Life Extension Magazine®
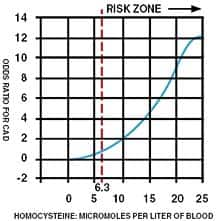
Several years ago, we made an alarming discovery! People who had previously been in safe ranges were calling us to report that their new blood tests revealed markedly higher homocysteine levels. We expected to see some elevation, as homocysteine levels are known to rise during normal aging. What startled us was the magnitude of increase that occurred in as little as one year. In some cases, this sharp rise in homocysteine was accompanied by the onset of a degenerative disorder (angina, stroke, renal impairment, aortic stenosis, memory loss, depression, etc.). In response to our findings, we alerted members about the importance of testing their blood annually to guard against unexpected surges in cardiovascular risk factors such as homocysteine, C-reactive protein, and low-density lipoprotein (LDL). Newly published studies are confirming the observations we first made in the late 1990s. In the latest study, a startling 100% of French elderly hospitalized patients showed higher than desired homocysteine levels, with 45% suffering from severe hyperhomocysteinemia (blood homocysteine greater than 18 µmol/L).1 Some physicians still define excess homocysteine as blood levels greater than 15-18 µmol/L. Published studies, on the other hand, indicate that keeping homocysteine below 7-8 µmol/L is ideal for reducing the risk of lethal diseases.2 In a three-year study of 600 Italian elderly hospitalized patients, the mean fasting homocysteine level was a frighteningly high 16.8 µmol/L. Patients with hyperhomocysteinemia in this study showed a greater prevalence of serious disease states, such as vascular and cognitive disorders.3 In a German paper titled Hyperhomo-cysteinemia In Advanced Age, the following conclusions where made as it relates to homocysteine and age-related disease:4
The Homocysteine “Hypothesis” of Degenerative Disease Throughout the 1980s, many doctors debunked the “hypothesis” that homocysteine was a risk factor for heart attack.5-7 Beginning in the mid-1990s, however, the findings from large human trials concluded that high homocysteine levels were associated with a significantly greater risk of heart attack and stroke. More recent studies not only confirm the cardiovascular dangers of homocysteine, but also its toxic effect on the brain. It turns out that high blood levels of homocysteine increase the incidence of depression, memory impairment, and even Alzheimer’s disease. The latest findings reveal that elderly people with common degenerative diseases frequently present with very high levels of homocysteine. The medical establishment woke up to the dangers of homocysteine when The New England Journal of Medicine and The Journal of the American Medical Association (JAMA) published articles suggesting that vitamin supplements be used to lower homocysteine levels.8-9 The evidence that homocysteine increased heart attack risk was substantial in 1981. It is regrettable that 22 years later, most doctors still do not recognize homocysteine as a toxic amino acid that should be reduced in the bloodstreams of all their aging patients. What Doctors Are Overlooking Some doctors now recognize the lethal role that homocysteine plays in the development and progression of common degenerative diseases. These doctors, however, seldom treat their patients in a scientific manner. For instance, patients with high homocysteine levels are sometimes told to take a folic acid supplement or moderate doses of vitamin B12, vitamin B6, and folic acid. Homocysteine blood levels are rarely checked again. These vitamin-prescribing doctors assume that homocysteine will be adequately lowered as long as the patient takes the recommended dose of vitamins, which is often the same dose recommended for all their patients. | |||||||
Original research conducted by Life Extension years ago, however, showed that there is a huge individual variability in the amount of B vitamins needed to keep homocysteine in optimal (safe) ranges. Life Extension found that a substantial percentage of members who were taking the recommended potencies of B vitamins were not achieving adequate homocysteine control. In these members with elevated homocysteine, higher doses of vitamin B6 and/or TMG (trimethylglycine) were needed to bring homocysteine down to the safe range. Doctors who fail to check their patients’ blood for homocysteine regularly and adjust the B vitamin dose accordingly are not going to reduce homocysteine to safe ranges in most of their patients. It is usually up to the patients themselves to take the initiative to inform their doctors that they intend to maintain their blood homocysteine levels below 7-8 µmol/L of blood. What Causes Homocysteine Overload
Another way the body rids itself of excess homocysteine is through the trans-sulfuration pathway, which is dependent on vitamin B6. As long as adequate levels of vitamin B6 are present, homocysteine is converted into beneficial cysteine in the body via this trans-sulfuration pathway. Those with moderate homocysteine elevation may respond to the daily intake of 800 mcg of folic acid, 600 mcg of vitamin B12, 100 mg of vitamin B6, and 500 mg of TMG. Life Extension has found that aging members sometimes require vitamin B6 in doses ranging from 250 to 1000 mg a day* and/or 1500 to 3000 mg of TMG a day to reduce homocysteine to a safe range (below 7-8 µmol/L of blood). Reducing consumption of foods that contain lots of methionine (such as red meat and chicken) can lower homocysteine. Scientific studies point to aging, vitamin deficiency, and chronic disease states (such as kidney failure) as common causes of hyperhomocysteinemia. Scientific Publications Recognize Homocysteine In the 1990s, however, the number of published studies swelled to 648, and most of them pointed to homocysteine as a culprit in the development of coronary artery disease and coronary thrombosis (heart attack).13 The last several years have seen an exponential increase in the number of scientific studies about homocysteine. From 2000 to the present, an astounding 553 papers were published about the significant role of homocysteine in the development of multiple age-related diseases.14 In fact, more studies have been published about homocysteine so far in 2003 than in the entire decade of the 1980s. An example of the kind of recently published research can be found in the August 22, 2003 issue of the journal Circulation Research. This study revealed how relatively low levels of homocysteine (10 µmol/L) can inflict massive damage to the arterial wall via several destructive molecular mechanisms.15
Folic Acid Is Not the Solution Life Extension discovered many years ago that high doses of folic acid by themselves do not sufficiently lower homocysteine levels. In one case, a member taking 20,000 mcg a day of folic acid saw his homocysteine level remain persistently high. A new study on kidney failure patients reveals just how critical vitamin B12 is in protecting against homocysteine overload. People suffering from end-stage kidney disease often manifest very high levels of homocysteine. In this study, doctors gave one group of patients oral supplements that contained 5000-6000 mcg of folic acid, 6-10 mcg of vitamin B12, and 5-10 mg of vitamin B6. The other group received a (1-mg) B-12 injection weekly in addition to the daily oral supplements.16 In the group receiving the weekly B-12 shot, homocysteine levels decreased by 32%, while the group receiving the oral folic acid, B12, and B6 did not show a change over the 8-16 week study period. What was so impressive about this study is that the subjects initially had normal blood levels of folic acid and vitamin B12. In response to the vitamin B12 injections, serum B12 levels increased more than 60-fold from 625 to 40,400 pmol/L, which resulted in a reduction of homocysteine by an average of 32%. This study showed a direct linear correlation between increased blood levels of vitamin B12 and decreased levels of homocysteine. One flaw in this study was that the oral dose of vitamin B12 (6-10 mcg) in the control group was very low. Most Life Extension members obtain over 600 micrograms a day of B12. The oral dose of vitamin B6 (5-10 mg/day) used in this study was also below the higher amounts (100 mg and above) taken by most Life Extension members. It is possible that if higher oral doses of vitamins B12 and B6 had been used in this study that compared B12 injections to folic acid-B12-B6 supplements, a reduction in homocysteine may have occurred. The value of higher oral doses of vitamins B12 and B6 was shown in another published study17 on end-stage kidney disease patients. Those receiving 100 mg of vitamin B6, 1000 mcg of vitamin B12, and 16,000 mcg of folic acid showed a 30% reduction in homocysteine levels—comparable to the 32% reduction seen in patients receiving weekly B12 shots. While most people can adequately lower homocysteine levels by increasing their oral intake of TMG and vitamins B12 and B6, elderly people sometimes suffer absorption difficulties and need weekly B12 shots that require a doctor’s prescription. For those whose homocysteine levels remain above 7-8 µmol/L despite taking the recommended oral doses of folic acid, TMG, and vitamins B12 and B6, a weekly 1-mg vitamin B12 shot is strongly recommended. | |||||
| Life Extension has consistently found that taking very large doses of folic acid does not lower homocysteine much more than moderate folic acid intakes. When members present with stubbornly high homocysteine levels, we have seen significant reductions when the dose of vitamin B6 and/or TMG is increased, but not when folic acid intake is increased. If homocysteine levels remain high despite higher intakes of TMG and B6, there is now the option of taking a weekly 1-mg vitamin B12 shot or trying very high sublingual doses of vitamin B12.
Why Homocysteine Blood Testing Is So Crucial As humans age, they sometimes need to increase their intake of homocysteine-lowering nutrients because their natural detoxification systems are no longer adequate. It is not possible to “guess” what one’s homocysteine levels may be. The only way to maintain safe ranges of homocysteine is to have your blood tested, follow the appropriate homocysteine-lowering program, and then retest your blood 30-90 days later to make sure you have reduced homocysteine to a safe range (below 7-8 µmol/L). Any other approach is the equivalent of throwing darts with your eyes blindfolded. Medicare Will Not Pay for Homocysteine Blood Testing Instead, Medicare classifies homocysteine as a “non-covered” test. We were told that Medicare always refuses to pay for the test because it considers the test “not medically reasonable and necessary.” The homocysteine blood test is FDA approved and the scientific literature conclusively links elevated homocysteine to increased risk of disease in the elderly. Yet Medicare denies payment for it. We believe that these kinds of illogical rules will accelerate Medicare’s collapse into insolvency in the not-too-distant future. | ||
Dramatic Reduction in Blood Testing Prices The retail price of a homocysteine blood test at a commercial laboratory is over $180. Life Extension members used to pay $85 for it. Under the new lower pricing structure, members of The Life Extension Foundation can obtain a homocysteine blood test for only $64. We are pleased to announce these price reductions because we know that blood tests provide a solid scientific basis for keeping Foundation members alive longer. I cannot tell you how many early-stage prostate cancers have been diagnosed because of the convenient mail-order blood-testing service we offer. I urge all Foundation members to have their blood tested at least once a year. If homocysteine levels are elevated, aggressive actions should be undertaken to lower it. After higher amounts of nutrients such as TMG and vitamin B6 have been taken for 30-90 days, another homocysteine blood test should be done to make sure levels are below 7-8 (µmol/L). While people often fear cancer more than cardiovascular disease, the irrefutable fact is that heart attack and stroke kill far more Americans than cancer and most other diseases combined. Overwhelming evidence suggests that keeping blood markers such as homocysteine in safe ranges can dramatically reduce the risk of vascular disease. I personally have been a beneficiary of having my blood tested and always recommend it on my media appearances. View information on the most important annual blood test for men and women to consider. For longer life, William Faloon. |
| References |
| 1. Salles-Montaudon N, Parrot F, Balas D, Bouzigon E, Rainfray M, Emeriau JP. Prevalence and mechanisms of hyperhomocysteinemia in elderly hospitalized patients. J Nutr Health Aging. 2003;7(2):111-6. 2. Robinson K, Mayer EL, Miller DP, Green R, van Lente F, Gupta A, et al. Hyperhomocysteinemia and low pyridoxal phosphate. Common and independent reversible risk factors for coronary artery disease. Circulation. 1995 Nov 15;92(10):2825-30. 3. Ventura P, Panini R, Verlato C, Scarpetta G, Salvioli G. Hyperhomocysteinemia and related factors in 600 hospitalized elderly subjects. Metabolism. 2001 Dec;50(12):1466-71. 4. Naurath HJ. Hyperhomocysteinemia in advanced age. Clin Chem Lab Med. 2001 Aug;39(8):695-7. 5. Wilcken DE, Reddy SG, Gupta VJ. Homocysteinemia, ischemic heart disease, and the carrier state for homocystinuria. Metabolism. 1983 Apr;32(4):363-70. 6. Mudd SH, Havlik R, Levy HL, McKusick VA, Feinleib M.A study of cardiovascular risk in heterozygotes for homocystinuria. Am J Hum Genet. 1981 Nov;33(6):883-93. 7. Rossouw JE, Labadarios D, Jooste PL, Shephard GS. Lack of a relationship between plasma pyridoxal phosphate levels and ischaemic heart disease. S Afr Med J. 1985 Apr 6;67(14):539-41. 8. Malinow MR, Duell PB, Hess DL, Anderson PH, Kruger WD, Phillipson BE, et al. Reduction of plasma homocyst(e)ine levels by breakfast cereal fortified with folic acid in patients with coronary heart disease. N Engl J Med. 1998 Apr 9;338(15):1009-15. 9. Tucker KL, Mahnken B, Wilson PW, Jacques P, Selhub J. Folic acid fortification of the food supply. Potential benefits and risks for the elderly population. JAMA. 1996 Dec 18;276(23):1879-85. 10. Verhoef P, Stampfer MJ, Buring JE, Gaziano JM, Allen RH, Stabler SP, et al. Homocysteine metabolism and risk of myocardial infarction: relation with vitamins B6, B12, and folate. Am J Epidemiol. 1996 May 1;143(9):845-59. 11. Stampfer MJ, Malinow MR, Willett WC, Newcomer LM, Upson B, Ullmann D, et al. A prospective study of plasma homocyst(e)ine and risk of myocardial infarction in US physicians. JAMA. 1992 Aug 19;268(7):877-81. 12. references no longer posted 13. references no longer posted 14. references no longer posted 15. Zeng X, Dai J, Remick DG, Wang X. Homocysteine mediated expression and secretion of monocyte chemoattractant protein-1 and interleukin-8 in human monocytes. Circ Res. 2003 Aug 22;93(4):311-20. 16. Elian KM, Hoffer LJ. Hydroxocobalamin reduces hyperhomocysteinemia in end-stage renal disease. Metabolism. 2002 Jul;51(7):881-6. 17. Bostom AG, Shemin D, Lapane KL, Hume AL, Yoburn D, Nadeau MR, et al. High dose-B-vitamin treatment of hyperhomocysteinemia in dialysis patients. Kidney Int. 1996 Jan;49(1):147-52. |