulation is called an atherosclerotic plaque. The plaque grows, and if it becomes unstable, it is vulnerable to acute rupture that exposes the contents of the plaque to blood. Platelets can then rapidly accumulate around this ruptured plaque, resulting in an acute blockage (or blood clot) on the inner surface of the blood vessel wall. This clot can become very large and occlude the vessel. Even small plaques, if they rupture, can interfere with blood flow and cause an acute heart attack. Alternatively, atherosclerotic plaques can grow to such a degree as to restrict blood flow severely, as was the case with former President Clinton. When blood flow within an artery is gravely compromised by a large plaque or blood clot, the cells of tissues that depend on blood flow from that artery become damaged or die. Coronary atherosclerosis cuts off the heart’s blood supply by occluding the heart’s arteries, thus stopping the oxygen supply to the heart and causing a heart attack. A stroke results when atherosclerotic processes cut off the oxygen supply to a portion of the brain. As you can see, therefore, much more is involved in the development of atherosclerosis than just high cholesterol and LDL. We must emphasize, however, that maintaining optimal LDL and cholesterol levels is an important component of an atherosclerosis-prevention program. Protecting Your Arterial Walls Other significant artery-damaging factors are high-normal levels of glucose, insulin, iron, homocysteine,16-24 and fibrinogen,25-28 and any level of C-reactive protein29-36 that is higher than optimal. Homocysteine is particularly dangerous because it can induce the initial atherosclerotic injury to the endothelium, then facilitate the oxidation of the fat and LDL that accumulate beneath the damaged endothelium, and finally contribute to the abnormal accumulation of blood components around the atherosclerotic plaque. Fibrinogen is a clotting factor that accumulates at the site of the endothelial lesion. Fibrinogen contributes to plaque buildup and can participate in the arterial blockage after an unstable atherosclerotic plaque ruptures. Glucose at high-normal levels may accelerate the glycation process that causes arterial stiffening, while high-normal fasting insulin inflicts direct damage to the endothelium. High levels of iron promote oxidation of LDL in the damaged endothelium, while low levels of testosterone (in men) appear to interfere with normal endothelial function. C-reactive protein is an inflammatory marker and directly damages the endothelium. Chronic inflammation, as evidenced by persistent high levels of C-reactive protein, not only creates initial injuries to the endothelium, but also accelerates the progression of existing atherosclerotic lesions. In response to a large number of published studies, enlightened people are taking charge of the health of their arteries. They are eating better, exercising regularly, and undergoing regular blood testing to identify the specific drugs, hormones, and dietary supplements they need to reduce their atherosclerotic risk factors. The News Media Can Endanger Your Arteries We do not want any of our members to become victims of news media hype about what may have caused Clinton’s arteries to clog. We suspect the former president’s heart problems were due to many of the atherogenic factors discussed in this editorial. Sad to say, most cardiologists are not even familiar with the multiple heart-attack risk factors that were long ago identified by the Life Extension Foundation. In this issue of Life Extension, we examine the pros and cons of statin drugs and provide rational strategies for using these drugs if natural approaches fail. We know that many Life Extension members with elevated LDL or cholesterol levels refuse to take statin drugs because of concern about side effects. The good news is that a patented dietary supplement has been developed that has shown remarkable effects in reducing LDL and cholesterol without side effects. For members who have excess LDL or cholesterol, this new supplement could help lower these artery-clogging factors to safe levels. For longer life, William Faloon |
| References |
| 1. Available at: http://www.americanheart.org/ presenter.jhtml?identifier=183. Accessed September 14, 2004. 2. Available at: http://www.nhlbi.nih.gov/guidelines/cholesterol/atglance.htm. Accessed September 14, 2004. 3. Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (2003). The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. NIH Publication No. 03–5233. Bethesda, MD: U.S. Department of Health and Human Services. 4. Available at:http://www.umm.edu/careguides/cholesterol/cholesterol_statins.html. Accessed September 14, 2004. 5. Suksompong S, Prakanratrana U, Chumpathong S, Sriyoschati S, Pornvilawan S. Neuropsychological alterations after coronary artery bypass graft surgery. J Med Assoc Thai. 2002 Sep;85 Suppl 3:S910-6. 6. Strauss B, Paulsen G, Strenge H, Graetz S, Regensburger D, Speidel H. Preoperative and late postoperative psychosocial state following coronary artery bypass surgery. Thorac Cardiovasc Surg. 1992 Apr;40(2):5964. 7. Scholz M, Nowak P, Blaheta R, et al. Relocalization of endothelial cell beta- catenin after coculture with activated neu- trophils from patients undergoing cardiac surgery with cardiopulmonary bypass. Invest Surg. 2004 May-Jun;17(3):143-9. 8. Dacey LJ, DeSimone J, Braxton JH, et al. Preoperative white blood cell count and mortality and morbidity after coronary artery bypass grafting. Ann Thorac Surg. 2003 Sep;76(3):760-4. 9. Wei M, Kuukasjarvi P, Laurikka J, et al. Relation of cytokines to vasodilation after coronary artery bypass grafting. World J Surg. 2003 Oct;27(10):1093-8. 10. Bergh C, Backstrom M, Jonsson H, Havinder L, Johnsson P. In the eye of both patient and spouse: memory is poor 1 to 2 years after coronary bypass and angioplasty. Ann Thorac Surg. 2002 Sep;74(3):689-93; discussion 694. 11. Fearn SJ, Pole R, Wesnes K, Faragher EB, Hooper TL, McCollum CN. Cerebral injury during cardiopulmonary bypass: emboli impair memory. J Thorac Cardiovasc Surg. 2001 Jun;121(6):1150-60. 12. Newman MF, Kirchner JL, Phillips-Bute B, et al. Longitudinal assessment of neurocognitive function after coronary-artery bypass surgery. N Engl J Med. 2001 Feb 8;344(6):395-402. 13. Phillips Bute B, Mathew J, Blumenthal JA, et al. Female gender is associated with impaired quality of life 1 year after coronary artery bypass surgery. Psychosom Med. 2003 Nov-Dec;65(6):944-51. 14. Rymaszewska J, Kiejna A. Depression and anxiety after coronary artery bypass graft- ing. Pol Merkuriusz Lek. 2003 Aug;15(86):193-5. 15. Blumenthal JA, Lett HS, Babyak MA, et al. Depression as a risk factor for mortality after coronary artery bypass surgery. Lancet. 2003 Aug 23;362(9384):604-9. 16. Montalescot G, Ankri A, Chadefaux-Vekemans B, et al. Plasma homocysteine and the extent of atherosclerosis in patients with coronary artery disease. Int J Cardiol. 1997 Aug 8;60(3):295-300. 17. Stampfer MJ, Malinow MR, Willett WC, et al. A prospective study of plasma homocysteine and risk of myocardial infarction in US physicians. JAMA. 1992 Aug 19;268(7):877-81. 18. Verhoef P, Stampfer MJ, Buring JE, et al. Homocysteine metabolism and risk of myocardial infarction: relation with vitamins B6, B12, and folate. Am J Epidemiol. 1996 May 1;143(9):845-59. 19. Robinson K, Mayer EL, Miller DP, et al. Hyperhomocysteinemia and low pyridoxal phosphate. Common and independent reversible risk factors for coronary artery disease. Circulation. 1995 Nov 15;92(10):2825-30. 20. Arnesen E, Refsum H, Bonaa KH, Ueland PM, Forde OH, Nordrehaug JE. Serum total homocysteine and coronary heart dis- ease. Int J Epidemiol. 1995 Aug;24(4):704-9. 21. Aronow WS, Ahn C. Association between plasma homocysteine and coronary artery disease in older persons. Am J Cardiol. 1997 Nov 1;80(9):1216-8. 22. Berwanger CS, Jeremy JY, Stansby G. Homocysteine and vascular disease. Br J Surg. 1995 Jun;82(6):726-31. 23. Bostom AG, Rosenberg IH, Silbershatz H, et al. Nonfasting plasma total homocysteine levels and stroke incidence in elderly persons: the Framingham Study. Ann Intern Med. 1999 Sep 7;131(5):352-5. 24. Bots ML, Launer LJ, Lindemans J, Hofman A, Grobbee DE. Homocysteine, atherosclerosis and prevalent cardiovascular disease in the elderly: The Rotterdam Study. J Intern Med. 1997 Oct;242(4):339-47. 25. Maresca G, Di Blasio A, Marchioli R, Di Minno G. Measuring plasma fibrinogen to predict stroke and myocardial infarction: an update. Arterioscler Thromb Vasc Biol. 1999 Jun;19(6):1368-77. 26. Acevedo M, Foody JM, Pearce GL, Sprecher DL. Fibrinogen: associations with cardiovascular events in an outpatient clinic. Am Heart J. 2002 Feb;143(2):277-82. 27. Thompson SG, Kienast J, Pyke SD, Haverkate F, van de Loo JC. Hemostatic factors and the risk of myocardial infarction or sudden death in patients with angina pectoris. European Concerted Action on Thrombosis and Disabilities Angina Pectoris Study Group. N Engl J Med. 1995 Mar 9;332(10):635-41. 28. Aspirin resistance increases risk of death. AHA. 2002 Mar 26;2002b. 29. Ridker PM, Stampfer MJ, Rifai N. Novel risk factors for systemic atherosclerosis: a comparison of C-reactive protein, fibrino- gen, homocysteine, lipoprotein(a), and standard cholesterol screening as predictors of peripheral arterial disease. JAMA. 2001 May 16;285(19):2481-5. 30. Lindahl B, Toss H, Siegbahn A, Venge P, Wallentin L. Markers of myocardial damage and inflammation in relation to long-term mortality in unstable coronary artery disease. FRISC Study Group. Fragmin during Instability in Coronary Artery Disease. N Engl J Med. 2000 Oct 19;343(16):1139-47. 31. Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of car- diovascular disease in women. N Engl J Med. 2000 Mar 23;342(12):836-43. 32. Kuller LH, Tracy RP, Shaten J, Meilahn EN. Relation of C-reactive protein and coronary heart disease in the MRFIT nested case-control study. Multiple Risk Factor Intervention Trial. Am J Epidemiol. 1996 Sep 15;144(6):537-47. 33. Mendall MA, Strachan DP, Butland BK, et al. C-reactive protein: relation to total mortality, cardiovascular mortality and cardio- vascular risk factors in men. Eur Heart J. 2000 Oct;21(19):1584-90. 34. Pasceri V, Willerson JT, Yeh ET. Direct proinflammatory effect of C-reactive protein on human endothelial cells. Circulation. 2000 Oct 31;102(18):2165-8. 35. Ridker PM, Buring JE, Shih J, Matias M, Hennekens CH. Prospective study of C- reactive protein and the risk of future car- diovascular events among apparently healthy women. Circulation. 1998 Aug 25;98(8):731-3. 36. Auer J, Berent R, Lassnig E, Eber B. C- reactive protein and coronary artery disease. Jpn Heart J. 2002 Nov;43(6):607-19. 37. MacDonald-Wicks L, Gibson LZ, Godfrey DM, et al. Oxidized LDL in newly diagnosed type 2 diabetes mellitus and impaired glucose tolerance. Asia Pac J Clin Nutr. 2004 13(Suppl):S65. 38. Ceriello A. Impaired glucose tolerance and cardiovascular disease: the possible role of post-prandial hyperglycemia. Am Heart J. 2004 May;147(5):803-7. 39. Calabresi L, Gomaraschi M, Franceschini G. Endothelial protection by high-density lipoproteins: from bench to bedside. Arterioscler Thromb Vasc Biol. 2003 Oct 1;23(10):1724-31. 40. Zulli A, Widdop RE, Hare DL, Buxton BF, Black MJ. High methionine and cholesterol diet abolishes endothelial relaxation. Arterioscler Thromb Vasc Biol. 2003 Aug 1;23(8):1358-63. 41. Ridker PM, Rifai N, Rose L, Buring JE, Cook NR. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N Engl J Med. 2002 Nov 14;347(20):1557-65. 42. Guize L, Benetos A, Thomas F, Malmejac A, Ducimetiere P. Cholesterolemia and total, cardiovascular and cancer mortality. Study of a cohort of 220,000 people. Bull Acad Natl Med. 1998 182(3):631-47. 43. Kashyap ML. Cholesterol and atherosclerosis: a contemporary perspective. Ann Acad Med Singapore. 1997 Jul; 26(4):517-23. 44. Gonzalez MA, Selwyn AP. Endothelial function, inflammation, and prognosis in cardiovascular disease. Am J Med. 2003 Dec 8;115 Suppl 8A:99S-106S. 45. Phillips GB, Pinkernell BH, Jing TY. The association of hypotestosteronemia with coronary artery disease in men. Arterioscler Thromb. 1994 May;14(5):701-6. 46. Migdalis IN, Kalogeropoulou K, Iiopoulou V, et al. Progression of carotid atherosclerosis and the role of endothelin in diabetic patients. Res Commun Mol Pathol Pharmacol. 2000 Jul-Aug;108(1-2):27-37. 47. Sundell J, Luotolahti M. Association between insulin resistance and reduced coronary vasoreactivity in healthy subjects. Can J Cardiol. 2004 May 15;20(7):691-5 48. Shah SV, Alam MG. Role of iron in atherosclerosis. Am J Kidney Dis. 2003 Mar;41(3 Suppl 1):S80-3. 49. Kraml P, Potockova J, Koprivova H, et al. Ferritin, oxidative stress and coronary atherosclerosis. Vnitr Lek. 2004 Mar;50(3):197-202. 50. Armaganijan D, Batlouni M. Serum ferritin levels and other indicators of organic iron as risk factors or markers in coronary artery disease. Rev Port Cardiol. 2003 Feb;22(2):185-95; discussion 197-201. 51. Minqin R, Watt F, Huat BT, Halliwell B. Correlation of iron and zinc levels with lesion depth in newly formed atherosclerot- ic lesions. Free Radic Biol Med. 2003 Mar 15;34(6):746-52. 52. Packard CJ, O’Reilly DS, Caslake MJ, et al. Lipoprotein-associated phospholipase A2 as an independent predictor of coronary heart disease. West of Scotland Coronary Prevention Study Group. N Engl J Med. 2000 Oct 19;343(16):1148-55. 53. Welin L, Eriksson H, Larsson B, et al Triglycerides, a major coronary risk factor in elderly men. A study of men born in 1913. Eur Heart J. 1991 Jun;12(6):700-4. 54. Carlson LA, Bottiger LE, Ahfeldt PE. Risk factors for myocardial infarction in the Stockholm prospective study. A 14-year fol- low-up focusing on the role of plasma triglycerides and cholesterol. Acta Med Scand. 1979 206(5):351-60. 55. Viles-Gonzalez JF, Fuster V, Corti R, Badimon JJ. Emerging importance of HDL cholesterol in developing high-risk coronary plaques in acute coronary syndromes. Curr Opin Cardiol. 2003 Jul;18(4):286-94. 56. Spieker LE, Sudano I, Hurlimann D, et al. High-density lipoprotein restores endothelial function in hypercholesterolemic men. Circulation. 2002 Mar 26;105(12):1399-402. 57. Phillips GB, Pinkernell BH, Jing TY. Are major risk factors for myocardial infarction the major predictors of degree of coronary artery disease in men? Metabolism. 2004 Mar;53(3):324-9. 58. Hak AE, Witteman JC, de Jong FH, Geerlings MI, Hofman A, Pols HA. Low levels of endogenous androgens increase the risk of atherosclerosis in elderly men: the Rotterdam study. J Clin Endocrinol Metab. 2002 Aug;87(8):3632-9. 59. Dobrzycki S, Serwatka W, Nadlewski S, et al. An assessment of correlations between endogenous sex hormone levels and the extensiveness of coronary heart disease and the ejection fraction of the left ventricle in males. J Med Invest. 2003 Aug;50(3-4):162-9. 60. Wu SZ, Weng XZ. Therapeutic effects of an androgenic preparation on myocardial ischemia and cardiac function in 62 elderly male coronary heart disease patients. Chin Med J (Engl). 1993 Jun;106(6):415-8. 61. Channer KS, Jones TH. Cardiovascular effects of testosterone: implications of the “male menopause”? Heart. 2003 Feb;89(2):121-2. 62. English KM, Steeds RP, Jones TH, Diver MJ, Channer KS. Low-dose transdermal testosterone therapy improves angina threshold in men with chronic stable angina: A randomized, double-blind, placebo- controlled study. Circulation. 2000 Oct 17;102(16):1906-11. 63. Phillips GB, Pinkernell BH, Jing TY. Are major risk factors for myocardial infarction the major predictors of degree of coronary artery disease in men? Metabolism. 2004 Mar;53(3):324-9. 64. English KM, Mandour O, Steeds RP, et al. Men with coronary artery disease have lower levels of androgens than men with normal coronary angiograms. Eur Heart J. 2000 Jun;21(11):890-4. 65. Woo KS, Chook P, Chan LL, et al. Long- term improvement in homocysteine levels and arterial endothelial function after 1- year folic acid supplementation. Am J Med. 2002 May;112(7):535-9. 66. Doshi S, McDowell I, Moat S, Lewis M, Goodfellow J. Folate improves endothelial function in patients with coronary heart disease. Clin Chem Lab Med. 2003 Nov;41(11):1505-12. 67. Doshi SN, McDowell IF, Moat SJ, et al. Folic acid improves endothelial function in coronary artery disease via mechanisms largely independent of homocysteine lowering. Circulation. 2002 Jan 1;105(1):22-6. 68. Paradisi G, Cucinelli F, Mele MC, Barini A, Lanzone A, Caruso A. Endothelial function in post-menopausal women: effect of folic acid supplementation. Hum Reprod. 2004 Apr;19(4):1031-5. 69. Pena AS, Wiltshire E, Gent R, Hirte C, Couper J. Folic acid improves endothelial function in children and adolescents with type 1 diabetes. J Pediatr. 2004 Apr;144(4):500-4. 70. Moat SJ, Lang D, McDowell IF, et al. Folate, homocysteine, endothelial function and cardiovascular disease. J Nutr Biochem. 2004 Feb;15(2):64-79. 71. Doshi S, McDowell I, Moat S, Lewis M, Goodfellow J. Folate improves endothelial function in patients with coronary heart dis- ease. Clin Chem Lab Med. 2003 Nov;41(11):1505-12. 72. Gokce N, Keaney JF Jr, Frei B, et al. Long- term ascorbic acid administration reverses endothelial vasomotor dysfunction in patients with coronary artery disease. Circulation. 1999 Jun 29;99(25):3234-40. 73. Jeserich M, Schindler T, Olschewski M, Unmussig M, Just H, Solzbach U. Vitamin C improves endothelial function of epicar- dial coronary arteries in patients with hypercholesterolaemia or essential hypertension—assessed by cold pressor testing. Eur Heart J. 1999 Nov;20(22):1676-80. 74. Deng YB, Xiang HJ, Chang Q, Li CL. Evaluation by high-resolution ultrasonography of endothelial function in brachial artery after Kawasaki disease and the effects of intravenous administration of vitamin C. Circ J. 2002 Oct;66(10):908-12. 75. Ling L, Zhao SP, Gao M, Zhou QC, Li YL, Xia B. Vitamin C preserves endothelial function in patients with coronary heart dis- ease after a high-fat meal. Clin Cardiol. 2002 May;25(5):219-24. 76. Singh N, Graves J, Taylor PD, MacAllister RJ, Singer DR. Effects of a ‘healthy’ diet and of acute and long-term vitamin C on vascular function in healthy older subjects. Cardiovasc Res. 2002 Oct;56(1):118-25. 77. Chin JP, Dart AM. HBPRCA Astra Award. Therapeutic restoration of endothelial function in hypercholesterolaemic subjects: effect of fish oils. Clin Exp Pharmacol Physiol. 1994 Oct;21(10):749-55. 78. Goodfellow J, Bellamy MF, Ramsey MW, et al. Dietary supplementation with marine omega-3 fatty acids improve systemic large artery endothelial function in subjects with hypercholesterolemia. J Am Coll Cardiol. 2000 Feb;35(2):265-70. 79. De Caterina R, Spiecker M, Solaini G, et al. The inhibition of endothelial activation by unsaturated fatty acids. Lipids. 1999 34 Suppl:S191-4. 80. Smith AR, Hagen TM. Vascular endothelial dysfunction in aging: loss of Akt-dependent endothelial nitric oxide synthase phosphory- lation and partial restoration by (R)-alpha-lipoic acid. Biochem Soc Trans. 2003 Dec;31(Pt 6):1447-9. 81. Jones W, Li X, Qu ZC, Perriott L, Whitesell RR, May JM. Uptake, recycling, and antioxidant actions of alpha-lipoic acid in endothelial cells. Free Radic Biol Med. 2002 Jul 1;33(1):83-93. 82. Zhang WJ, Frei B. Alpha-lipoic acid inhibits TNF-alpha-induced NF-kappaB activation and adhesion molecule expression in human aortic endothelial cells. FASEB J. 2001 Nov;15(13):2423-32. 83. Morcos M, Borcea V, Isermann B, et al. Effect of alpha-lipoic acid on the progression of endothelial cell damage and albu- minuria in patients with diabetes mellitus: an exploratory study. Diabetes Res Clin Pract. 2001 Jun;52(3):175-83. 84. Kunt T, Forst T, Wilhelm A, et al. Alpha- lipoic acid reduces expression of vascular cell adhesion molecule-1 and endothelial adhesion of human monocytes after stimulation with advanced glycation end prod- ucts. Clin Sci (Lond). 1999 Jan;96(1):75-82. 85. Hernandez-Diaz S, Martinez-Losa E, Fernandez-Jarne E, et al. Dietary folate and the risk of nonfatal myocardial infarction. Epidemiology. 2002 Nov;13(6):700-6. 86. Czeizel E, Kalina A. Public health control of hyperhomocysteinemia and its consequences. Orv Hetil. 2003 Oct 5;144(40):1981-9. 87. Mostafa S el-D, Garner DD, Garrett L, Whaley RF, el-Sekate M, Kiker M. Beneficial effects of vitamin C on risk factors of cardiovascular diseases. J Egypt Public Health Assoc. 1989;64(1-2):123-33. 88. Brouwer IA, Zock PL, Wever EF, et al. Rationale and design of a randomised controlled clinical trial on supplemental intake of n-3 fatty acids and incidence of cardiac arrhythmia: SOFA. Eur J Clin Nutr. 2003 Oct;57(10):1323-30. 89. Harris WS, Park Y, Isley WL Cardiovascular disease and long-chain omega-3 fatty acids. Curr Opin Lipidol. 2003 Feb;14(1):9-14. 90. Yam D, Bott-Kanner G, Genin I, Shinitzky M, Klainman E. The effect of omega-3 fatty acids on risk factors for cardiovascular diseases. Harefuah. 2001 Dec;140(12):1156-8, 1230. 91. Gao TL, Huang YZ. Effects of lipoic acid on reperfusion induced arrhythmias and myocardiac action potential alterations induced by free radical generating system. Sheng Li Xue Bao. 1991 Apr;43(2):149-55. 92. Coombes JS, Powers SK, Hamilton KL, et al. Improved cardiac performance after ischemia in aged rats supplemented with vitamin E and alpha-lipoic acid. Am J Physiol Regul Integr Comp Physiol. 2000 Dec;279(6):R2149-55. 93. Vodoevich VP. Effect of lipoic acid, biotin and pyridoxine on blood content of saturated and unsaturated fatty acids in ischemic heart disease and hypertension. Vopr Pitan. 1983 Sep-Oct;(5):14-6. 94. Shih JC. Atherosclerosis in Japanese quail and the effect of lipoic acid. Fed Proc. 1983 May 15;42(8):2494-7. 95. Pollice PF, Rosier RN, Looney RJ, Puzas JE, Schwarz EM, O’Keefe RJ. Oral pentoxifylline inhibits release of tumor necrosis factor-alpha from human peripheral blood monocytes : a potential treatment for aseptic loosening of total joint components. J Bone Joint Surg Am. 2001 Jul;83-A(7):1057-61. 96. Neuner P, Klosner G, Schauer E, et al. Pentoxifylline in vivo down-regulates the release of IL-1 beta, IL-6, IL-8 and tumour necrosis factor-alpha by human peripheral blood mononuclear cells. Immunology. 1994 Oct;83(2):262-7. 97. Boldt J, Brosch C, Piper SN, et al. Influence of prophylactic use of pentoxifylline on postoperative organ function in elderly car- diac surgery patients. Crit Care Med.. 2001 May;29(5):952-8. 98. McCarty MF. Interleukin-6 as a central mediator of cardiovascular risk associated with chronic inflammation, smoking, dia- betes, and visceral obesity: down-regulation with essential fatty acids, ethanol and pentoxifylline. Med Hypotheses. 1999 May;52(5):465-77. 99. Bruynzeel I, van der Raaij LM, Willemze R, Stoof TJ. Pentoxifylline inhibits human T-cell adhesion to dermal endothelial cells. Arch Dermatol Res. 1997 Mar;289(4):189-93. 100. Lenoble Giovannangeli M. New aspects of the pharmacology of pentoxifylline. J Mal Vasc. 1989;14 Suppl A:35-41. 101. Hansen PR, Holm AM, Qi JH, Ledet T, Rasmussen LM, Andersen CB. Pentoxifylline inhibits neointimal formation and stimulates constrictive vascular remod- eling after arterial injury. J Cardiovasc Pharmacol. 1999 Nov;34(5):683-9. 102. Hofbauer R, Frass M, Gmeiner B, Handler S, Speiser W, Kapiotis S. The green tea extract epigallocatechin gallate is able to reduce neutrophil transmigration through monolayers of endothelial cells. Wien Klin Wochenschr. 1999 Apr 9;111(7):278-82. 103. Deana R, Turetta L, Donella-Deana A, et al. Green tea epigallocatechin-3-gallate inhibits platelet signalling pathways triggered by both proteolytic and non-proteolytic agonists. Thromb Haemost. 2003 May; 89(5):866-74. 104. Kawai K, Tsuno NH, Kitayama J, et al. Epigallocatechin gallate attenuates adhe- sion and migration of CD8+ T cells by binding to CD11b. J Allergy Clin Immunol. 2004 Jun;113(6):1211-7. 105. Wheeler DS, Catravas JD, Odoms K, Denenberg A, Malhotra V, Wong HR. Epigallocatechin-3-gallate, a green tea- derived polyphenol, inhibits IL-1 beta- dependent proinflammatory signal trans- duction in cultured respiratory epithelial cells. J Nutr. 2004 May;134(5):1039-44. 106. Ahmed S, Wang N, Lalonde M, Goldberg VM, Haqqi TM. Green tea polyphenol epigallocatechin-3-gallate (EGCG) differen- tially inhibits interleukin-1 beta-induced expression of matrix metalloproteinase-1 and -13 in human chondrocytes. J Pharmacol Exp Ther. 2004 Feb;308(2):767-73. 107. Dona M, Dell’Aica I, Calabrese F, et al. Neutrophil restraint by green tea: inhibition of inflammation, associated angiogenesis, and pulmonary fibrosis. J Immunol. 2003 Apr 15;170(8):4335-41. 108. Flaten H, Hostmark AT, Kierulf P, et al. Fish-oil concentrate: effects on variables related to cardiovascular disease. Am J Clin Nutr. 1990 Aug;52(2):300-6. 109. von Schacky C, Angerer P, Kothny W, Theisen K, Mudra H. The effect of dietary omega-3 fatty acids on coronary atherosclerosis. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 1999 Apr 6;130(7):554-62. 110. Daviglus ML, Stamler J, Orencia AJ, et al. Fish consumption and the 30-year risk of fatal myocardial infarction. N Engl J Med. 1997 Apr 10;336(15):1046-53. 111. Kremer JM. n-3 fatty acid supplements in rheumatoid arthritis. Am J Clin Nutr. 2000 Jan;71(1 Suppl):349S-51S. 112. James MJ, Gibson RA, Cleland LG. Dietary polyunsaturated fatty acids and inflammatory mediator production. Am J Clin Nutr. 2000 Jan;71(1 Suppl):343S-8S. 113. De Caterina R, Massaro M. Effects of diet and of dietary components on endothelial leukocyte adhesion molecules. Curr Atheroscler Rep. 1999 Nov;1(3):188-95. 114. Yaqoob P, Calder PC. N-3 polyunsaturated fatty acids and inflammation in the arterial wall. Eur J Med Res. 2003 Aug 20;8(8):33754. 115. German JB, Lokesh B, Kinsella JE. The effect of dietary fish oils on eicosanoid biosynthesis in peritoneal macrophages is influenced by both dietary N-6 polyunsaturated fats and total dietary fat. Prostaglandins Leukot Essent Fatty Acids. 1988 Oct;34(1):37-45. 116. De Caterina R, Spiecker M, Solaini G, et al. The inhibition of endothelial activation by unsaturated fatty acids. Lipids. 1999 34 Suppl:S191-4. 117. Khalfoun B, Thibault F, Watier H, et al. Docosahexaenoic and eicosapentaenoic acids inhibit in vitro human endothelial cell production of interleukin-6. Adv Exp Med Biol. 1997 400B:589-97. 118. Das UN. Beneficial effect(s) of n-3 fatty acids in cardiovascular diseases: but, why and how? Prostaglandins Leukot Essent Fatty Acids. 2000 Dec;63(6):351-62. 119. James MJ, Gibson RA, Cleland LG. Dietary polyunsaturated fatty acids and inflammatory mediator production. Am J Clin Nutr. 2000 Jan;71(1 Suppl):343S-348S. 120. De Caterina R, Spiecker M, Solaini G, et al. The inhibition of endothelial activation by unsaturated fatty acids. Lipids. 1999 34 Suppl:S191-4. 121. Chrubasik S. Evidence for antirheumatic effectiveness of Herba Urticae dioicae in acute arthritis: a pilot study. Phytomedicine. 1997 (4):105-8. 122. Riehemann K, Behnke B, Schulze-Osthoff K. Plant extracts from stinging nettle (Urtica dioica), an antirheumatic remedy, inhibit the proinflammatory transcription factor NF-kappaB. FEBS Lett. 1999 Jan 8;442(1):89-94. 123. Obertreis B, Ruttkowski T, Teucher T, Behnke B, Schmitz H. Ex-vivo in-vitro inhibition of lipopolysaccharide stimulated tumor necrosis factor-alpha and interleukin-1 beta secretion in human whole blood by extractum urticae dioicae foliorum. Arzneimittelforschung. 1996 Apr;46(4):389-94. 124. Straub RH, Scholmerich J, Zietz B. Replacement therapy with DHEA plus corticosteroids in patients with chronic inflammatory diseases – substitutes of adrenal and sex hormones. Z Rheumatol. 2002 59:Suppl 2:108-118. 125. Kawano H, Yasue H, Kitagawa A, et al. Dehydroepiandrosterone supplementation improves endothelial function and insulin sensitivity in men. J Clin Endocrinol Metab. 2003 Jul;88(7):3190-5. 126. Barret-Connor E, Knaw KT, Yen SSC. A prospective study of dehydroepiandrosterone sulfate, mortality and cardiovascular disease. N Engl J Med. 1986 Dec 11; 315:1519-24. 127. Casson PR, Anderson RN, Herrod HG, et al. Oral dehydroepiandrosterone in physiologic doses modulates immune function in postmenopausal women. Am J Obstet Gynecol. 1993 Dec;169(6):1536-9. 128. Simoncini T, Mannella P, Fornari L, Varone G, Caruso A, Genazzani AR. Dehydroepiandrosterone modulates endothelial nitric oxide synthesis via direct genomic and nongenomic mechanisms. Endocrinology. 2003 Aug;144(8):3449-55. 129. Blackwell GJ, Radomski M, Moncada S. Inhibition of human platelet aggregation by vitamin K. Thromb Res. 1985 Jan 1;37(1):103-14. 130. Seyama Y, Hayashi M, Takegami H, Usami E. Comparative effects of vitamin K2 and vitamin E on experimental arteriosclerosis. Int J Vitam Nutr Res. 1999 Jan;69(1):23-6. 131. Kipper-Galperin M, Galilly R, Danenberg HD, Brenner T. Dehydroepiandrosterone selectively inhibits production of tumor necrosis factor alpha and interleukin-6 [correction of interlukin-6] in astrocytes. Int J Dev Neurosci. 1999 Dec;17(8):765-75. 132. Haden ST, Glowacki J, Hurwitz S, Rosen C, LeBoff MS. Effects of age on serum dehydroepiandrosterone sulfate, IGF-I, and IL-6 levels in women. Calcif Tissue Int. 2000 Jun;66(6):414-8. 133. Reddi K, Henderson B, Meghji S, et al. Interleukin 6 production by lipopolysaccharide-stimulated human fibroblasts is potently inhibited by naphthoquinone (vitamin K) compounds. Cytokine. 1995 Apr;7(3):287-90. 134. Available at: http://www.nhlbi.nih.gov/public/Aug00/sept00.pdf. Accessed September 14, 2004. 135. Landmesser U, Hornig B, Drexler H. Endothelial function: a critical determinant in atherosclerosis? Circulation. 2004 Jun 1;109(21 Suppl 1):II27-33. 136. Endemann DH, Schiffrin EL. Endothelial dysfunction. J Am Soc Nephrol. 2004 Aug;15(8):1983-92. 137. Chang HJ, Chung J, Choi SY, et al. Endothelial dysfunction in patients with exaggerated blood pressure response during treadmill test. Clin Cardiol. 2004 Jul;27(7):421-5. 138. Tu L, Wei W, Liu X, Deng Y, Yu S. Endothelial function and carotid artery wall thickening in patients with early essential hypertension. J Tongji Med Univ. 1999 19(4):288-90, 303. 139. Rodriguez-Porcel M, Lerman LO, Herrmann J, Sawamura T, Napoli C, Lerman A. Hypercholesterolemia and hypertension have synergistic deleterious effects on coronary endothelial function. Arterioscler Thromb Vasc Biol. 2003 May 1;23(5):885-91. 140. Najemnik C, Sinzinger H, Kritz H. Endothelial dysfunction, atherosclerosis and diabetes. Acta Med Austriaca. 1999;26(5):148-53. 141. Maggi FM, Raselli S, Grigore L, Redaelli L, Fantappie S, Catapano AL. Lipoprotein remnants and endothelial dysfunction in the postprandial phase. J Clin Endocrinol Metab. 2004 Jun;89(6):2946-50. 142. Saini HK, Arneja AS, Dhalla NS. Role of cholesterol in cardiovascular dysfunction. Can J Cardiol. 2004 Mar 1;20(3):333-46. 143. Fan J, Watanabe T. Inflammatory reactions in the pathogenesis of atherosclerosis. J Atheroscler Thromb. 2003;10(2):63-71. 144. Dart AM, Chin-Dusting JP. Lipids and the endothelium. Cardiovasc Res. 1999 Aug 1;43(2):308-22. 145. Kusterer K, Pohl T, Fortmeyer HP, et al. Chronic selective hypertriglyceridemia impairs endothelium-dependent vasodilatation in rats. Cardiovasc Res. 1999 Jun;42(3):783-93. 146. Liu L, Zhao SP, Gao M. Influence of postprandial hypertriglyceridemia on the endothelial function in elderly patients with coronary heart disease. Hunan Yi Ke Da Xue Xue Bao. 2002 Jun 28;27(3):259-62. 147. Toikka JO, Ahotupa M, Viikari JS, et al. Constantly low HDL-cholesterol concentration relates to endothelial dysfunction and increased in vivo LDL-oxidation in healthy young men. Atherosclerosis. 1999 Nov 1;147(1):133-8. 148. Campuzano R, Moya JL, Garcia-Lledo A, et al. Endothelial dysfunction and intima-media thickness in relation to cardiovascular risk factors in patients without clinical manifestations of atherosclerosis. Rev Esp Cardiol. 2003 Jun;56(6):546-54. 149. Ambrose JA, Barua RS. The pathophysiology of cigarette smoking and cardiovascular disease: an update. Am Coll Cardiol. 2004 May 19;43(10):1731-7. 150. Poreba R, Skoczynska A, Derkacz A. Effect of tobacco smoking on endothelial function in patients with coronary arteriosclerosis. Pol Arch Med Wewn. 2004 Jan;111(1):27-36. 151. Puranik R, Celermajer DS. Smoking and endothelial function. Prog Cardiovasc Dis. 2003 May-Jun;45(6):443-58. 152. Campia U, Sullivan G, Bryant MB, Waclawiw MA, Quon MJ, Panza JA. Insulin impairs endothelium-dependent vasodilation independent of insulin sensitivity or lipid profile. Am J Physiol Heart Circ Physiol. 2004 Jan;286(1):H76-82. 153. Furuta M, Tsunoda K, Arita M, Nanjo K, Sanke T. Endothelium-dependent vasodilation in type II diabetes mellitus. Rinsho Byori. 2003 Nov;51(11):1111-5. 154. Higashi Y, Yoshizumi M. Endothelial function. Nippon Rinsho. 2003 Jul;61(7):1138-44. 155. Shinozaki K, Kashiwagi A, Masada M, Okamura T. Molecular mechanisms of impaired endothelial function associated with insulin resistance. Curr Drug Targets Cardiovasc Haematol Disord. 2004 Mar;4(1):1-11. 156. Najemnik C, Sinzinger H, Kritz H. Endothelial dysfunction, atherosclerosis and diabetes. Acta Med Austriaca. 1999;26(5):148-53. 157. Jarvisalo MJ, Raitakari M, Toikka JO, et al. Endothelial dysfunction and increased arterial intima-media thickness in children with type 1 diabetes. Circulation. 2004 Apr 13;109(14):1750-5. 158. Bakker SJ, IJzerman RG, Teerlink T, Westerhoff HV, Gans RO, Heine RJ. Cytosolic triglycerides and oxidative stress in central obesity: the missing link between excessive atherosclerosis, endothelial dysfunction, and beta-cell failure? Atherosclerosis. 2000 Jan;148(1):17-21. 159. Blann AD, Bushell D, Davies A, Faragher EB, Miller JP, McCollum CN. von Willebrand factor, the endothelium and obesity. Int J Obes Relat Metab Disord. 1993 Dec;17(12):723-5. 160. Yu YR, Li HL, Yu HL, Wang C, Pu S. The relationship between insulin resistance and endothelium-dependent vasodilatation in obese subjects. Zhonghua Yi Xue Za Zhi. 2003 Sep 10;83(17):1467-70. 161. Lyon CJ, Law RE, Hsueh WA. Minireview: adiposity, inflammation, and atherogenesis. Endocrinology. 2003 Jun;144(6):2195-200. 162. Mitu F, Mitu M. Physical exercise and vascular endothelium. Rev Med Chir Soc Med Nat Iasi. 2003 Jul-Sep;107(3):487-93. 163. Edwards DG, Schofield RS, Lennon SL, Pierce GL, Nichols WW, Braith RW. Effect of exercise training on endothelial function in men with coronary artery disease. Am J Cardiol. 2004 Mar 1;93(5):617-20. 164. Higashi Y, Yoshizumi M. Exercise and endothelial function: role of endothelium-derived nitric oxide and oxidative stress in healthy subjects and hypertensive patients. Pharmacol Ther. 2004 Apr;102(1):87-96. 165. Gokce N, Vita JA, Bader DS, et al. Effect of exercise on upper and lower extremity endothelial function in patients with coronary artery disease. Am J Cardiol. 2002 Jul 15;90(2):124-7. 166. De Caterina R, Lenzi S. The role of LDL in the origin and progression of atherosclerosis: pathobiological concepts on the origin and development of atherosclerotic lesions and the role of the endothelium. G Ital Cardiol. 1998 Feb;28(2):158-67. |
It was long ago established that consumption of cold-water fish reduces the risk of heart attack.1 In fact, just two to three servings of fish a week may protect against many diseases, including arthritis, stroke, certain cancers, and a host of inflammation-related disorders.2-9 When scientists sought to discover which components of fish are responsible for preventing heart attacks, they found that the oil plays a critical role. Cold-water fish oil is high in omega-3 fatty acids that function in multiple ways to reduce cardiovascular disease risk.10 Based on the published scientific evidence about fish oil, a lawsuit was filed against the FDA in 1994 by Durk Pearson and Sandy Shaw, seeking to force the agency to allow the following health claim on fish oil supplement labels: “Consumption of omega-3 fatty acids may reduce the risk of coronary heart disease.” The FDA rejected this one-sentence claim and a multi-year litigation battle ensued. In their lawsuit, Durk and Sandy pointed out that consumers would benefit by learning of the value of fish oil in protecting against heart disease. They also argued that the FDA lacked the constitutional authority to ban this truthful health claim. The FDA contended that this health claim was not adequately backed by scientific studies and that the agency had the legal authority to ban these kinds of health claims. Seven years of extensive litigation ensued as the FDA asserted that it had the sole authority to dictate what Americans could read on the label of fish oil supplements. After an onslaught of irrefutable scientific evidence was presented, including articles published in the most prestigious scientific journals in the world, the FDA capitulated and said it would permit the following claim: “Consumption of omega-3 fatty acids may reduce the risk of coronary heart disease. FDA evaluated the data and determined that although there is scientific evidence supporting the claim, the evidence is not conclusive.” Life Extension Challenges FDA On Fish Oil Health Claim The Life Extension Foundation Buyers Club, Inc., and Wellness Lifestyles, Inc., filed a health claim petition against the FDA on June 23, 2003. The petition urged the FDA to reconsider its permitted health claim for omega-3 fatty acids and coronary heart disease risk, and to allow the following revised claim: “Consumption of omega-3 fatty acids may reduce the risk of coronary heart disease.” To substantiate this position, a massive document enumerating the scientific studies backing the benefits of omega-3 fatty acids was filed, along with legal arguments supporting the constitutional right to disseminate this truthful information. Also included in the petition was a calculation of how many American lives were needlessly being lost because of the FDA’s restriction of this simple health claim. Epidemiological data were presented showing that if all Americans regularly took fish oil supplements or ate about two cold-water fish meals a week, it would prevent about 150,000 deaths a year. Life Extension further argued that during the seven years it took to litigate this case against the FDA, Americans suffered over 1 million preventable sudden-death heart attacks. The Political Battle Over What Americans Eat For nearly two decades, the FDA protected the economic interests of companies selling high-fat and high-cholesterol foods by making it illegal to promote a healthy diet as a way of preventing heart disease. Heart attack rates were three times higher in the 1950s than in the 1990s. The FDA’s censorship of healthy dietary information caused tens of millions of Americans to unnecessarily succumb to cardiovascular and other diseases. FDA Capitulates To Scientific Reality According to Acting FDA Commissioner Dr. Lester M. Crawford, “Coronary heart disease is a significant health problem that causes 500,000 deaths annually in the United States. This new qualified health claim for omega-3 fatty acids should help consumers as they work to improve their health by identifying foods that contain these important compounds (EPA and DHA).” The FDA now permits the following statement to be printed on the label of fish oil supplements: “Supportive but not conclusive research shows that consumption of EPA and DHA omega-3 fatty acids may reduce the risk of coronary heart disease.” The FDA went on to recommend that consumers not exceed more than 3 grams per day of EPA and DHA omega-3 fatty acids, with no more than 2 grams per day derived from a dietary supplement. Life Extension argues that many scientific studies show that higher amounts of EPA and DHA are often needed to obtain optimal benefits, such as reduction of triglycerides and prevention of restenosis (re-occlusion of a blocked artery).11 This battle over what can be stated about fish oil began back in 1994. While the FDA’s announcement of a broader health claim represents a significant legal victory, Life Extension is still not satisfied with the FDA’s latest health claim on fish oil supplements. We reiterate our position that evidence from peer-reviewed scientific publications supporting the benefit of EPA and DHA supplements in reducing heart attack risk is conclusive and not merely “supportive” as the FDA contends. Life Extension congratulates attorney Jonathan Emord for the hundreds of hours of productive work he has put into this case over the past ten years. Jonathan filed the initial lawsuit against the FDA on behalf of Durk Pearson and Sandy Shaw that resulted in a precedent-setting legal victory against FDA censorship. Jonathan then prepared the petition on behalf of Life Extension and Wellness Lifestyles that resulted in the FDA allowing this new expanded health claim to be made about the protective effect of fish oils against cardiovascular disease. It is unfortunate that Bill Clinton, as president in 1994, did not take actions he had the authority to take. President Clinton could have ordered the FDA to allow truthful, non-misleading health claims on dietary supplements. If Bill Clinton personally followed the scientifically-based diet and supplement program that the FDA was suppressing at that time, perhaps he could have avoided his recent coronary bypass surgery. |
| References |
| 1. FTC Press Release, November 29, 2000. “FTC Reaches Record Price-fixing Settlement to Settle Charges of Price-fixing in Generic Drug Market.” 2. Price quoted by Hollywood Discount Pharmacy in Hollywood, Florida on Jan 15, 2002. 3. Associated Press, October 4, 2001. “Drugmaker to pay $875 million fine.” 4. Robert Pear (New York Times News Service). “Health spending jumps 6.9%—Main factors: hospitals and drug costs, managed care resistance, The Herald, Tuesday, January 8, 2002. 5. Faloon William, “Dying from Deficiency,” Life Extension magazine, October 2001. 6. National Vital Statistics Reports, Vol. 48, No.11. 7. Wall Street Journal, December 24, 2001, pp- A3, “Schering Fines Could Total $500 Million.” 8. http://www.cnn.com/HEALTH/9804/14/drug.reaction/Chicago CNN. “Study: Drug reactions kill an estimated 100,000 a year,” April 14, 1998. 9. David Willman, “The Rise and Fall of the Killer Drug Rezulin,” Life Extension magazine, 10. http://news.ft.com/ft/gx.cgi/ftc?pagename= View&c=Article&cid=FT3HZ3AFMWC &live=true&tagid=IXLHT5GTICC&subheading=heal By David Firn in London, “More deaths linked to Bayer’s Lipobay,” January 18, 2002. 19:44 | Last Updated: January 18 2002 19:48 11. Calder PC. n-3 fatty acids and cardiovascular disease: evidence explained and mechanisms explored. Clin Sci (Lond). 2004 Jul;107(1):1-11. |