Life Extension Magazine®

Ever thought about growing a new brain? It may not be as far-fetched as you think. Dr. Fred H. Gage of the Salk Institute in La Jolla, California has discovered baby brain cells deep inside the adult brain that can grow up to become new brain. His work is blowing apart the notion that a damaged brain or spinal cord can't be fixed. LIFE EXTENSION magazine's contributor, Terri Mitchell, interviews Dr. Gage.
Terri Mitchell: For years we've been told that the brain can't regenerate, can't recover. But you have data from the 1960s saying it can. What's the story?
Fred Gage: As early as 1962, a fellow named Joe Altman did experiments where he put a chemical into rodents that highlights dividing cells. Surprisingly, he could see brain cells were forming in adults, and it looked like they were becoming neurons.
TM: What happened between 1960 and now?
FG: There was a burst of activity around that time, and people were trying to replicate his results. But the technology wasn't good enough to convince everyone. Also, even though they saw it, they thought it was just a phenomenon left over from leeches and other animals.
TM: So how did we get from there to where we are now?
FG: About 1983, Fernando Nottebom at Rockefeller observed that birds sing new songs every season. He also found that their singing new songs coincided with them making new brain cells. Apparently, every season, brain cells were dying and new ones were being born in the brains of these birds. But it wasn't until the 1990s that we had the technology that would enable us to examine the question of whether or not new neurons were being created in adults. While the concept has been around for awhile in several different fields, the proof wasn't demonstrated until recently.
TM: How did this come about?
FG: It had been established in animals, and in the '80s a couple of groups showed it occurring in primates, but it was really controversial because of the methods being used. So we decided that we would try to figure out a way to find out if new cells were being made in humans. That seemed to be the most important question.
So about 1996, we decided to try using a chemical that marks dividng cells called bromodeoxyuridine (BrdU). If you put it into the blood stream, it will go everywhere in the body. When DNA is undergoing synthesis, BrdU slips in and becomes a red flag that the cell has divided. And because it's in the blood-stream maybe an hour-you can see all the cells that underwent cell division during that hour. You can even come back years later and look for that tagged cell and find out what the cell became, where it went, and what it did.
TM: So you put BrdU into humans?
FG: No, we didn't have to. It was already being used in cancer patients. They use it diagnostically. What they do is give it, and then do a biopsy of a tumor, section through it and count the number tagged cells. This gives you a fix on how rapidly the tumor was growing.
TM: This is useful for testing whether a cancer therapy is working or not-
FG: Right. But BrdU goes throughout the whole body. So the first thing we did a couple of years ago was try to get autopsy material from people who may have been in a clinical trial, and were treated with BrdU.
TM: If you found enough people with BrdU showing up in their brains-it would mean new cells were being created there.
FG: Yes. So we got sections of the brain from people and we stained for BrdU, and we were surprised to see that there were dividing cells. But the key is not just dividing cells, but that they actually turn into neurons. Unfortunately, the sections we got from these old storage banks were not adequate to do the modern technique to prove with double labeling that the newly-formed cells had turned into neurons. So we realized that we needed fresh tissue and that's when one of my fellows went back to his university and got involved in clinical trials where cancer patients were being given BrdU. Some of these people agreed to let us look at their brains if they died. We finally got five patients, and we were able to confirm that in the area we were examining, new cells were being born and they were turning into neurons.
TM: How old were these people?
FG: They were between 55 and 72 years old, so they weren't young patients. Nevertheless, we were able to say, one, that there is cell division in the human, and two, that some of the cells give rise to neurons, and this seems to persist hroughout life even in unhealthy people.
TM: But this only occurs in certain areas, is this right?
FG: Absolutely. One of the problems through the years was that scientists looked at the brain and saw that neurons were not getting smaller or less in number-things seemed pretty static. But several groups, including ours, have been able to show that if you go to certain areas of the brain, and take cells out, you can put them in a petri dish and make them grow. They're called stem cells.
TM: In other words, they are baby brain cells. But isn't "stem cell" a term usually used for cells in bone marrow that turn into blood cells-are you saying the brain has stem cells too?
FG: They fit the criteria for stem cells, so, yes, the brain has undifferentiated cells that "grow up" to become neurons and glial cells and all the different cells in the brain. The discovery of these dividing cells helped people to overcome the idea that the brain can't make new neurons. The trick in understanding how the brain could make new neurons came with the identification in the adult brain areas where these baby cells persist throughout life. Before, the thinking had been that you couldn't replace brain cells because of all their elaborate connections and what they store-they're such differentiated and complex cells.
TM: You would lose all your memories if you replaced your brain cells-
FG: Your memories, your motor function-if you were to have a turnover of the cells that control motor function, that would be quite a distressing event.
TM: So how is it that the brain will allow new cells in?
FG: We're just beginning to generate hypotheses about this. But we
think that only parts of the brain where storage of information is not an issue are the ones that can renew. The hippocampus is one of those areas. It's believed that the
hippocampus is integral to forming new memories. We think a memory is formed when a lot of information is linked together. Think about the fact that when you have a memory, you won't think of just one thing, but you'll think of a voice, link it to a face, and maybe a smell or emotion. These signals come through different areas of the cortex and then converge in the hippocampus where they're integrated and then farmed back to the cortex in the form of a memory. But the idea is that the hippocampus is a critical way station for excitatory information coming in-not a storage site. It's a processor of information, and as such, it and the other areas like it, are vulnerable to overstimulation so there is a selective advantage to having these areas be able to renew themselves. There's no downside to renewing them because you're really not asking these cells to store information-you're only asking them to process.
TM: So the brain may keep reserves of baby cells in case these processor areas get damaged?
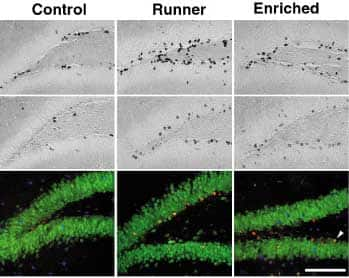
Photomicrographs of BrdU-positive cells one
day (top panel) and four weeks (middle panel),
in control (left row) in runner (middle row) and
enriched environment (right row).
In the bottom panel, confocal fluorescent images
of BrdU (red) and NeuN (green) indicating neuronal
phenotype. Arrow shows BrdU-labeled neurons
(orange is red plus green).
FG: Yes, the neurotransmitter that conveys new information is glutamate, and at too high a level it is actually toxic to brain cells. So, the brain may have "back-up" cells just in case.
TM: What do stem cells do?
FG: A stem cell is a cell that self-renews-that means when it divides, it creates another stem cell and also can become a mature cell. That's one feature. The second feature of a stem cell is that it can turn into different types of brain cells. And we've proven that you can take stem cells out of the brain, put them in culture, transplant them back into an adult brain, and they'll pick up where they left off. We haven't done this in humans yet. But given the similarities between the work we've seen in animals and humans to date, I think we're confident that what we're dealing with is a cell that will act this way.
TM: You said that you can take the stem cells out, put them in a petri dish and make them turn into neurons and other brain cells. How do you get them to grow?
FG: By using certain things called "growth factors". One of the factors we use is fibroblast growth factor.
TM: That's surprising-why use a factor that makes skin grow when you're trying to make brain cells grow?
FG: Fibroblast growth factor was originally discovered as a factor that stimulates fibroblasts (skin cells) to growth, but now everybody knows that fibroblast growth factor has very strong potential in the central nervous system. It's not unusual for growth factors that are originally discovered in, say, the liver or someplace else to also affect other organs. If there are receptors for this factor, it doesn't matter what type of cell it is. Wherever there are receptors for the factor, the factor will go. It turns out that both in the adult and developing brain there are receptors for fibroblast growth factor, and it plays a really important role in neural development. There are now 16 known fibroblast growth factors.
TM: So you've been able to take baby brain cells out of the brain, douse them with growth factor and reawaken them, is that right?
FG: Right, and I think the big challenge to all of this, both in the culture dish and in the brain, is to understand what are the factors involved in keeping them alive and in a primitive state. And then we want to know what are the factors that cause them to differentiate into "adult" cells. And how do they make a choice between becoming a neuron or a glial cell, for example? And when they become a neuron, what are the factors that make them become one type of neuron versus another? How do they hook up so that they can function? It's one thing to say you're a neuron; it's another to say you're wired for action. So one of the big challenges is figuring out which factors help make the baby cells make choices.
TM: Would it be more important to make neurons than glial cells?
FG: Not necessarily. Glial cells are support cells, and they make many of the important factors themselves. Of course you would like to know how to make neurons, but you also want to be able to create an appropriate balance between the different cell types. For example, the connections between cells require myelination which is dependent on a type of glial cell. This cell allows for appropriate electrical conduction. So while I think it's okay to divide things up into saying you're going to make an acetylcholine neuron or a dopamine neuron, the reality is that at the end of the day you need an appropriate balance of the different cell types.
TM: So the idea here is that during fetal development and probably to a certain extent after birth, baby brain cells are exposed to growth factors, and then something shuts them off-is that right?
FG: In most places. Or the growth factors take on a different role. For example, fibroblast growth factor can still affect adult cells-it induces them to survive rather than divide. So you can shift the function. But the effect of fibroblast growth factor on stem cells that persist only in certain areas such as the hippocampus, is to send a signal to them to proliferate. So, again, just like a growth factor can have different effects on different types of cells, it can also have two different functions in the same cell-a survival factor or a growth factor, depending on the state of the cell when it meets up with the factor.
TM: We've talked about taking stem cells out of the brain, putting them in a petri dish and making them grow. What about making them grow in the adult brain?
FG: We can do it. In animals. The problem with humans is you can't monitor what's happening. Obviously, the factors we use will stimulate baby brain cells to proliferate in the brain. What we don't know yet is whether or not this will have good or bad effect on function.

with other mice, stimulation with different objects or
"toys" and choices of different foods.
TM: You did some very interesting experiments with mice and environmental stimulation. Can you tell me about that?
FG: One of the surprises came several years ago when a post-doc named Gerd Kempermann took mice and put them in an enriched environment-by that I mean lots of toys and more animals to interact with. After 45 days, he went back and counted the number of cells in the brain that were dividing and had become neurons. Just by shifting the environment, he could increase the total number of brain cells in certain parts of the brain.
TM: By how much?
FG: Fifteen percent which is 50,000 cells per area which is a substantial amount relative to the size of the animal's brain. Those were young animals-two or three months old. But we also took animals that were eighteen, twenty months old-who had spent their whole lives in a bare cage-and shifted them into an enriched environment. Two things happened. One, we found that neurogenesis (new neurons) is occurring even in these old animals (albeit at a slower rate). And, two, that new cell growth coincides with increased performance of certain tasks.
TM: So the stimulating environment actually increased the survival of brain cells?
FG: Right, and then the next thing that happened is that a researcher named Henrietta van Praag said, 'I wonder what element of this enriched environment is really important.'
The area of the brain where the neurogenesis took place is the hippocampus which is important for learning and memory, so maybe just by learning something, you could activate this process. So she set up training schedules for a batch of new mice, and they were trained to do certain tasks. Some of them were put in a complex environment, some had minimal exercise, and some had a running wheel placed in the cage so they had free access exercise. And the surprise was that there was a dramatic increase in cell proliferation in the animals with the running wheel. They would run six to ten hours a day (but it's actually not running because it's all ball bearings so it's almost like walking). This was a big surprise. What we saw is that having the running wheel nearly doubled the number of dividing cells. And at the end of four weeks, we looked at how many of these newly-born cells became neurons. We saw that the increase in neurons was equivalent to the animals that had the enriched environment. The enriched environment did not increase the pool of dividing cells, but provoked greater survival of those cells, whereas running actually increased the entire pool. The same number of cells died, but the net effect was that the runners ended up with as many cells as the enriched environment group. And both groups were about double the cells of the control group.
TM: Any ideas about why this occurred?
FG: Either the activity induces a change in the brain that causes the cells to divide and survive, or a factor is coming in from the blood stream since we know that new blood vessels form in the brain when you exercise.
TM: You have ideas for something called a "biological pump." What is that all about?
FG: You could take a cell and genetically engineer it to make something like fibroblast growth factor and transplant it into the brain. There it would pump out factors.
TM: You would engineer a brain cell?
FG: No. You could take a skin cell that normally doesn't do anything but sit there, and put the gene in it for fibroblast growth factor, for example, grow it in culture, and then transplant it into the brain. It would sit there and pump out factors.
TM: Have you done this?
FG: Yes, and we've found, for example, in spinal cord injuries, if you engineer skin cells to make a growth factor called NT3, you can stimulate proliferation of new cells, and what appears to be myelination of growing axons. And those new cells are coming from populations of cells in the spinal cord that don't normally give rise to neurons. (ed. note: myelin is a coating around nerve cells. Axons are the "arms" of nerves that connect with other nerves).
TM: In essence, you're saying that you've found in the spinal cord what you've discovered in the brain, i.e., reservoirs of baby cells that have the potential to "grow up" and become mature, functioning cells.
FG: A clarifying point here is that while the birth of neurons only occurs in two areas-the hippocampus and the olfactory bulb, new cells are formed in other parts of the nervous system but they usually turn into glial cells or turn into nothing. They're just sort of being born and dying off. It's a bit of a conundrum. What we're trying to figure out right now is how to train those cells to become things they don't normally become. Now, you don't want them to become things they shouldn't become. For example, this is interesting with regard to epilepsy. It has been shown recently in experimental animals that if you induce epilepsy (and by the way, the hippocampus is very much a target for epilepsy and a lot of focal seizures begin in the hippocampus)-if you induce epilepsy, there's a massive increase in new cells in the ihbaby cellly zone, but they migrate to the wrong place and create a layer of neural cells. This is highly abnormal, and it causes bad wiring. This kind of thing highlights the idea that while new brain cells are a great thing, one needs to be aware that control and regulation of those cells is key.
TM: This line of research where you are engineering cells is a different line of research than your work with growth factors-
FG: Yes, we haven't yet mixed those two stories, although we are thinking about ways we could activate brain cells to make the factors themselves by sending in a bio-engineered cell.
TM: You are working with a very interesting vector to deliver messages into the mature brain-messages such as 'make growth factors'. You've gutted a human immunodeficiency virus (HIV) and you're using its shell as a way of getting into brain DNA.
FG: Right. The starting virus is HIV, and this work is being done together with Dr. Inder Verma here at the Salk Institute. After we engineer it to cut out the bad stuff, there's only about 15% HIV left. We only need a few HIV proteins-ones that allow it to get into non-dividing cells. This is the trick because mature brain cells, unlike other cells in the body, don't divide. The thing that sets HIV apart from other viral vectors is that it can get into these grown-up, non-dividing cells. That's why we use it.
TM: And what do you put inside the HIV that you deliver into mature brain cells?
FG: Therapeutic genes. In other words, genes that would correct a deficiency in that cell. Hemophiliacs, for example, lack certain genes that could be delivered with a viral vector. Once a gene is inserted into DNA, it can manufacture something that the cell needs, but doesn't currently have.
TM: Dopamine, for example, could be delivered into the brains of people with Parkinson's disease-
FG: Experimentally, people are doing that now. However, what area you put it in, how much you put in- questions that might be characterized as trivial-are not so trivial when you think about it because if you give too much L-dopa, you create problems. These questions have to be answered before we can say we have viable therapy.
TM: How long will one of these genes continue to operate? Will it stop at some point?
FG: That's one of the things that's been a problem with gene therapy. Some viruses get shut off with time. So far the lentiviruses seem to stay on for at least a year, year-and-a-half (ed. note: lentiviruses are the family of viruses to which HIV belongs). But nobody has done it in humans yet, and there is a question as to whether or not you mount an immune response at some point. Another virus that seems to have staying power is adeno-associated virus (AAV).
TM: What's the difference in what you're doing and fetal tissue transplants?
FG: With a viral vector, what you're doing is putting a gene into the cells that helps them do new things. With fetal tissue, you're substituting or adding more cells, asking these ihfetallc cells to grow up and function. But actually, it seems funny to say this, but fetal cells are already quite mature-committed to becoming one thing or another-so you can't get them to divide so easily. Unless you isolate just that small population of remaining cells, divide them up and train them to become mature fetal cells, then graft them. The problem with fetal tissue as it stands right now is getting enough tissue. In Sweden, where fetal cell transplant is successful, they're using nine to twelve fetuses per patient. No matter what your philosophy is about that, it's just not practical. The idea of using stem cells to create new populations of cells is that you can grow a lot of cells from just a few cells because stem cells are truly young cells.
All the strategies are somewhat different, and it's too early to tell at this point what's right or what's wrong, what's better or worse-the important thing is that people are going down multiple pathways to find rational approaches to brain regeneration.
TM: When does the brain begin to deteriorate?
FG: It doesn't, actually. It's really amazing. Normal, healthy brains don't decline and degenerate. If you go to certain areas of the brain in healthy individuals, the numbers of neurons don't really change. You're not losing brain cells, but you may lose net functional capacity of existing brain cells-which is a different story than saying the cells are gone.
TM: So the cells are there, but they're not acting right?
FG: Right, studies where they count the number of cells are showing that there aren't big differences in the number of brain cells in older people. Disease is a different story. You can see with the naked eye cell loss in Alzheimer's and Parkinson's diseases. The idea that the brain just starts shrinking and dying with age just isn't true.
TM: These factors that you've talked about may be critical in maintaining or restoring the functional capacity of brain cells-in essence reversing the effects of aging, is this right?
FG: Right. But one thing I must emphasize. Clearly, people are showing that physical and mental activity has a role in maintaining brain cells. So you could theorize that areas of the brain that receive input from outside stimuli, in turn "talk" to the areas where baby cells are made.
TM: So the age-reversing factors might be naturally made in the brain if a person were to exercise and keep active?
FG: Yes.
TM: What are you working on currently?
FG: We're trying to figure out how to maintain brain cells in a proliferating state, how to get them to go to the right place, how to make them become the type of cell we want them to become. Certainly we would like to make neurons in area where they're not normally made. One of the goals is to get cells to divide and migrate to places of damage or degeneration. But right now we have our hands full just trying to figure out what factors do what with regard to brain cells.
TM: Any chance we're going to get to see some of this applied to humans soon?
FG: Some of our collaborators are proposing a clinical trial now because the safety seems to be quite clear.
TM: When did you start doing this research?
FG: 1969.
TM: Very interesting. Thank you for taking the time, Dr. Gage. We look forward to learning more about your research as it further develops.
Eds. note: as exciting as Dr. Gage's work is, he's not a clinician, and cannot treat patients, and we must respect his right to continue his research without interruption.
