| 
Last year, the scientists spearheading the Genesis Experiment, underwritten by the Life Extension Foundation, explained details of their pending research, and what they hoped to find. Here are the results. By Michael G. Darwin, Sandra R. Russell, and Steven B. Harris Gerontology is, at the present time, an infant discipline and an unintegrated one. In contrast to (say) genetics or evolutionary biology, we still do not have a workable theory of aging that both explains our data and excludes the other theories. Instead, there are a number of competing theories of aging that have yet to be integrated into a reasonably comprehensive and exclusive explanation of aging.
This lack of a unifying theory of aging poses problems for people who are getting old and dying now. The most important problem is that most research being done by experimental gerontologists is focused on unraveling the mechanisms of aging and development (in fact, Mechanisms of Ageing and Development is the name of one of the leading scientific publications in the field). Thus, this research will typically be rather far removed from clinical applications.
To put it bluntly, most scientists are working on things that may translate to anti-aging products that won't be available for 30 years. Or 50 years.
Undoubtedly, there are more empirical treatments that could be of considerable use in extending our lives. The problem is that the only way to have any idea if these possible anti-aging therapies really work is to do life span studies in a relevant animal model. This kind of research, however, is not very "clean," or elegant from a scientific standpoint. Because it doesn't elucidate a mechanism, it's not the kind of thing that gets you a Ph.D. Nor is it usually "hot stuff" for publication in academic journals, because most of the things one tries empirically to influence aging are not going to work.
For these reasons, such wild experimentation-where the experiments are really experiments, instead of nearly sure things-do not constitute the kind of research most scientists turn to in order to advance their careers.
For these reasons, the Life Extension Foundation has decided to pick up the ball that government and industry has dropped, and is funding studies aimed at achieving rejuvenation...in other words, partial reversal of aging or degenerative changes associated with aging. These are interventive studies which, without fully understanding the mechanisms of aging, seek to find influences on aging anyway by means of shrewd hunches.
We know this is possible. We know, for example, that dietary restriction, the most promising method to slow aging in mammals right now-and the one that has been the focus of thousands of experiments-was discovered by a shrewd hunch (and partly by accident) in experiments with rats done in 1935.
Even after three generations of scientific study, we still do not know how dietary restriction works. Yet surely there must be more ways to influence the aging process than restricting the diet. More hunches need to be tried.
The first of the Life Extension pilot project studies attempting to influence aging has now been completed. Called the Genesis Experiment, it is a classic example of research that would not typically be possible to do in a university or industry setting.
As laid out in our first article on the beginnings of the Genesis Experiment last year, we proceeded on the basis that adult animals (including humans) can be "re-populated" with missing cell types. The most common example of this is the administration of bone marrow intravenously for patients who have undergone chemotherapy or radiation treatment for cancer. In this case, the cells find their own way to where they need to go. And in fact, among the theories of aging, two state that aging occurs in part or in whole because of a decline in the population of young viable cells in the body.
We wanted to find out if the administration of "youthful" stem cells-the cells that give rise to the various differentiated tissues of the body-could extend life span in aged animals. This could be considered similar to cell therapy in humans, whereby humans have for years been given cells from animal fetuses. However, our goal was to use animals that are inbred, and thus identical in their tissue makeup, to eliminate cell rejection.
We divided a group of Fischer 344 rats into three groups containing 10, 10 and nine animals. One group (experimental) was given prepared fetal tissue by intraperitoneal administration (into the abdominal cavity). The live fetal cells, which had been prepared and gently separated in a nutrient buffer so that they were able to pass through a needle, came from fetuses removed from five females euthanized painlessly at 10 days of gestation.
Another group (control) was given the vehicle solution in which the fetal cells had been prepared (Hank's Balanced Salt Solution) in the same manner as the experimental group, but with no cells.
A third group (also a control) was given the same solution containing a similar mass of separated cells from adult liver and spleen tissue, which had been obtained from a non-pregnant female of the same age as those used to provide the fetal tissues.
The purpose of this last group was to serve as a control on the sterile technique and the preparation technique used to generate the injected fetal tissue cell suspension material. The spleen of adult animals also contains many immune cells, including stem cells. If we saw any effects on life span, we wanted to be able to say they were due to fetal cells, and not just stem cells that could also be obtained from a younger adult animal.
By 310 days into the study (7.2 months after injection), all the already aged animals were dead. The longest lived animal died at 921 days (30.2 months) of age, the human equivalent of about 90 years. It is also reasonably close (given our relatively small number of animals) to the maximum life span of Fischer 344 rats fed lab chow diets whenever they like (called an "ad libitum" diet).
How did the experimental groups do? They did very much the same as the group overall, and they died at close to the average time one would expect for Fischer 344 rats starting from advanced ages (for which life expectancy would be expected to be a little longer than average). The average ages at death were 801, 829, and 814 days for the three experimental groups. The differences were not significant.
Genesis Life Span Curve
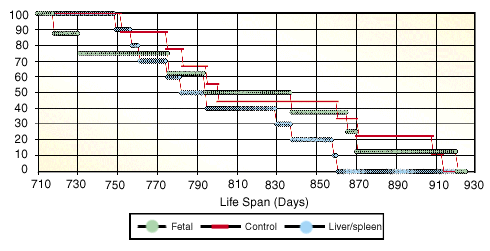 The genesis Experiment showed slight differences in the average life spans of the three groups of test animals, but the differences and the groups themselves were too small to be statistically significant. New tests will include larger groups of animals.
Our conclusion, therefore, is that the treatment as we tried it, with just one injection of fetal tissue, didn't work. The fetal cells themselves didn't seem to harm the animals, but they didn't help them, either. Since a shot of fetal cells is claimed to help humans for much longer than seven months, we would have expected to see results perhaps from a single injection in rats with less than seven months to live. Yet we did not.
The fetal cell rejuvenation business is a tough one. It's a lot easier to convince wealthy older people paying a lot of money for fetal cell injections that it works, than it is to see results in a laboratory under carefully controlled conditions. And now we can also see why university scientists are loathe to do experiments like this one. Even if they are done well, they can return data that aren't exciting.
But all is not lost, and it should be emphasized that we are just at the beginning of these experiments. We can confidently try models in the future in which we utilize more injections, and injections of cells from different times in fetal development. Our animals are doing as expected in a very good lab environment, and our experiments are evidently being done under conditions of adequate sterility, so that we are not causing obvious harm by tissue injection.
For the remainder of their life spans after injection, our rats did as well as rats in the best facilities elsewhere. All this is important for future research plans.
Some of the staff at 21st Century Medicine had not worked with rodents before, and were quite amazed at the differences exhibited among the various animals as they aged. The animals died naturally at widely different ages, from 718 to 921 days. They died from widely different cancers, from lymphoma to bowel cancer. Visually, some of these normally white animals appeared old and yellowed long before others did. And yet these animals are all genetically identical. Moreover, they were housed the same way, and ate the same food.
What causes these differences? We don't know. We do know that some aging differences between animals, from arthritis to graying and inactivity, happen in all rodent experiments, even those using inbred (identical) strains. Some animals simply age more quickly, and die far sooner than others, even if they have the same genes and environment.
So what is there, besides genetics and environment, that can influence aging and length of life? Evidently, there is an element in aging that has much to do with random chance. In the case of cancer, this idea by now should not surprise, for we now know that cancer arises due to random "hits" on the genes of certain cells. Although you can influence the odds by how you live, getting cancer is still much like losing at roulette.
Far more strangely, observations on inbred animals that are housed identically show that the aging process itself also contains some of this randomness. Perhaps this comes from random hits to genes in mitochondria, the powerhouse organelles of the cells where most free radicals are generated. Again, we don't know. This is a fertile area for future research, and one that has had too little attention paid to it.
We are now planning the second of the rejuvenation experiments. It is currently anticipated that more of these experiments will evaluate the effects of growth hormone supplementation on aging in Fischer 344 rats. Unlike the pilot Genesis Experiment, the Growth Hormone Study will probably include laboratory evaluations of blood and more detailed measurements of the animals' physiological response to the treatment.
In the first Genesis Experiment, the only endpoints were cause and time of death. Effects of treatment on lean body mass, total body fat, blood chemistries, immune function and other parameters of interest were not determined. However, as other agents and approaches to slowing or reversing aging in the aged adult animal are evaluated, there will be more detailed monitoring.
|