Food Reactions (Allergies, Intolerances & Sensitivities)
Food Reactions (Allergies, Intolerances & Sensitivities)
Last Section Update: 06/2024
Contributor(s): Shayna Sandhaus, PhD; Cristina Mathewman, PhD; Chancellor Faloon, Health & Wellness Author; Akash Goel, MD; Shanti Albani, ND; Scott Fogle, ND
1 Defining Food Reactions
Summary & Quick Facts
- There are several types of potential reactions to foods or food components.
- Food allergies are mediated by the immune system (eg, immunoglobulin E [IgE] or T cells)
- Food intolerances are non-immune mediated (eg, enzyme deficiency).
- Food sensitivities are adverse reactions to foods that are not better described as allergies or food intolerances. Some alternative medicine practitioners believe that IgG antibodies contribute to food sensitivities. Sometimes, “food sensitivity” is used as an umbrella term to refer to any non-allergy food reaction.
- IgE-mediated food allergies can be life-threatening and should be investigated by an allergist as soon as they are suspected.
- Microbiome imbalance, suboptimal gut function, some medications, and genetics are contributing factors.
- Certain dietary and lifestyle changes may help alleviate symptoms of some food reactions and improve overall health.
- Supplementation with nutrients such as vitamin D, flavonoids, probiotics, zinc, and digestive enzymes may help ease symptoms of some food reactions.
An adverse food reaction is any symptom caused by eating a specific food or food component. These reactions can range from mild to severe and may manifest in a variety of ways, including1-3:
- Digestive issues (eg, bloating, gas, diarrhea, constipation, abdominal pain)
- Skin rashes (eg, hives, eczema, psoriasis, acne)
- Headaches or migraines
- Fatigue or lethargy
- Joint pain or stiffness
- Brain fog or difficulty concentrating
- Mood changes (eg, anxiety, depression, irritability)
- Respiratory issues (eg, wheezing, coughing, nasal congestion)
- Insomnia or other sleep disturbances
- Muscle pain or weakness
- Anaphylaxis (a severe and potentially life-threatening systemic allergic reaction)
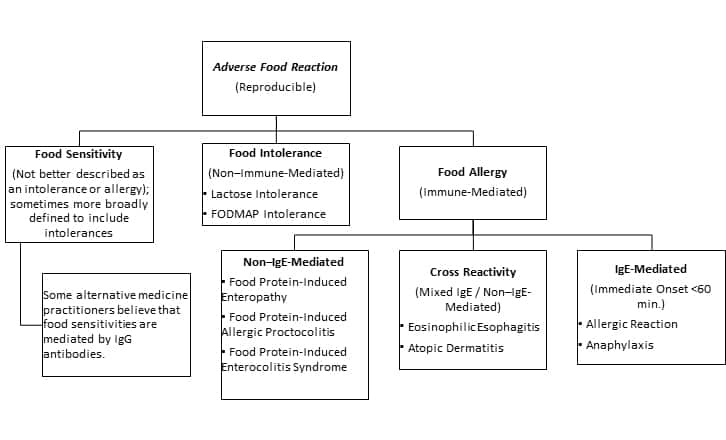
Many people may believe they have a food allergy when they experience discomfort or adverse symptoms after eating certain foods.1,4 However, it is important to recognize that there are several types of food reactions, each defined by how the body reacts to a specific food or food component. While some reactions may be immediate and severe, others may be more subtle or delayed.
The scientific literature and popular media are riddled with inconsistent terminology related to potential reactions to foods or food components. To minimize confusion, we at Life Extension use the following definitions as consistently as possible throughout this Protocol:
- “Food allergy” – Food allergy is an adverse reaction to food that is mediated by the immune system and is reproducible. Food allergies can be life-threatening. The common conception of “food allergy” refers to a rapid reaction mediated by immunoglobulin-E (IgE), though other types of allergic reactions to foods can occur.
- “Food intolerance” – Food intolerance is a gastrointestinal adverse and reproducible reaction to food that does not involve an immune reaction. Food intolerances are generally thought to result from difficulty digesting certain food components. Examples of food intolerances include lactose intolerance, histamine intolerance, and gastrointestinal reactions to fermentable oligosaccharides, disaccharides, monosaccharides, and polyols (FODMAPs).
- “Food sensitivity” – We use “food sensitivity” to refer to food reactions that are not better described as immune-mediated “allergies” or non–immune-mediated “intolerances.” Some innovative medical practitioners believe that IgG antibodies may mediate adverse reactions to foods that are sometimes described as food sensitivities. Sometimes, “food sensitivity” is used as an umbrella term to refer to any non-allergy food reaction.
- “Celiac disease” – Celiac disease is an autoimmune reaction triggered by the protein gliadin in gluten, the molecule that gives bread its soft texture. While some people may be allergic to gluten, celiac disease is not classified as an allergy. In celiac disease, the body mistakenly attacks the intestinal lining when gluten is ingested. Readers interested in celiac disease should refer to Life Extension’s Celiac Disease & Non-Celiac Gluten Sensitivity Protocol for more information; celiac disease is not covered in detail in this Food Reactions Protocol.
2 Causes & Contributing Factors
Food Allergies
Food allergies can be categorized as follows5:
- IgE-mediated allergies, also called hypersensitivities, that result in significant release of inflammatory factors such as histamine, leukotrienes, and cytokines
- Non–IgE-mediated food allergies where T cells of the immune system release inflammatory chemicals in response to signals other than those mediated by IgE
- Mixed-type food allergies, which have features of both IgE- and non–IgE-mediated allergies
The primary differences between these types of allergies are the part of the immune system involved in reacting to an allergen and often the timing of the response.6 Estimates of the prevalence of food allergies among U.S. adults range from about 3.5% to 10%.3,7
IgE-mediated food allergy. IgE-mediated food allergies are generally thought of as true or classical allergies. IgE-mediated food allergy symptoms occur when the immune system mistakenly identifies a food component (often a protein) as a harmful substance and produces IgE antibodies specific to the food allergen. This first exposure to an allergen is called “sensitization.” When the food is eaten again, the IgE antibodies recognize the allergen and signal immune cells called mast cells, eosinophils, and basophils. The mast cells and eosinophils release histamine and other compounds in a process called degranulation. The release of these immune-mediator compounds causes problematic and bothersome symptoms such as swelling, itching, congestion, and inflammation. These reactions may be mild but can also be life-threatening—such as in the case of anaphylactic shock, which requires immediate medical attention.5-8 If one suspects an IgE-mediated food allergy they should seek testing and guidance from an allergist as soon as possible to avoid these life-threatening reactions.
Common foods that may trigger IgE-mediated allergies in susceptible individuals include8:
- Peanuts
- Tree nuts
- Milk (different from lactose intolerance)
- Eggs
- Soy
- Wheat (different from gluten intolerance or celiac disease)
- Fish
- Shellfish
- Sesame
- Red meat (caused by sensitization to a compound called alpha-gal via tick bites)
In fact, potentially serious allergies to some of the above-mentioned allergens are common enough that the U.S. Food and Drug Administration (FDA) mandates that products containing the following allergens disclose as much on product labeling: milk, eggs, fish, shellfish, tree nuts, peanuts, wheat, soybeans, and sesame.
There are many hypotheses as to why allergies develop. For instance, one possibility is that allergies are a misdirected immune response that is meant to protect the body against parasites and other harmful invaders. However, in the developed world, where parasites are less of a threat, this same immune response can cause harm by leading to the development of allergies.9
- Loss of oral tolerance. Loss of naturally
occurring oral tolerance appears to be another contributing factor to food
allergies.10 The natural response to antigens in food is immune
tolerance, but some people gradually become less tolerant over time. This
issue causes sensitization to certain food antigens that leads to an
IgE-mediated food allergy.11
Oral immunotherapy may be recommended to circumvent the loss of oral tolerance. Oral immunotherapy involves administering food protein to an individual with a food allergy through oral ingestion to help increase the reaction threshold to a specific food allergen. The initial dosage is very low and is then increased carefully under the supervision of a qualified allergist to ensure safety. This process progressively restores oral tolerance for many individuals by promoting desensitization.10 A maintenance dosage helps sustain desensitization, but standardized maintenance dosages and schedules are still being established as of mid-2024. Studies are ongoing to determine the safety and efficacy of oral immunotherapy for the purpose of restoring oral tolerance. Oral immunotherapy should not be attempted without the supervision of a qualified healthcare provider.
The gut microbiota plays a vital role in establishing and maintaining oral tolerance to food allergens through mechanisms involving immune regulation, short-chain fatty acid (SCFA) production, and maintaining mucosal barrier integrity. Dysbiosis (ie, gut microbiome imbalance) is linked to increased susceptibility to food allergies, while probiotics and prebiotics may offer therapeutic benefits. Ongoing research continues to unravel the intricate connections between the gut microbiome and immune responses to dietary antigens.12-14
- Cross-reactivity of allergens. The IgE antibodies that the body develops against one antigen can sometimes also recognize other proteins through a process called “cross-reactivity.” For example, the proteins in cashews might be structurally similar to related nuts, such as pistachios, and cause an allergic reaction. In oral allergy syndrome, also known as pollen-food allergy syndrome (PFAS or PFS), IgE antibodies produced against plant pollens cross-react with proteins in raw fruits, vegetables, and nuts, which leads to itching and swelling of the mouth, lips, face, tongue, and throat soon after eating. However, the allergens in foods that IgE antibodies recognize are often heat sensitive (heat-labile) proteins, which means that cooked fruit or vegetables generally do not cause a reaction.8
Non–IgE-mediated food allergy. Non–IgE-mediated food allergies occur when the body’s immune system reacts to certain proteins in food as if they were harmful substances, thus triggering an inflammatory response. Unlike an IgE-mediated food allergy, which involves the production of IgE antibodies that bind to the allergen and trigger a reaction, non–IgE-mediated reactions involve a different type of immune cell called T cells.15,16 These T cells are activated by the allergen and release different immune signaling molecules, which cause inflammation and various symptoms. Non–IgE-mediated reactions are not usually life-threatening but can still cause significant discomfort and health problems.17 Typical non–IgE-mediated food allergies often affect infants and young children; these include food protein-induced enterocolitis syndrome (FPIES), food protein-induced enteropathy (FPE), and food protein-induced allergic proctocolitis (FPIAP). Celiac disease is a immune-mediated (non-IgE) food reaction that affects adults and children.3
Mixed-type food allergies. Mixed-type food allergies involve both IgE-mediated and T cell-mediated immune responses.18 Symptoms may include hives, swelling, itching, anaphylaxis, vomiting, diarrhea, abdominal pain, and eczema. When a food allergy involves both IgE-mediated and non–IgE-mediated immune responses, it can be more difficult to diagnose and manage because the timing of the reaction may be delayed.19 In addition, the specific symptoms often vary and may not always be immediately apparent. A few common triggers for mixed-type food allergies include milk, peanuts, eggs, shellfish, and wheat.18
-
Eosinophilic gastrointestinal disorders. Eosinophilic
gastrointestinal disorders (EGIDs) are a group of chronic digestive system
disorders characterized by an abnormal accumulation of eosinophils, a type
of white blood cell, in the gastrointestinal tract. These conditions can
affect any part of the gastrointestinal tract, from the esophagus to the
rectum. Examples of EGIDs include eosinophilic esophagitis (EoE), allergic
eosinophilic gastroenteritis, eosinophilic colitis, and eosinophilic
enteritis. Among EGIDs, EoE is by far the most common. Although EoE is
often diagnosed in infancy, the rate of diagnosis in adults is increasing.6,20,21
Symptoms of EGIDs include vomiting, gastrointestinal pain, swallowing difficulties, diarrhea, loss of appetite, and acid reflux that does not respond to antacids.8,22 Over time, the inflammation and damage that EGIDs cause can lead to the thickening of gastrointestinal tissues and difficulty moving food through the gastrointestinal system.20
Food Intolerance
In contrast to immune-mediated food allergies, food intolerances occur when the digestive system struggles to process a specific food or food component in an amount that is normally tolerated by most people.23 Food intolerances are not the result of an immune reaction; instead, they involve an abnormal digestive response. The amount of food ingested is directly linked to the severity of symptoms—as opposed to allergic reactions that can be triggered by even a very small amount of the allergen—and the specific food or food component causes the same type of symptoms with each exposure.8,24 The occurrence of food intolerances may be due to a lack of certain digestive enzymes that promote the absorption and breakdown of food in the gut.23 The classic example is lactose intolerance due to a deficiency of the lactase enzyme. Typical symptoms of food intolerance often manifest as bloating, gas, diarrhea, and abdominal pain.6,25
Carbohydrate intolerance. When certain carbohydrates are not digested and absorbed properly, they have an osmotic effect, causing water to accumulate in the small bowel. This can lead to bloating and drives food and water into the colon where bacteria ferment the carbohydrates, leading to excessive production of gas (eg, carbon dioxide, hydrogen, methane) that can cause flatulence and intestinal pain. These kinds of easily fermented carbohydrates are referred to as FODMAPs, an acronym that stands for fermentable, oligo-, di-, monosaccharides, and polyols.23,25
Gastrointestinal symptoms (eg, diarrhea) only develop under certain conditions, including the accumulation of malabsorbed carbohydrates at a rate that exceeds bacterial fermentation, thus causing an overload in the colon and digestive upset. Efficient bacterial fermentation is also disrupted by certain medications (eg, antibiotics) and the presence of inflammatory disease. In the absence of these circumstances, small amounts of malabsorbed carbohydrates may not cause symptoms of intolerance.
- Lactose intolerance. Lactose intolerance is a common adverse reaction to food that occurs when the body is unable to digest lactose, a type of sugar found in milk and other dairy products.26-28 This happens when the body produces insufficient amounts of lactase, the enzyme that breaks down lactose in the small intestine. Symptoms are generally proportionate to dose (ie, the more dairy a person consumes, the worse the symptoms may be). The prevalence of lactose intolerance in the United States is about 36% of the population. However, the prevalence varies substantially around the world, with lower prevalence in much of Europe (generally 10–40%) and much higher prevalence in the Middle East (about 50–90%) and central Africa (roughly 80–100%).29,30 The most common treatment for lactose intolerance is the avoidance of dairy foods and lactase enzyme supplementation.
- Fructose intolerance. Fructose intolerance is an adverse reaction to foods that contain fructose, a type of sugar found in many fruits, vegetables, and sweeteners such as honey or high-fructose corn syrup. The condition occurs when the body cannot efficiently absorb fructose in the small intestine, possibly due to a deficiency in fructose transporters. For this reason, fructose intolerance is usually referred to as fructose malabsorption, as the symptoms are caused by the inability to transport fructose rather than break it down.25 A hereditary mutation of the enzyme aldolase B can also cause fructose sensitivity, although this is rare.31 Fructose intolerance is usually diagnosed via breath tests. Supplementation with enzymes such as xylose isomerase may be used for the treatment of fructose intolerance, but the condition is generally managed by avoiding foods that contain fructose to minimize symptoms.31,32 Foods with high concentrations of fructose include honey; dried fruits; fruit juices; sweeteners like agave syrup and high-fructose corn syrup; sweet sauces such as barbeque sauce and some salad dressings; and some whole fruits such as pears, figs, and mangoes.33
- Sucrose intolerance. Congenital sucrase-isomaltase deficiency causes sucrose intolerance. Sucrose is a natural sugar found in a wide variety of fruits, vegetables, and nuts; table sugar is sucrose as well. Individuals may also experience a mild negative reaction to isomaltose, a carbohydrate released during the digestion of starch.25 Sucrose intolerance is diagnosed via duodenal biopsy.34 Since many foods contain these carbohydrates, it is often necessary to take a sacrosidase (sucrase) enzyme supplement (Sucraid).35,36 Estimates as to the prevalence of sucrose intolerance in adults vary geographically, ethnically, as well as among studies and with diagnostic method. One study reported an incidence of sucrose malabsorption of about 34% using hydrogen-methane breath tests and almost 27% using the 13C-sucrose breath test.34 Another study indicated that the incidence of sucrase-isomaltase deficiency, which leads to sucrose intolerance, ranges from 0.2% in North America to 10.0% in Greenland Inuit populations. These variations highlight the complexity and potential underdiagnosis of sucrose intolerance in the adult population.37
Pharmacological or chemical food intolerance. Pharmacological or chemical food intolerances occur when a person experiences an adverse reaction to certain foods due to the presence of additives or naturally occurring chemicals—as opposed to a protein as in most other types of reactions—in the foods that affect the body.6,38 Some chemicals that may cause symptoms of a pharmacological food intolerance include caffeine, histamine, and other biogenic amines, which are a specific type of molecule that is usually made when amino acids (ie, the building blocks of protein) are broken down by enzymes.
- Caffeine. Caffeine is a common stimulant found in foods and drinks such as coffee, tea, energy drinks, and chocolate. However, some people may experience negative effects, including headaches, sleep disturbances, and jitters after consuming caffeine, particularly in large amounts. Caffeine intolerance can be caused by an inability to metabolize or process caffeine effectively, or intake of excessive amounts, leading to these adverse reactions. Caffeine is broken down in the liver by a group of enzymes known as cytochrome P450 enzymes, and genetic variations that affect the activity of these enzymes may cause slower or faster caffeine metabolism. The rate of caffeine metabolism affects the risk of caffeine-related side effects; slow metabolizers are more likely to experience caffeine intolerance.39 Genetic tests, such as those offered by 23andMe, provide a report for caffeine metabolizer status, as a gene variant called CYP1A2 provides an indication of how fast the liver metabolizes caffeine.40 Certain medications, such as statins, antidepressants, and antiepileptic drugs, may also induce or inhibit these enzymes, leading to significant drug interactions or unanticipated adverse reactions to caffeine.41,42
- Histamine and biogenic amines. Histamine is a chemical messenger that acts as a signal for allergic and inflammatory reactions.43 However, many foods contain histamine. Histamine intolerance occurs when the body is unable to break down histamine efficiently, either due to a deficiency of diamine oxidase (DAO; an enzyme in the gut that breaks down ingested histamine) or an overload of histamine in the diet.44,45 Reduced DAO levels or activity can result from genetic variations, medication use, or certain medical conditions, such as inflammatory bowel disease or celiac disease.
- Flushing or reddening of the skin
- Skin rashes, burning, tingling, and swelling
- Low blood pressure
- Headache
- Diarrhea
- Vomiting
- Sweating
- Heart palpitations
- Nasal congestion
- Respiratory failure or asthmatic symptoms
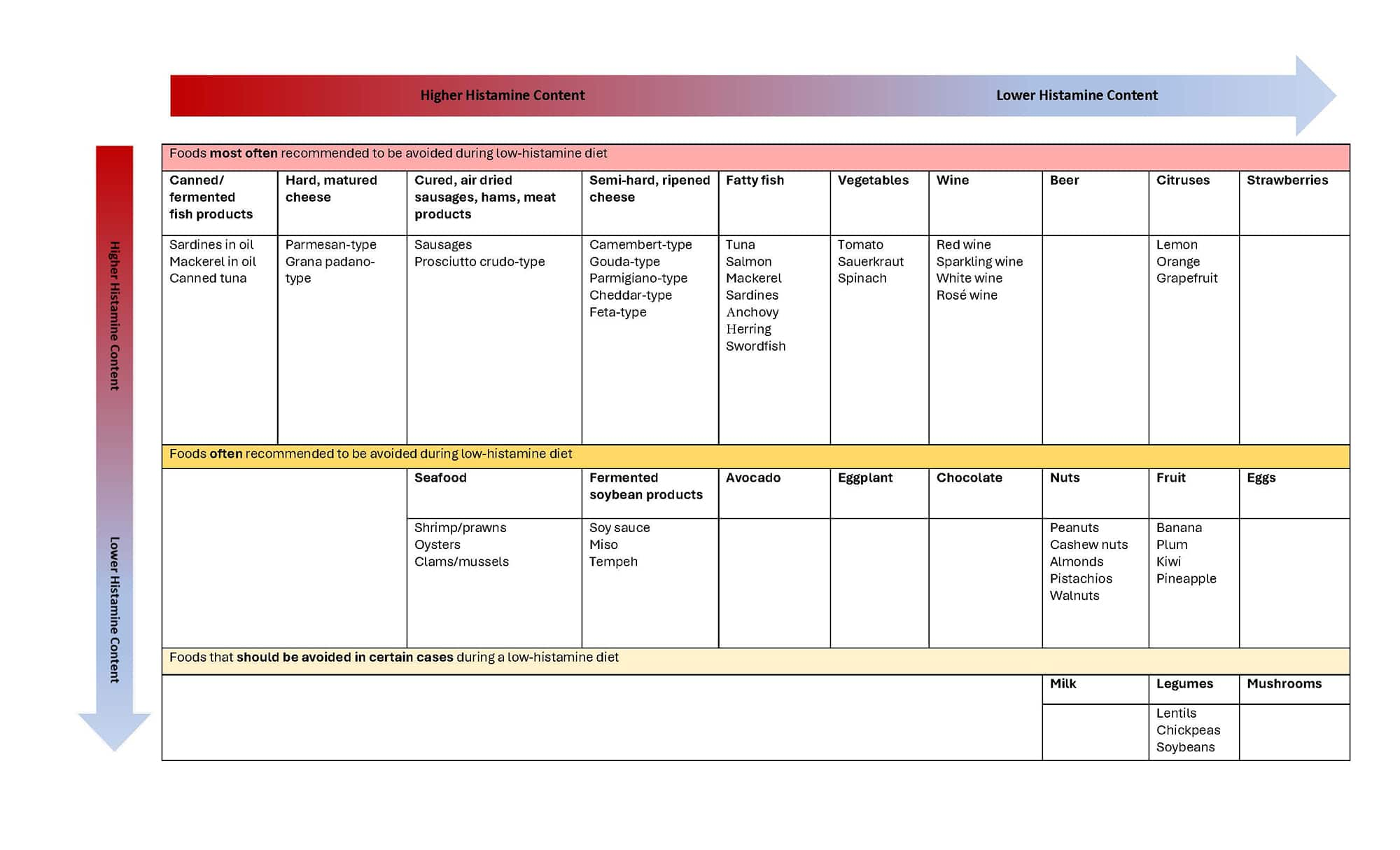
- Aged cheeses, such as cheddar, Swiss, and blue cheese
- Fermented foods, such as sauerkraut, kimchi, and pickles
- Cured meats, such as pepperoni, salami, and bacon
- Certain types of fish, such as tuna, mackerel, and anchovies
- Soy products, such as tofu and soy sauce
- Certain fruits, such as bananas, avocados, and raspberries
- Nuts and seeds, such as peanuts and pumpkin seeds
The prevalence of histamine intolerance worldwide is estimated to be around 1–3% of the population, although a lack of data and diagnostic tools make it difficult to determine the real prevalence. Even those without histamine intolerance may experience histamine toxicity after consuming large amounts of histamine. Foods that contain large amounts of histamine (see list below), or those that inhibit DAO activity (eg, alcohol), can also worsen symptoms of histamine intolerance.46 Scombroid fish poisoning, or histamine fish poisoning, is a syndrome similar to an allergic reaction that is caused by consuming fish in the Scombridae family (most frequently tuna or mackerel) that has not been properly refrigerated. Improper refrigeration allows bacteria to begin to break down the fish and form histamine, which can lead to histamine-related symptoms.47
Some symptoms of histamine intolerance include45,48:
Foods containing high histamine levels include46:
|
|
|
|---|---|
|
|
|
|
|
|
|
Expectorants/mucolytics |
ambroxol |
| Non-steroidal anti-inflammatory drugs (NSAIDs) |
aspirin, ibuprofen |
| H2 receptor blockers | cimetidine |
|
|
|
| Prokinetics | metoclopramide |
| Anti-infectives | clavulanic acid, isoniazid, cefuroxime, cefotiam, pentamidine, chloroquine, doxycycline, neomycin B, acriflavine, D-cycloserine |
|
Bronchodilators |
aminophylline, theophylline |
|
Diuretics |
amiloride, furosemide |
|
Antidepressants |
amitriptyline, monoamine oxidase inhibitors |
|
Anxiolytics |
diazepam, barbiturates |
|
Antipsychotics |
haloperidol |
|
Cytostatics |
cyclophosphamide |
|
Antihypertensives |
verapamil, dihydrazine, alprenolol |
|
Cardiotonics |
dobutamine, dopamine |
|
Opioids |
pethidine, morphine, codeine |
|
Analgesics |
metamizole |
|
General anesthetics |
thiopental |
|
Muscle relaxants |
pancuronium, alcuronium, D-tubocurarine |
|
Antiarrhythmics |
propafenone, verapamil, quinidine |
|
H1 receptor blockers |
promethazine |
|
|
|
|
Radiocontrast agents |
Iodine-containing radiocontrast agents |
Table 2. Foods to avoid during a low-histamine diet49
Other biogenic amines such as tyramine, putrescine, and cadaverine may also trigger symptoms of histamine intolerance.46 Putrescine and cadaverine are produced when the amino acids arginine and lysine, respectively, are broken down. Tyramine is produced from the breakdown of the amino acid tyrosine. Deficiencies or inhibition of the monoamine oxidase (MAO) enzymes can lead to the buildup of tyramine in the body. Elevated levels of biogenic amines can also inhibit DAO, thus increasing the amount of histamine in the body.50
Relevant genetic tests are available from direct-to-consumer companies like 23andMe or through a physician. For example, testing for variations in genes affecting DAO (eg, AOC1) or MAO (often coupled with COMT, another important enzyme) activity may be insightful.
Other Contributing Factors
Many other factors may contribute to the development of adverse reactions to food, including:
Autoimmune reactions. Some food-triggered immunological reactions may be autoimmune in nature. In these cases, symptoms can occur when the immune system mistakenly attacks the body's own tissues in response to consuming a specific food or food component. Autoimmune conditions that may be triggered by food include celiac disease, Crohn's disease, and ulcerative colitis.51,52
Impaired gut barrier function. Dysfunction of the gut barrier, also sometimes referred to as “leaky gut,” develops when junctions between cells that line the gut, called tight junctions, loosen or become damaged. Tight junctions are small gatekeeping gaps located in the intestinal lining that promote nutrient transfer while preventing harmful compounds from being transported into the blood. Weak tight junctions allow harmful microorganisms (eg, Candida yeast), toxins, and undigested food particles to leak through the intestinal wall and into the bloodstream.53 This can potentially cause systemic symptoms similar to those associated with food sensitivities.
Factors that contribute to impaired gut barrier function include exposure to reactive food components (eg, gluten), dysbiosis, bacterial or fungal overgrowth in the gut, certain medications (eg, NSAIDs), and chronic inflammatory states, such as celiac disease and inflammatory bowel disease.54 Chronic inflammation disrupts nerve signaling along the gut-brain axis and contributes to microbiome imbalance, which creates the ideal environment for microbial growth that can lead to impaired gut barrier function and reduced nutrient absorption.55
Microbial overgrowth and gluten sensitivity are potential contributing factors in leaky gut because these health issues induce the release of a protein called zonulin.54 Increased blood levels of zonulin have been associated with the gradual loosening of tight junctions.56,57 This allows harmful particles to leak into the bloodstream where they can lead to an overactive immune response and intestinal problems, such as flatulence, abdominal cramping, and bloating.53
Occludin is a transmembrane protein that participates in maintaining tight junction integrity. Some researchers have suggested that measurement of serum IgG or IgA antibodies against occludin may provide insight into gut barrier health. However, this approach needs to be validated in rigorous studies.58
Another potential concern in the context of leaky gut is the possibility that lipopolysaccharide (LPS) could enter the bloodstream through a dysfunctional intestinal barrier and cause systemic inflammation. LPSs are large molecules consisting of a lipid and polysaccharide that make up a bacterial toxin, also sometimes called an endotoxin. LPS is a major component of the outer membrane of gram-negative bacteria. Emerging evidence suggests that LPS may induce an inflammatory response that can contribute to the development of atherosclerosis.59-62
Some labs are now offering new innovative testing to assess levels of zonulin, occludin, and LPS in the blood.
Gut microbiome. The microbiome, which includes bacteria, viruses, and fungi living in and on the human body, plays a crucial role in many aspects of human health, including digestion, immunity, and metabolism. Recent research suggests the gut microbiome may also be involved in the development or prevention of adverse reactions to food.63 Healthy gut microbes can help maintain the intestinal mucus layer, while adherence to a “Western-style” diet that is low in fiber may promote the growth of microbes that damage the mucus layer.64 Promoting a healthy microbiome through a balanced diet, probiotics, and other interventions (eg, digestive enzyme supplementation) may help prevent or manage food intolerances or sensitivities. However, more research is needed to further clarify the multifaceted association between the microbiome and the immune system in the development of adverse reactions to food.26
Some medications. Certain medications can affect the body's ability to digest food and impact gut function. In particular, proton pump inhibitors (PPIs, a class of drugs used to treat gastroesophageal reflux and peptic ulcer) may contribute to the progression of eosinophilic esophagitis by hindering the digestion of food allergens, contributing to impaired gut barrier function that leads to the absorption of undigested food allergens, and causing microbiome imbalance.65 Similarly, anti-acid medications may neutralize or inhibit stomach acid, allowing undigested food allergens and components of other drugs with an increased capacity to induce a reaction to enter circulation.66 As a result, people may experience medication-induced intolerances or encounter difficulties with the speed at which their gut processes food. These problems arise due to the medication's impact on the digestive system, which can disrupt the natural balance of digestive enzymes and bacteria. It is important to consult a doctor if adverse reactions to medication develop or you experience problems with digesting food while taking medication.8
Mental health. Certain disordered eating behaviors, such as anorexia nervosa and bulimia nervosa, can affect how the body responds to food. In some cases, individuals with these conditions may experience psychosomatic reactions to food, leading to feelings of nausea or finding certain foods unappetizing. It is believed that these reactions stem from the person's psychological relationship with food, rather than a physical intolerance to the food itself.6,23,67
Diet. Some observational evidence suggests increased consumption of ultra-processed foods is associated with an increased risk of certain gastrointestinal disorders, like Crohn’s disease and ulcerative colitis.68-70 It has been suggested that various additives (eg, excessive salt, artificial sweeteners, and dyes like red 40) in ultra-processed foods might alter the microbiome and promote intestinal inflammation.70
Environmental factors. Environmental conditions can play a significant role in adverse food reactions. Exposure to environmental pollution, such as tobacco smoke, exhaust, and microplastics, can all disrupt the epithelial barrier (protective lining) in the gastrointestinal tract and potentially contribute to the development of food allergies.71 In addition, exposure to perfluoroalkyl and polyfluoroalkyl substances (PFASs) is also problematic, as these substances belong to a class of organic compounds that appear to exert an immunotoxic effect and are persistent in the environment due to a robust carbon-fluorine structure that is not susceptible to degradation. For some people, exposure to PFASs leads to an increased incidence of self-reported food allergies.72
Genetics and family history. Your genetics influence whether your body produces enough of the enzymes needed to digest food or whether your body is likely to mistake food for a foreign invader. These factors contribute to the development of food intolerances or food allergies.73
Age. Over time, the body may produce fewer enzymes or encounter difficulties digesting certain foods efficiently. This can lead to adverse reactions, such as lactose intolerance in adults.8 The risk of other sensitivities, such as to caffeine, may also increase with age.74
Exocrine pancreatic insufficiency. Exocrine pancreatic insufficiency (EPI) is not a food reaction but a condition that can mimic the symptoms of food reactions and should be considered in individuals who believe they are experiencing such symptoms. Symptoms of EPI include abdominal pain, gas and bloating, diarrhea, stomach pain, and steatorrhea (fatty, foul-smelling stool), which can overlap with food reaction symptoms and may occur concomitantly. EPI occurs when the pancreas does not produce enough digestive enzymes (lipase, amylase, and protease) to properly break down food. The primary cause of reduced enzyme production is inflammation of the pancreas, which can result from various conditions such as celiac disease, diabetes, inflammatory bowel disease, and pancreatic cancer.75
Salicylates. Salicylates, which are chemicals produced by plants as part of their defensive strategy against insects and disease, are found in a wide variety of foods. Most people can consume salicylates without experiencing any adverse effects and even gain anti-inflammatory and other health benefits from them. However, some people may be sensitive to salicylates. In these individuals, salicylates may cause symptoms that mimic food reactions. Common symptoms of salicylate intolerance include stuffy nose, sinus infections, nasal polyps, diarrhea, gastrointestinal inflammation/colitis, and hives. A trial of a salicylate-free or reduced diet may help determine if someone is hypersensitive.76
Sulfites. Sulfites are chemicals used to preserve food, drinks, and medications, and are naturally found in grapes and aged cheeses. Sulfites are also added to foods like dried fruit and wine to prevent spoilage. Sensitivity to sulfites can also be confused with food reactions. Typical symptoms include hives, swelling of skin, stuffy nose, hypotension (low blood pressure), flushing, diarrhea, wheezing, and coughing. Sulfites may even cause airway constriction in asthmatic patients. Foods high in sulfites include dried fruit, wine, apple cider, canned vegetables, pickled foods, potato chips, beer, tea, and some baked goods.77
Lectins. Lectins have gained attention recently due to claims that they cause food reactions in some individuals. These proteins, which plants produce to protect themselves from pests and pathogens, are found in a variety of foods, particularly in the nightshade family such as tomatoes, peppers, potatoes, and eggplant. The theory proposes that some individuals may have difficulty breaking down lectins, leading to impaired digestion, nutrient absorption, gastrointestinal inflammation, disruption of the gut microbiome, and potentially contributing to leaky gut and systemic inflammation, which could trigger autoimmune issues.
However, lectin sensitivity remains a controversial topic with limited scientific research.78 Most people can consume lectin-containing foods without any problems. Nonetheless, some individuals report significant improvements in gastrointestinal and systemic health after adopting a lectin-free diet. To determine if lectins are the cause of their symptoms, individuals can try a lectin-free diet for 10–14 days and observe any changes. If no improvement is noticed, other potential causes should be considered.
3 Food Reaction Assessment & Diagnosis
Food intolerances, allergies, and sensitivities can be difficult to diagnose, and may also cause a range of symptoms, from mild discomfort to severe allergic reactions. In addition to performing a physical examination and considering an individual’s medical history, a doctor may also use the results of diagnostic tests to identify the underlying cause(s) of adverse food reactions. Understanding the tests that are currently available can help individuals and healthcare providers make informed decisions about diagnosis and treatment, ultimately leading to improved quality of life for those affected by these conditions.73
It is vital to understand that there is no perfect allergy, intolerance, or sensitivity test that can identify all the ways a food may be causing reactions. The different tests available all provide different information and look at different ways an individual may be adversly reacting to food. For example, one person may show positive for IgE reactions to milk but not show elevated IgG/IgA reactions for milk and not have intolerances (eg, lactose intolerance). Another person who also knows they react to milk may show negative for IgE allergy reactions but postive for IgG/IgA sensitivities and also be lactose intolerant. Yet another person may show negative for IgE, IgG, and IgA reactions, but is lactose intolerant and will have signficant gastrointestinal symtoms. Bottom line is no one test will provide all the answers; each test has its pros and cons and each test provides different information as to potential reasons for food reactions.
Common Tests
Skin prick and atopy patch tests. Skin prick and atopy patch tests are common diagnostic tests for food allergies (and environmental allergies) that are administered under the care of a board certified allergist.73 The skin prick test involves placing a small amount of food allergen on the skin by pricking the skin with a needle containing the allergen. If the person is allergic to the food, a raised bump or hive will appear at the site. This test can help identify trigger foods in those with IgE-mediated food allergies, and the severity of the reaction to the prick test typically matches the severity of the food allergy.6,38
However, it is important to note that there are some limitations to the skin prick test. For instance, the test may not detect non–IgE-mediated food allergies. Furthermore, a positive skin prick test result does not always predict that an adverse reaction will occur upon eating a certain food, or vice versa. Additionally, the methods and testing supplies used for skin prick tests are not standardized across testing kits, which can lead to inconsistent results upon re-testing. Skin prick tests may not be suitable for individuals with severe eczema or those who have recently taken antihistamines, as these factors can affect the accuracy of the test. In people with extensive inflammatory conditions affecting the skin, such as eczema, allergy tests administered via routes other than the skin may be recommended.79-82
The atopy patch test involves holding potential food allergens on the skin for 48–72 hours, typically by applying a patch or bandage containing the allergens. Then, the test is “read” by looking for a reaction on the skin where the patch was placed. This test is useful for the identification of food reactions that take longer to develop, such as non–IgE-mediated and mixed-type allergies.6 The patch testing method may also be used in some cases where a skin prick test is negative, but the clinician retains suspicion of allergy.83
While both the skin prick and atopy patch tests are useful in identifying potential triggers of food allergies, they cannot always predict whether a person will react to a certain food when ingested and should not be used alone when designing elimination diets.20
Oral food challenge test. Food challenge tests are an important diagnostic tool for identifying food intolerances, sensitivities, and allergies, but are not often used due to time constraints. This test involves slowly consuming increasing amounts of the suspected food under medical supervision and monitoring for any adverse reaction(s).84 The food challenge test may be used when skin and blood tests are inconclusive, such as to confirm the diagnosis of non–IgE-mediated food allergies, which can be challenging to diagnose using other tests. It is also used to determine if an individual has outgrown a food allergy or to identify the threshold at which a reaction occurs.85
Elimination and reintroduction. Elimination diets are one of the best ways to identify foods that are responsible for causing adverse reactions, in particular those food reactions that are not thought to be IgE mediated. However, they are challenging to complete given most people’s busy lifestyles and the significant preparation needed. When used as a test for food reactions, foods that are suspected to cause adverse reactions are eliminated from an individual’s diet for 2–4 weeks. There are three general levels of food elimination86:
- Level 1: Simple elimination diet (eg, gluten-free and dairy-free)
- Level 2: Moderate intensity elimination diet (eg, low-FODMAP)
- Level 3: The few-foods diet (strict diet for a limited duration)
The elimination diet level varies depending on how many suspected foods are being eliminated. After the elimination phase, individual foods are added back into the diet one at a time. If a reaction occurs, the food that caused an adverse reaction is removed and the diet is returned to the elimination phase to allow the body time to recover from the reaction. Once the symptoms of the last reaction subside, the next potential trigger food is reintroduced. Elimination diets continue through these elimination, reintroduction, and recovery phases until all suspected foods are tested.6
While these diets are easy in theory, consultation with a clinician experienced in the management of food reactions is recommended during this process as the elimination of certain foods may result in nutritional deficiencies, and they are meant to be more of a short-term tool rather than a long-term solution. There is also a potential for inaccurate self-diagnosis of food reactions if an elimination diet is not performed with the guidance of an experienced clinician.6
One potential elimination diet, designed primarily to address gastrointestinal symptoms, is the low-FODMAP diet. This diet eliminates high-FODMAP foods, such as dairy, grains, and certain fruits and vegetables, with the goal of finding which specific FODMAP foods trigger adverse reactions. This allows doctors and registered dietitians to design a customized diet for individuals with food sensitivities or reactions without sacrificing nutrition.6,87
Serum immunoglobulin E (IgE) tests. The total immunoglobulin E (IgE) blood test looks at the total levels of IgE antibodies in the blood. This test can help determine whether an individual’s immune system is more primed for allergic responses. However, the total IgE test cannot identify which specific food may be responsible for the presence of high IgE levels in the blood or the allergic reaction.6
The radioallergosorbent test (RAST) is an allergen-specific IgE test that looks for IgE antibodies in the blood that recognize an allergen-specific IgE, such as the protein found in cod, egg whites, or soybeans. While this test is useful for determining if there is potential for a food to cause a reaction, it is not as useful in diagnosing or predicting future reactions to food. However, the RAST is helpful, standardized, and can help guide other tests such as picking foods to test for an elimination and reintroduction test or a food challenge test.38,88
While some allergists are using the RAST in their practice, more have switched to using newer IgE testing such as ImmunoCap specific IgE tests (which utilize fluorescence enzyme immunoassay [FEIA] technology) and other types of enzyme-linked immunosorbent assays (ELISAs). ImmunoCAP assays represent the “next-generation” of allergen-specific testing. These newer testing methods, like ImmunoCAP, may better reflect the potency of IgE-mediated allergic reactions than older methods.89
A more specific version of the allergen-specific IgE test is the component resolved diagnosis (CRD) test. This test can identify IgE antibodies that are specific for individual allergenic molecules.6 For example, if milk shows positive, the testing continues to examine individual components of milk (eg, lactalbumin, lactoglobulin, and casein) that may be trigging the allergy. However, CRD testing is not often used clinically in the routine diagnosis and management of food allergies.90
Although there are a number of ways that IgE levels can be measured, IgE tests may not always detect an allergen-specific IgE. While IgE testing can be helpful, other tests may need to be performed to determine the cause of food reactions.
Hydrogen breath testing. Hydrogen breath tests are a diagnostic tool sometimes used to identify food intolerances, particularly those related to lactose and fructose. These tests involve drinking a solution containing lactose or fructose, followed by the measurement of hydrogen levels in the breath. If the body is unable to digest lactose or fructose, the undigested sugars will inappropriately ferment, producing excess hydrogen gas, which is then expelled through the breath. Elevated levels of hydrogen in the breath indicate that the individual may be intolerant to the specific sugar tested.25,27,28
It is important to note that hydrogen breath tests are not always reliable and can produce false-positive or false-negative results. Additionally, they may not be suitable for individuals with certain conditions (eg, irritable bowel syndrome [IBS]) or inflammatory bowel disease) as these conditions can affect the accuracy of the test. However, careful reading of results can often provide helpful and actionable information. In many cases, a healthcare provider may recommend a combination of different diagnostic tests to accurately diagnose food intolerances.25
Endoscopy and biopsy. For some diseases, endoscopy and biopsy of the affected gastrointestinal tissue is needed for a diagnosis. Endoscopy is a medical procedure that involves the use of an endoscope (ie, a long, thin, flexible tube with a camera and light at the end) to examine the inside of the body. During an endoscopy, the doctor uses the camera to look for abnormalities or signs of disease, such as inflammation, bleeding, or tumors. A doctor can also obtain tissue samples (biopsies) or perform procedures such as removing polyps. Diseases that may require an endoscopy, and in some cases a biopsy, include EoE, food protein-induced syndromes, and celiac disease.27,88 In extreme cases, some food intolerances can be diagnosed through a biopsy in which enzyme levels in the tissue are measured.27,45 In most cases of food sensitivity, endoscopy and a biopsy are not necessary or indicated for diagnosis. In some cases of food reactions, such as EoE, an esophageal biopsy is necessary for diagnosis, but this is not a standard diagnostic procedure for food sensitivity.91
Additional Testing Options
Immunoglobulin G (IgG) tests. Blood tests to measure immunoglobulin G (IgG) antibodies directed at a panel of food antigens have been suggested by some innovative medicine practitioners as a method of identifying potential triggers of food sensitivities.
IgG antibodies are critical components of the immune system produced by B cells. They participate in protection against microbial pathogens, vaccination-induced immunity, and ensuring tolerance of nonpathogenic antigens. At the same time, they can also contribute to the pathogenesis of some autoimmune and inflammatory diseases. Their function is complex and context-dependent.92
The body normally produces IgG antibodies to food antigens upon typical dietary exposure. At the center of the research around IgG food sensitivity testing is the kind of immune response these food-directed IgG antibodies trigger: tolerance (non-reactive/non-inflammatory) or intolerance (reactive or inflammatory). The research on this topic is complex and incomplete, but it is clear that the type of response orchestrated by IgG antibodies varies depending on several factors (eg, interindividual genetic variability; route, dose, and duration of antigen exposure; and underlying inflammatory conditions).
There are four subclasses or isotypes of IgG: IgG1, IgG2, IgG3, and IgG4. The different IgG isotypes exert variable influences over the immune response to or tolerance of antigens. For example, there is robust evidence that IgG4 antibodies mitigate IgE-mediated allergic responses. This effect arises in part due to IgG4 blocking of IgE receptor binding and to an anti-inflammatory effect mediated through IgG4 signaling via Fc-gamma receptor II-beta (FcγRIIβ).93-95 IgG isotypes 1 and 3 have often been described as pro-inflammatory given their greater ability to activate the complement immune response (IgG2 only weakly activates complement and IgG4 does not activate complement). However, more recent evidence has clarified that IgG1–3 (and IgG1 in particular) can also counteract the inflammatory allergic response to antigens, in part through FcγRIIβ signaling in some cell types. IgG isotypes 1, 3, and 4 have been reported to have higher binding affinities for FcγRIIβ than IgG2.92,94 Interestingly, oral immunotherapy for food allergies triggers an increase in IgG1–4 levels, suggesting that increasing IgG production (of all isotypes) is characteristic of a shift from reactive to tolerant immunity to a particular food antigen.93,94 Research is ongoing to better characterize the roles of IgG1–3 in immune tolerance to food antigens typically encountered in the diet.
Advocates of IgG blood testing historically recommended testing all four IgG subclasses or only IgG4. However, as the protective role of IgG4 in the context of food allergies became clearly established in the literature, advocates of IgG food sensitivity testing began suggesting assessment of IgG isotypes 1–3 only. Both types of tests remain available on the market as of mid-2024.
Proponents of IgG testing point to several published reports of benefits of IgG-guided elimination diets in conditions such as irritable bowel syndrome and migraine headache,88,96-104 and some alternative medicine clinicians perceive value in IgG testing for food sensitivities (the food reactions not related to intolerances or allergies), particularly to guide elimination diets. These clinicians also sometimes suggest that elevated blood IgG levels may provide insight into a patient’s gut health. An example of when these clinicians may feel IgG testing is justified is when a patient reports ongoing perceived adverse food reactions despite having ruled out specific allergies or intolerances. In these cases, identifying the foods the patient frequently eats via IgG testing may aid the clinician in constructing a therapeutic trial elimination diet. Conversely, clinicians who do not advocate the use of IgG testing may prefer to pursue dietary changes guided by other means, such as a food diary or simply the elimination of one or a few foods often perceived to be problematic in association with the patient’s specific concern. Other clinicians may prefer to help the patient improve adherence to a generally healthy dietary pattern, such as the Mediterranean diet. The preferred approach will depend on the clinician’s experience.105-110 Some researchers and clinicians have suggested that IgG antibodies may cause or contribute to non–IgE-mediated food sensitivities in some cases. In particular, some evidence for potential IgG involvement in the context of EoE has emerged in recent years. Several studies have found that blood IgG testing helped guide the design of elimination diets as well. Elimination diets based on IgG testing have been shown to improve symptoms of diarrhea-predominant irritable bowel syndrome (IBS-D) and migraine in small studies. One study that enrolled and placed people with migraines on an elimination diet based upon IgG testing found that the IgG-guided elimination diet reduced the number of migraine-like headaches at four weeks, but not 12 weeks.
Basophil and mast cell activation tests. Basophil activation tests involve the blood collection of basophils, one of the immune cells responsible for allergic responses. After collection, the basophils are exposed to specific allergens and the degree of basophil reaction is measured.111
Mast cell activation tests are similar to basophil activation tests, except the serum (clear part of the blood) of an individual is added to mast cells in a laboratory. Mast cells—a type of immune cell that plays a role in immediate allergic reactions—also help regulate how the immune system responds to certain parasites and bacteria. This test is positive if the mast cells that were treated with serum activate when they are exposed to allergens.111
Both the basophil and mast cell activation tests may help predict whether an individual will react to a certain food. However, more research is needed to standardize these tests and make them more widely available.90,111
Genetic screening. Genetic screening is a relatively new approach to diagnosing food intolerances and allergies. This test analyzes an individual's DNA to identify any genetic variations that are associated with an increased risk of developing a food intolerance or allergy. For instance, genetic testing can help determine whether an individual is at risk for developing celiac disease.51,112 Genetic testing may also identify individuals with histamine, fructose, or lactose intolerance, although this is not often done in clinical practice.23,45 However, more direct-to-consumer genetic testing has become available in recent years.
Although genetic testing can provide useful information about an individual's genetic risk for food intolerances and allergies, it does not definitively predict whether a person will develop an intolerance or allergy. This means that having a genetic variation that is associated with a certain intolerance or allergy does not necessarily indicate that the individual will develop the condition; however, it does reveal an increased probability for developing the condition.112 Furthermore, many of the genes that may contribute to the development of adverse food reactions are still unknown.113 For these reasons, genetic testing may be used along with other diagnostic approaches to provide a more comprehensive understanding of an individual's risk.111,114
Gut barrier testing. There is no standardized test to measure how permeable, or “leaky,” a person’s gut is. There are tests in development that are designed to determine if different compounds and molecules can “leak” out of the gut and into the bloodstream. These include blood tests that look for bacterial components in the blood and urine tests that look for different types of sugars that have escaped the gut. In particular, gut barrier panel tests detect specific markers that contribute to the regulation of gut barrier function or indicate its permeability, such as zonulin, occludin, Candida albicans, and LPSs. The results of these panels provide a means of assessing the presence of leaky gut and monitoring gut barrier function. Biopsies and endoscopies can also help determine whether the gut barrier is damaged.115 Some of these tests are still in experimental stages and others are available commercially. A qualified healthcare practitioner can help decide whether gut barrier testing is appropriate in any given case.
While there are many tests available to diagnose the causes of adverse food reactions, some causes are difficult or impossible to diagnose definitively with one test. There is no one test that can test for all food allergies/sensitivities/reactions/intolerances because all the different tests are assessing different ways the body can react to foods. Each test provides different information on how the immune system and body react. For some conditions, such as histamine intolerance, there are no tests currently available to diagnose them. Only after the elimination of other causes is histamine intolerance considered as a diagnosis.23,45 A licensed health professional can also help determine the type and combination of diagnostic procedures that can lead to an accurate diagnosis.
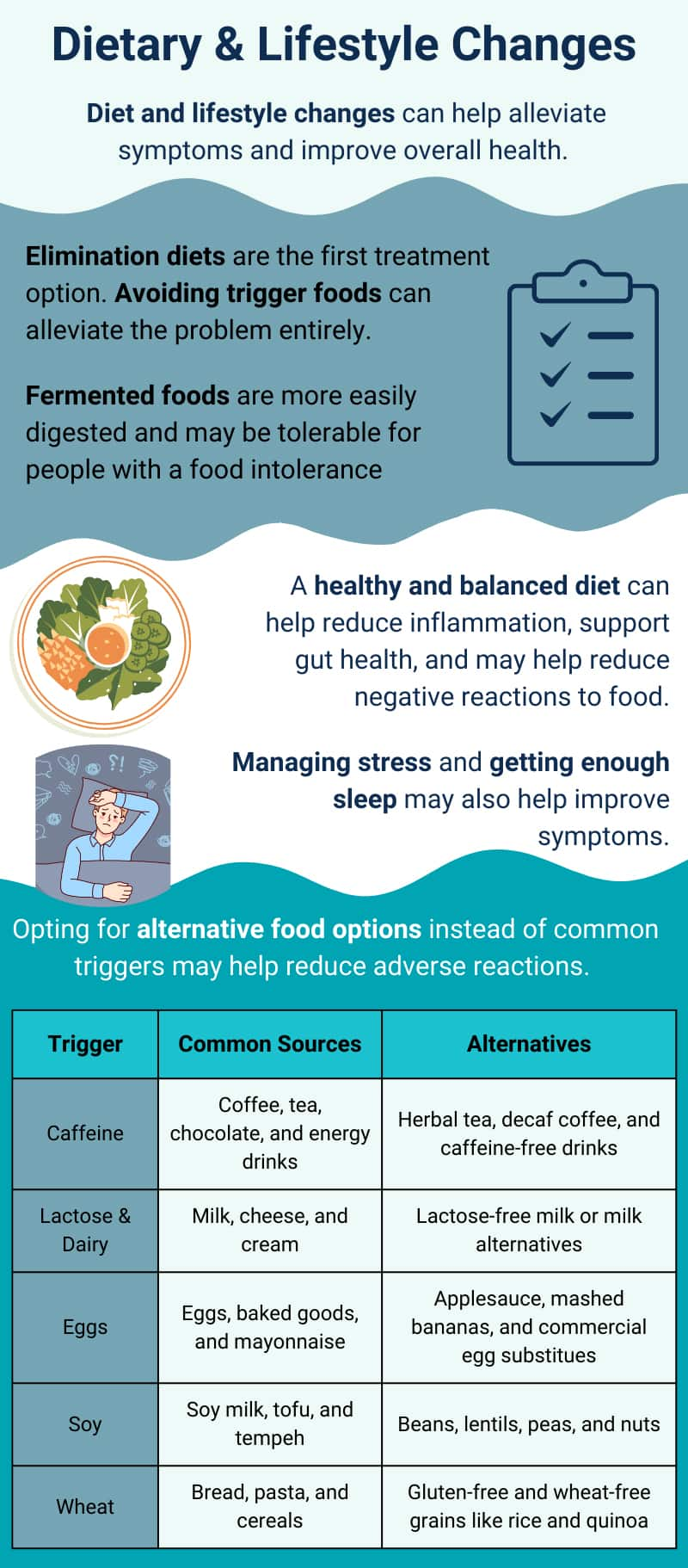
4 Dietary & Lifestyle Changes
Food sensitivities, intolerances, and allergies can cause a range of unpleasant symptoms and can be challenging to diagnose and treat. However, making certain dietary and lifestyle changes can help alleviate symptoms and improve overall health. Here are some useful tips:
The first line of treatment for food reactions is to eliminate trigger foods from the diet. In many cases, this addresses the entirety of the problem, and no further treatment (medical or otherwise) is necessary. However, avoidance may be difficult for those with sensitivities to common foods or ingredients. Peanuts, for example, can contaminate foods that are processed in the same facility. This could even eliminate foods that do not contain peanuts from the diet of people with peanut allergies.5 In addition, the exclusion of certain foods can lead to vitamin and nutrient deficiencies, especially in children. For example, an individual with lactose intolerance may need to supplement their diet with calcium and potassium, as well as vitamins B12 and D, as dairy products are a major source of these nutrients.6,28,116
When deciding to eliminate or limit foods in the diet, the severity of the reaction that typically occurs should be considered. Individuals with non–immune-mediated intolerances might be able to handle small amounts of trigger foods, as the severity of the reaction often depends upon how much of the trigger food is ingested. For example, many lactose intolerant people can tolerate up to 12 grams of lactose (equivalent to about 200–250 mL, or about 8 fluid ounces of milk) at a time without experiencing symptoms when taken with other foods.25 Some studies suggest fermented dairy products, like yogurt, may be more easily digested than other kinds of dairy foods due to their bacterial content. The bacteria may contain lactase enzymes that support lactose digestion, but individuals may react differently, and additional research is required.25,117-119
For immune-mediated sensitivities such as food allergies or celiac disease, it is often necessary to completely remove trigger foods from the diet. Consuming gluten with celiac disease can cause irreparable damage to the gut which means all gluten-containing foods must be avoided. Food allergies may also result in anaphylaxis and potentially death. Furthermore, some foods that are related to known trigger foods should be avoided because they may cause a reaction due to cross-reactive IgE antibodies.38
As it is often difficult to avoid and replace common foods associated with adverse reactions, we have prepared a table of common triggers and some potential alternatives:
| Table 3.Common food reaction triggers and potential alternatives | ||
|---|---|---|
| Trigger | Common Sources | Potential Alternatives |
| Caffeine |
Coffee, tea, chocolate, energy drinks, and some medications |
Herbal tea, decaf coffee, and caffeine-free soft drinks |
| Lactose and Dairy |
Milk, cheese, yogurt, ice cream, butter, cream, and many baked goods, sauces, and processed foods |
Lactose-free milk or milk alternatives such as soy milk, almond milk, coconut milk, or oat milk |
| Eggs |
Eggs, baked goods, pasta, mayonnaise, custard, meringue, and many processed foods |
Egg substitutes such as applesauce, mashed banana, silken tofu, or commercial egg replacers Alternative protein sources such as legumes, nuts, and seeds |
| Fructose |
Many fruits such as apples, pears, mangoes, and watermelon Honey, agave, and high-fructose corn syrup in many processed foods and sweetened beverages |
Lower-fructose fruits such as strawberries, oranges, bananas, avocadoes, kiwi, and pineapple |
| Peanuts |
Peanuts, peanut butter, baked goods, and many processed foods |
Alternative protein sources such as beans, lentils, peas, and soy products Nut butters made from almonds, cashews, or sunflower seeds |
| Soy |
Soy milk, tofu, tempeh, soy sauce, miso, edamame, and many processed foods |
Alternative protein sources such as beans, lentils, peas, and nuts Coconut aminos instead of soy sauce |
| Raw fruits or vegetables |
Pineapples, mangos, cashews, apples, pears, peaches, plums, cherries, kiwi, bananas, melons, tomatoes, celery, carrots, and potatoes |
Cooked or canned fruits and vegetables Alternative fresh fruits and vegetables that are not in the same plant family as the trigger food |
| Wheat |
Bread, pasta, cereal, crackers, baked goods, beer, soy sauce, salad dressings, sauces, and gravies Non-wheat grains that contain gluten: barley, rye, triticale, bulgur, and couscous |
Gluten intolerance: Gluten-free grains such as rice, quinoa, buckwheat, and oats (certified gluten-free) Wheat allergies: Wheat-free grains such as rice, corn, or quinoa Coconut aminos instead of soy sauce |
Adopting a healthy and balanced diet can also help decrease negative reactions to food. Consuming a variety of fruits, vegetables, whole grains, lean proteins, and healthy fats can help support gut health and reduce inflammation. It may also be helpful to incorporate probiotics and prebiotics into the diet to promote the growth of beneficial gut bacteria that help properly break down food. Fermented foods may also offer gastrointestinal benefits for people who suffer from food sensitivities. Some research shows that kefir, a fermented drink produced from kefir grains and cow, sheep, or goat milk, may be tolerable for individuals who are lactose intolerant. The bacteria in kefir consume lactose which may make it easier for the body to digest.120 Research regarding the impact of fermented foods on food allergy, gut microbiome, and immune system modulation is ongoing.
Lastly, making lifestyle changes such as reducing stress levels and getting enough sleep may also help improve symptoms and overall health.121,122 Getting enough sleep is also important as research has shown that sleep deprivation may increase reaction severity to allergen challenge in individuals with peanut allergy, for example.123
In summary, making dietary and lifestyle changes can help alleviate symptoms and improve overall health for individuals with food sensitivities, intolerances, and allergies. Identifying and avoiding trigger foods, adopting a healthy and balanced diet, and making lifestyle changes can all contribute to improved symptoms and overall well-being.
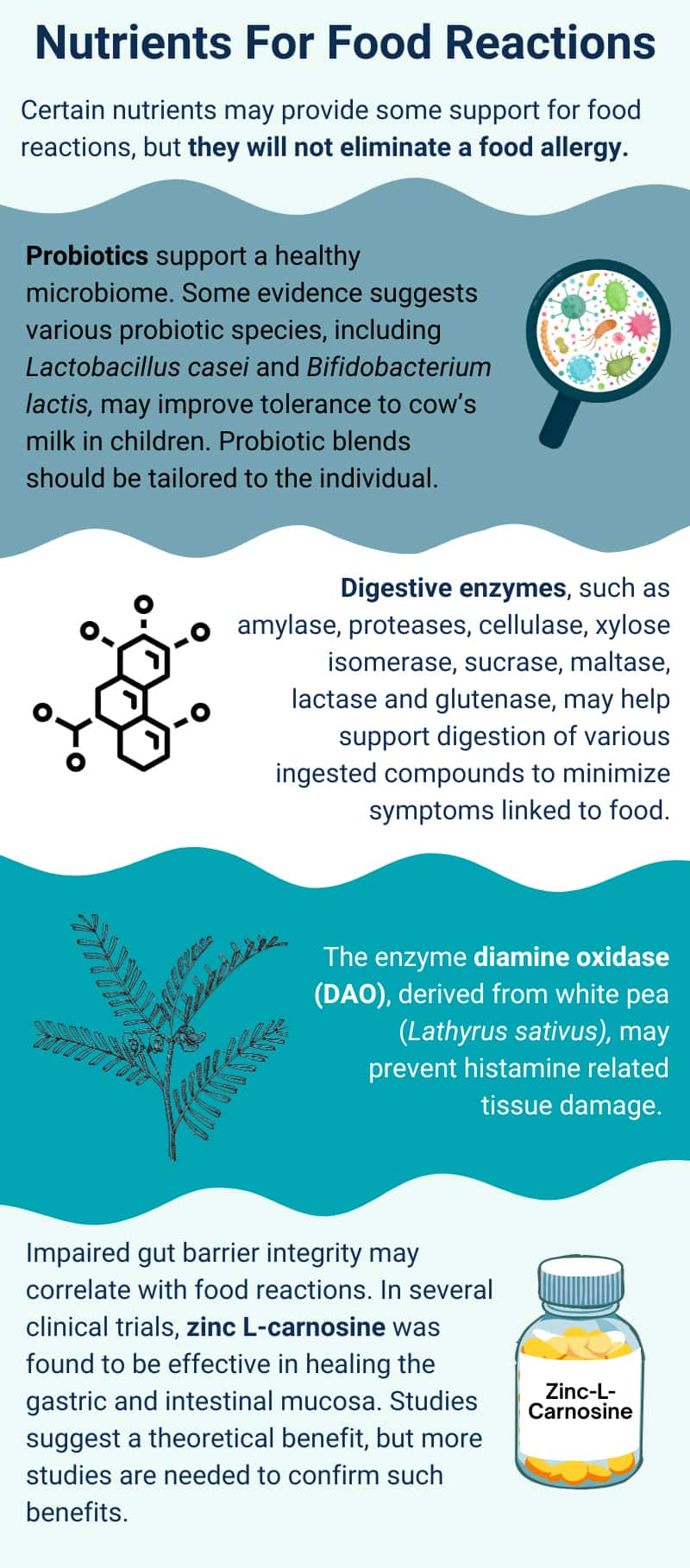
5 Nutrients
Caution: The nutrients described here will not eliminate a true food allergy. Individuals with diagnosed food allergies should work closely with their allergist or immunologist and never assume that any supplement will allow them to consume foods to which they are allergic.
Supporting the Gut Microbiome
Probiotics. Changes in the gut microbiota of modern humans have probably contributed to the increasing prevalence of food allergies.124 Observational studies have identified quantitative and qualitative differences in the gut microbiota between people with food allergies and those without. As a result, supporting the health and diversity of the gut microbiome is rational for those with food allergies and sensitivities.125 However, because there are numerous strategies through which the microbiome can be targeted, evidence is generally preliminary for any given intervention, such as probiotic supplementation. Nevertheless, taking steps to support the gut microbiome is probably wise for individuals with food sensitivities. One such step is probiotic supplementation. However, as of late 2023, most research on probiotics in the context of food allergies has involved children, primarily those with cow’s milk or peanut allergies. In this context, research is promising though preliminary.126,127
Various probiotic species, including Lactobacillus casei, Bifidobacterium lactis, L. rhamnosus, and/or B. bifidum, have been reported to potentially induce immune tolerance in children with a cow milk allergy.128,129 A trial enrolled 61 children aged 1–18 years who had a cow’s milk allergy and allocated one group to oral immunotherapy plus probiotic supplementation and the other group to oral immunotherapy plus placebo. Supplementation with Lactiplantibacillus plantarum YIT 0132 led to slightly improved tolerance to cow’s milk after 24 weeks.130 In a multicenter, randomized, controlled trial, 151 children with a cow’s milk allergy and atopic dermatitis who were being treated with an elimination diet received daily supplementation with a placebo or 1 billion bacteria from the strains L. rhamnosus ŁOCK 0900, L. rhamnosus ŁOCK 0908, and L. casei ŁOCK 0918. The participants taking the probiotics were found to have significantly improved atopic dermatitis symptoms at three months compared with participants receiving placebo.131 A cohort study that evaluated 330 children with a cow’s milk allergy found that L. rhamnosus GG supplementation along with extensively hydrolyzed casein protein was associated with reduced functional gastrointestinal disorder prevalence compared with casein alone.132 Other evidence also suggests L. rhamnosus GG supplementation may benefit children with an allergy to cow’s milk.133,134
Of note, no one probiotic works for everyone due to individual genetic/biochemical differences. While one person may feel improved with one strain of probiotic another may feel worsened. Trial and error is sometimes needed to find the best probiotic blend for each individual.
Digestive Support
Enzymes. Enzymes in the digestive tract promote nutrient absorption and digestive function. For certain food intolerances and sensitivities, enzymes available in supplement form may help minimize symptoms by improving digestion of the trigger compound. These include amylase, proteases, cellulase, xylose isomerase, sucrase, maltase, lactase, and glutenase, which may help support the digestion of various ingested compounds like complex carbohydrates, plant-based proteins, sugar, lactose, and gluten.135 Lactase is useful for people with lactose intolerance, while gluten-degrading enzymes have been explored for those with gluten sensitivities and celiac disease.136,137 It is important to note that as of late 2023, the only well-accepted treatment for celiac disease is a gluten-free diet. However, these digestive enzymes may help minimize symptoms linked to food sensitivities that are associated with gastrointestinal diseases (eg, IBS).135
One specific enzyme, AN-PEP (ie, a prolyl endoprotease derived from Aspergillus niger), has been studied in vitro and shown to degrade gluten. AN-PEP was shown to effectively degrade gluten in the pH range of the stomach, compared with other commercially available protease supplements. In this in vitro study, AN-PEP effectively degraded nine immunogenic gluten peptides, whereas five commercially available digestive enzyme supplements did not.138 In 18 subjects with self-reported gluten sensitivity, taking AN-PEP along with a gluten-containing porridge significantly reduced gluten levels in the stomach and duodenum compared with placebo. This study used two doses of AN-PEP: a low-dose and a high-dose, which provided double the enzymatic activity of the low dose. The dosages were based on enzymatic activity. Both dosage regimens were effective, and the effects did not differ significantly between the two dosages.139 Other evidence from a small clinical study also supports the potential of AN-PEP to degrade gluten in the stomach.140 However, another small clinical study found that people with celiac disease who took AN-PEP and introduced gluten under controlled conditions did not exhibit significantly different celiac disease quality scores or gut lining health upon close examination (histology) compared with those who took a placebo.141 More research is needed to clarify the effectiveness of AN-PEP supplementation in the context of gluten sensitivity.
As discussed elsewhere in this Protocol, histamine and other biogenic amines may contribute to food intolerance symptoms. The concept of “histamine intolerance” has been put forth by some researchers as a potential contributor to symptoms that may be described as food sensitivities, such as gastrointestinal discomfort, headache, skin flushing, sneezing or runny nose, and asthma. Diamine oxidase (DAO) is the main enzyme that breaks down histamine in the gut (ie, food histamine), and histamine intolerance is thought to be characterized by a relative lack of DAO enzyme activity.46
Studies have investigated the effects of supplemental DAO derived from pig kidney and have suggested some potential benefits (eg, improved gastrointestinal symptoms, urticaria [hives] symptoms, and migraines) for people thought to be histamine intolerant.142-144 However, more recently, a form of DAO derived from white pea (Lathyrus sativus) was shown to have higher specific activity than animal-derived DAO and prevented histamine-related tissue damage.145,146
Omega-3 Fatty Acids
Omega-3 polyunsaturated fatty acids (PUFAs) have been shown to influence immune system responses and inflammatory pathways. Preclinical research suggests omega-3 supplementation can suppress allergic responses and improve symptoms.173 Additionally, PUFA-derived pro-resolving mediators, such as resolvins, lipoxins, maresins, and protectins, are anti-inflammatory agents that may have beneficial, protective effects on inflammatory states, including potentially allergies and asthma.174
It has been suggested that maintaining a healthy omega-6:omega-3 ratio (closer to 4:1, instead of the more common 20:1 in typical Western diets) may be an effective strategy for reducing inflammation, allergies, and autoimmune reactions.175 In a small clinical study, individuals with a peanut allergy were shown to have higher levels of arachidonic acid (omega-6) and lower levels of the omega-3 PUFAs eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) compared with healthy controls. Screening also demonstrated an inverse correlation between specific IgE levels and total PUFA concentration.176 These findings provide evidence of the role that PUFA consumption plays in the occurrence, progression, and possible treatment of chronic allergic disorders and food reactions; hopefully, future studies will elucidate the role of omega-3 PUFAs in food reactions and related conditions.
Polyphenols
Polyphenols, antioxidants found in fruits and vegetables, as well as plant-based foods and beverages like tea and wine, are known for their anti-inflammatory properties and have been studied in the context of a variety of health concerns. Their antioxidant activity helps reduce inflammation and may potentially support the treatment of some food reactions.177-179 Polyphenols may also potentially help improve symptoms of inflammatory bowel disease.180,181 Furthermore, polyphenols have recently been described as important prebiotics that can positively affect the microbiome. When ingested, prebiotic polyphenols promote the growth of beneficial bacteria and the production of important metabolites.182
It has been suggested that polyphenols could “mask” allergens and food triggers by binding to proteins in the gut and preventing immune cells from detecting them.183,184 For example, the polyphenol epigallocatechin gallate (EGCG) from green tea has been shown to bind to gluten and block its recognition by gut immune cells in vitro.183,185 Numerous other polyphenols have been shown to interact with potentially immunogenic food proteins, but the clinical effects of polyphenols on food reactions needs to be further studied.183
In relation to food allergies, there is some preliminary research suggesting certain polyphenols may have anti-inflammatory properties and may help reduce allergic reactions.186
Green tea polyphenols. A 2013 pilot study found that treatment with 400–800 mg EGCG daily improved symptoms of ulcerative colitis with few side effects. This was a small study that involved 20 people, thus future research with a larger group of participants is needed to provide further evidence regarding whether green tea polyphenols can be used in the treatment of other food reactions and related conditions.187
Resveratrol. Resveratrol is a polyphenol found in grapes, berries, and red wine. A small study performed in 2015 found that treatment of ulcerative colitis patients with 500 mg resveratrol daily for six weeks improved disease severity and patient quality of life. However, the study size was small, with 50 participants.188
Quercetin. Quercetin is one of the most common flavonoids found in a wide variety of foods, including bell peppers, red onions, cranberries, lettuce, and coriander. Quercetin has been shown in vitro to stabilize the cell membrane of mast cells and basophils, thus preventing them from releasing excess pro-inflammatory mediators.189 Animal studies suggest quercetin may help improve allergic rhinitis, atopic dermatitis, and other allergic-related conditions190; however, more studies are needed to determine whether quercetin is beneficial in food reactions and other related conditions.
Zinc
Zinc is an important mineral that plays a central role in immune function in the intestinal lining and throughout the body. Proper zinc signaling helps control mast cell, T-cell, B-cell, eosinophil, and basophil activity, limiting inflammatory and allergic immune activity.191,192 Zinc deficiency has been implicated as a contributor to allergic and inflammatory diseases.191-193 In fact, patients with eczema and asthma—conditions closely associated with food reactions—have consistently been found to have lower zinc status than healthy individuals.194,195 This may be due in part to zinc’s effects on T-cell populations: laboratory research has indicated zinc signaling promotes regulatory T cells and T-helper 1 (Th1) cells while zinc deficiency skews the immune response by increasing T-helper 2 (Th2) cell activity.191,193 Excessive Th2 activity is seen in allergic conditions, including IgE-mediated food allergies.7,196 Zinc supplementation may help restore Th1/Th2 balance.191
Various zinc transporters work with zinc-binding molecules called metallothioneins to regulate zinc absorption, its movement into and out of cells, and its distribution within cells, controlling zinc homeostasis and preserving balanced immune activity.192,197 Inflammation and infection have been shown to disrupt zinc homeostasis by altering expression of zinc transporters. Because zinc deficiency can cause poor inflammation control and increased allergic reactivity, this suggests there may be a vicious cycle involving zinc depletion and allergy/inflammation.191
Zinc supplementation may restore balanced immune function in the intestinal wall, and clinical research has indicated it reduces gut inflammation and improves health in patients with inflammatory bowel disease.198 Some preclinical and clinical evidence suggests zinc supplementation may improve gut barrier function by supporting tight junctions between cells in the intestinal lining.199-201 By reducing gut leakiness,201 zinc supplementation has the potential to limit immune reactions to foods. In animal research, zinc deficiency suppressed immune tolerance in the gut,202 demonstrating another possible mechanism by which low zinc status may increase the likelihood of food reactions.
A vegan diet is often deficient in zinc.201 Zinc absorption is enhanced by citrate and dietary protein, especially animal protein. On the other hand, calcium and phytates (found in plant fiber) interfere with its uptake.193,197 Fermentation and sprouting can increase dietary zinc availability by reducing phytate.197 Zinc oxide has been found to be less effective for raising zinc levels than zinc salts, such as zinc gluconate, zinc citrate, and zinc acetate, while zinc salts appear not to vary substantially in their bioavailability.197,203 Importantly, PPIs interfere with normal zinc absorption, and certain diuretics and antihypertensive medications can deplete zinc.197,201
It is important to note that supplementing with 50 mg zinc per day or more over a long period of time can lead to zinc toxicity and related immune dysfunction due to disruption of copper and iron metabolism. Symptoms may include nausea, vomiting, diarrhea, and muscle cramping.197
Propionyl-L-carnitine
Research suggests that propionyl-L-carnitine (PLC), a derivative of L-carnitine, can help restore intestinal microvasculature and reduce mucosal inflammation in the context of ulcerative colitis.204 In a multicenter phase 2 trial, individuals with mild-to-moderate ulcerative colitis were given 1 gram per day PLC, 2 grams per day PLC, or a placebo to evaluate the impact of PLC on symptom severity. Each study participant was also taking stable oral medication. The results showed that 72% (57 of 79) of the individuals who took either 1 or 2 grams PLC daily had a marked, clinical response compared with those who took the placebo, with the highest response being observed for the participants who took 1 gram per day.205 Furthermore, 55% (22 of 40) of the participants in the 1 gram group experienced remission. These results indicate PLC is a beneficial adjuvant treatment for mild-to-moderate ulcerative colitis. PLC’s role in ameliorating food reactions and related conditions has yet to be studied, but this appears to be an area of research that should be explored.
Vitamin D
Vitamin D plays an important role in immune function and anti-inflammatory pathway modulation, with research showing that elevated levels of vitamin D in the blood are associated with stronger immune responses and decreased production of inflammatory cytokines.206,207 Furthermore, vitamin D produced from sun exposure activates antimicrobial proteins; helps protect the gut microbiome; enhances gut barrier function; and balances mast cell, dendritic cell, and T-cell activity.208
Insufficient levels of vitamin D are associated with immune-mediated inflammatory processes in inflammatory bowel disease, as individuals with these conditions typically have lower than normal levels.207 Although vitamin D supplementation may support improved health for people with intestinal diseases (eg, inflammatory bowel disease), the effect of supplementation in people with food allergies or sensitivities has not yet been elucidated; but research in this area is warranted.207,208
6 Medical Treatment of Food Reactions
Food allergy can cause serious and potentially life-threatening complications.23,209 If you believe you are experiencing an allergic reaction to food, seek medical attention immediately.
There are several types of medications that may be used to treat food reactions.23 However, the use of off-label drugs for the management of food sensitivities, allergies, or intolerances should only be undertaken with the guidance of a qualified health professional.210 In addition, it is important to address the root cause of the sensitivity or allergy rather than rely on medication to manage symptoms.
Note: individuals with celiac disease should refer to Life Extension’s Protocol on Celiac Disease and Non-Celiac Gluten Sensitivity.
Treatment of Food Allergies
While avoidance of trigger foods is the primary strategy for the treatment of food allergies, there are several approved and off-label treatments that may help reduce food allergy symptoms if the trigger food is ingested.38 People with true food allergies should always consult with a qualified allergist regarding the best treatment approach for them.
Epinephrine. EpiPen auto-injectors administer epinephrine (Adrenaline), a hormone and neurotransmitter, as an emergency treatment for anaphylaxis caused by food allergies.38 Individuals who have experienced or are at risk for anaphylaxis should carry an EpiPen at all times to rapidly decrease the body’s allergic reaction to a food allergen.
Off-label anti-IgE antibodies. Two anti-IgE monoclonal antibodies sometimes used off-label to treat food allergies are omalizumab (Xolair) and dupilumab (Dupixent).22,211,212
Oral immunotherapies. Peanut (Arachis hypogaea) allergen powder (Palforzia) is an oral immunotherapy drug recently approved by the FDA to prevent anaphylaxis in children aged 4 to 17 years with peanut allergies.213,214 Importantly, those being treated must still strictly avoid peanuts in their diets; the drug is intended to prevent severe reactions in case of accidental exposure. Oral immunotherapy is described further in the “Emerging Therapies” section.
Antihistamines and corticosteroids. These well-known medications, including antihistamines like diphenhydramine (Benadryl) and cetirizine (Zyrtec) and corticosteroids like prednisone, may be used to treat inflammatory reactions caused by food allergies.215,216 Antihistamines are generally used for milder allergic reactions, while corticosteroids are generally used short term and then tapered off to reduce inflammation following a severe allergic reaction.
Allergen immunotherapy. Allergen immunotherapy involves administering gradually increasing doses of an allergen to an allergic individual over a period of several months or years. The aim of allergen immunotherapy is to desensitize the individual's immune system to the allergen by gradually building up tolerance. While it may help reduce symptoms, allergen immunotherapy requires consistent exposure to allergens to maintain tolerance. These kinds of treatments are typically only offered in specialized clinics under close medical supervision due to the potential for severe allergic reactions during treatment.38
- Oral immunotherapy. Oral immunotherapy typically involves the daily oral administration of an allergen extract under close medical supervision. Some research shows that oral immunotherapy is effective at desensitizing individuals to certain food allergens, such as peanuts, milk, and eggs, but there is a risk of severe allergic reactions during treatment and the therapy likely must be continued indefinitely to maintain desensitization.217,218 One oral immunotherapy drug, Palforzia, has been FDA-approved to reduce the risk of severe allergic reactions to peanuts (see above).219
- Epicutaneous immunotherapy. For epicutaneous immunotherapy (EPIT), an allergen extract is administered through a patch applied to the skin. This relatively new therapy is currently being evaluated for the treatment of peanut allergies.218,220 EPIT appears to be effective at desensitizing individuals to peanuts, but the therapy must be continued long-term to maintain desensitization.220,221
- Sublingual immunotherapy. Sublingual immunotherapy (SLIT) involves the administration of gradually increasing doses of the allergen extract in the form of allergy drops or tablets under the tongue. This therapy has been used for decades to treat respiratory allergies, such as hay fever, and has also been studied for use in treating food allergies. SLIT is effective at desensitizing individuals to certain food allergens. SLIT is typically administered at home and is considered safer and more convenient than other forms of immunotherapy.218
Treatment of Food Intolerances
Avoiding the food or ingredient that causes an adverse reaction is the main form of treatment for food intolerances. However, as most intolerances are due to a deficiency of a particular enzyme, another common treatment is to add an enzyme supplement to the diet. The two most frequently recommended enzyme supplements are lactase and sacrosidase, which are used to treat lactose and sucrose intolerance, respectively.27,36 Some people may also find relief from certain symptoms of food intolerance by using over-the-counter medications, as recommended by a health professional, for acid reflux or diarrhea medication for gastrointestinal symptoms.
Treatment for Eosinophilic Esophagitis (EoE)/Eosinophilic Disorders (EGID)
Recent clinical evidence shows that eliminating animal milk alone may be a beneficial starting point for people who have EoE. Individuals following a one-food elimination diet (1FED) of animal milk for about six weeks experienced marked improvement of EoE symptoms and a substantial reduction of eosinophil (immune cell) levels in the blood, with 34% experiencing complete symptom remission (histological remission).107 Individuals who did not respond to the 1FED were able to proceed to a six-food elimination diet (6FED; eliminating animal milk, wheat, egg, soy, fish and shellfish, and peanut and tree nuts) to improve the possibility of experiencing EoE symptom improvement or histological remission; of those who proceeded to the 6FED, 43% experienced complete remission.
In addition to recommending dietary changes, medication may also be prescribed to manage symptoms, decrease inflammation, and reduce tissue damage. Common medications used to treat EoE include PPIs, which can help reduce acid reflux, and corticosteroids that target inflammation in the esophagus.
Dupilumab, which was FDA approved in 2022 for the treatment of EoE, is an antibody that targets interleukin (IL)-4 and IL-13, two immune proteins involved in eosinophilic-related inflammation.222 Dupilumab also appears to be potentially useful for the treatment of other EGIDs, thus this particular medication may offer promise as an approach for non-EoE EGIDs.
In extreme cases, endoscopic dilation may be necessary to widen the narrowed areas of the esophagus or intestine.223
7 Emerging Therapies
Treatment for Histamine Intolerance
There are no drugs specifically approved for the treatment of histamine intolerance, as it is not yet a recognized medical condition by many healthcare providers. However, some medications may be used off-label to help manage symptoms associated with histamine intolerance.
- Second generation antihistamines that have less sedating effects, such as loratadine (Claritin), fexofenadine (Allegra), and cetirizine, are commonly used to treat allergic reactions and may also help alleviate some symptoms of histamine intolerance, such as itching, hives, and runny nose. However, they may not be effective for all symptoms associated with histamine intolerance, such as digestive issues.23
- Corticosteroids, which suppress overactive immune responses, may also be useful in treating symptoms of histamine intolerance. However, high-dose corticosteroid use can suppress the immune system over time and therefore these drugs are generally used short term for acute inflammation crises. Lower dosages (eg, 5mg a day of prednisone) are more appropriate for longer term use and generally do not cause significant side effects.23,224
- Additionally, some people with histamine intolerance may benefit from medications that help stabilize mast cells, such as cromolyn sodium or ketotifen.8 These medications can help prevent the release of histamine and other inflammatory compounds from mast cells, which can reduce symptoms such as hives, itching, and gastrointestinal discomfort.
For people with histamine intolerance, medication should not be relied upon as the primary form of treatment. The most effective approach typically involves identifying and eliminating trigger foods from the diet. Working with a healthcare provider or registered dietitian to ensure proper nutrition and management of any related symptoms or conditions is another important approach.
Allergies
Emerging therapies for allergies focus on reducing the immune system’s overactive response to allergens. Currently the outcomes for emerging therapies are disparate, but each therapeutic route focuses on the basic principles of desensitization.210
Anti-IgE strategies. Anti-IgE antibodies were originally approved for the treatment of asthma and chronic spontaneous urticaria, although they may also be used off-label in the treatment of food allergies.225 As these antibodies bind to all IgE molecules, they are not specific for one allergen or trigger. Anti-IgE antibodies can be used as a pretreatment before immunotherapy, such as oral immunotherapy, to encourage desensitization to an allergen.220 Omalizumab is one such anti-IgE antibody that has shown promise in the treatment of food allergies both as a monotherapy or in conjunction with oral immunotherapy.226 Ligelizumab is another anti-IgE antibody that, as of late 2023, is in phase 3 clinical trials for the treatment of peanut allergies.227
Another IgE-related strategy for food allergy treatment is to prevent the immune response that leads to IgE production. Dupilumab is an antibody that binds to a specialized structure called IL-4 receptor. When immune proteins bind to this receptor in response to an allergen, the production of IgE begins to increase. Dupilumab disrupts this interaction and prevents the production of IgE by blocking immune proteins from binding to the IL-4 receptor. Dupilumab is approved for the treatment of several conditions such as asthma and atopic dermatitis, and several clinical trials are ongoing to determine whether it is useful in the treatment of food allergies.22,212
Eosinophilic Esophagitis (EoE) and Eosinophilic Disorders (EGID)
Complex allergic disorders such as EoE and EGID can be difficult to treat, but several emerging therapies are under investigation.
Antibody treatments. As of mid-2023, a number of antibodies are being developed for the treatment of eosinophilic disorders, particularly EoE, including:
- Lirentelimab. Lirentelimab (AK002) is an antibody that targets the sialic acid-binding immunoglobulin-like lectin (Siglec)-8 receptor, a protein expressed on the surface of eosinophils and mast cells, with low expression on basophils. Siglec-8 is an important immune protein that tells eosinophils, mast cells, and basophils that they are no longer needed to fight off an infection. The overactivity of immune cells (eg, eosinophils, mast cells, and basophils) is associated with allergic reactions and overactive immune responses. Lirentelimab showed promising results in reducing inflammation caused by eosinophils and improving symptoms of EoE in a phase 2 clinical trial.228 Lirentelimab has been granted orphan drug status for eosinophilic gastritis, eosinophilic duodenitis, and eosinophilic esophagitis.229
- RPC4046. RPC4046 is an IL-13 antibody that was evaluated in a phase 2 clinical trial. The result of this trial found that RPC4046 treatment lowered eosinophil counts and improved symptoms in individuals with steroid-resistant EoE.230,231 An open-label extension of this trial found that continued treatment with RPC4046 for up to one year was well tolerated and led to continued improvement and/or maintenance of endoscopic, histologic, and clinical measures of EoE disease compared with baseline.232
- Reslizumab. Reslizumab (Cinqair) is an anti–IL-5 antibody that is approved for the treatment of eosinophilic asthma.233 Thus far, results in EoE have shown only moderate benefit.234
- Benralizumab. Benralizumab (Fasenra) is an antibody that is used for the treatment of asthma by blocking the IL-5Rα receptor and disrupting the IL-5 pathway,234,235 which is involved in eosinophil activity and survival. Disrupting this pathway can help reduce the production of IgE. As of late 2023, benralizumab is undergoing phase 3 trials to investigate its efficacy in the treatment of EoE and eosinophilic gastritis and gastroenteritis.234
- Dupilumab. Dupilumab is an antibody that targets IL-4 and IL-13 and has already been approved for use in EoE, asthma, atopic dermatitis, and chronic rhinosinusitis with nasal polyps. It is currently being evaluated for other eosinophilic conditions.
- Cendakimab. Cendakimab (CC-93538) is an anti–IL-13 antibody that is undergoing clinical trials for the treatment of EoE.236 As of early 2024, an ongoing late-stage (phase 3) trial is investigating the efficacy of 48 weeks of treatment with CC-93538 in adults and adolescents with eosinophilic esophagitis. The estimated completion of the study is late 2024.237
Other Therapies in Development
- Etrasimod. Etrasimod (Velsipity) is a selective sphingosine-1-phosphate (S1P) receptor modulator that prevents white blood cells from leaving the lymph nodes to cause inflammation. It was first developed for the treatment of ulcerative colitis and, as of late 2023, is in phase 2 clinical trials to evaluate whether it is also useful for the treatment of EoE.236,238
- IRL201104. IRL201104 is a peptide drug derived from an immune-regulating protein produced by the bacteria Mycobacterium tuberculosis. This peptide drug alters how the immune system recognizes antigens, and (as of mid-2024) it had undergone a phase 2 study for EoE, with some promising initial results.239 The FDA granted orphan drug designation to IRL201104 in January 2024.240
8 Frequently Asked Questions
What are some tests for food reactions?
It can be hard for doctors to accurately diagnose food reactions. Several testing approaches may be used in any given case, and often more than one test may be needed to make the diagnosis since there is no one perfect test for food reactions. A qualified healthcare professional can help ensure the right tests are chosen for particular symptoms. Some approaches include:
- Skin prick and atopy patch tests by an allergist
- Oral food challenge test by an allergist, although less common
- Elimination and reintroduction
- Serum immunoglobulin E (IgE) tests, often done by allergist
- Serum immunoglobulins IgG and IgA may be recommended by innovative integrative doctors
- Hydrogen breath testing, usually done by a gastroenterologist
- Endoscopy and biopsy by gastroenterologists
What causes food reactions?
Food reactions can be an allergy, sensitivity, or an intolerance. Food allergies are mediated by the immune system mistakenly reacting against a food or food component, either from IgE antibodies or a T cell inflammatory cascade.5,6 Food intolerances, on the other hand, are not related to an immune reaction but rather to abnormal or incomplete digestion.24
Food sensitivities are sometimes associated with IgG and IgA antibodies or other factors such as sensitivities to chemicals like histamine and salicylates, or to “leaky gut” or similar conditions.
Which foods are commonly associated with food reactions?
Many foods may trigger food reactions, and trigger foods can be different from person to person. The best way to figure out which foods may be problematic for you is to undergo testing with the guidance of a qualified clinician. However, the following foods or food components are often associated with food reactions73:
- dairy (lactose)
- eggs
- peanuts
- soy
- raw fruits or vegetables
- wheat
- caffeine
- fructose
Can food reactions develop over time?
Yes, food reactions may develop over time, such that foods you did not used to be sensitive to may become problematic. Food allergies usually first emerge during childhood, but sometimes the first occurrence of a reaction may be in adulthood or even old age.8
Unfortunately, the exact reasons for developing food reactions are difficult to determine and are complex. Preliminary research suggests that some environmental chemicals can act as haptens, which are small molecules that bind to proteins in the body. Once bound to proteins, haptens can alter their structure, potentially confusing the immune system and leading to abnormal autoimmune-like reactions or inflammatory responses. It is also postulated that increased oral exposure to some environmental compounds may interfere with the development of oral tolerance to food proteins, predisposing individuals to food protein allergies. Studies are needed to test these hypotheses.241,242
If you begin to notice bothersome symptoms after eating a certain food or food component—even if it did not used to cause you any problems—it may be a good idea to discuss food sensitivity or intolerance testing with a qualified healthcare provider.
Do food reactions ever go away?
In some cases, food reactions may go away or become less troublesome over time. However, if you have a history of severe reactions to foods, make sure you do not try to re-introduce the food if you have avoided it for a long time without first talking with your doctor. Immune-mediated allergies are less likely to resolve over time than non–immune-mediated intolerances.
Can you reverse food reactions?
There are treatments available for both food allergies (immune-mediated) and food intolerances (not immune-mediated). Their effectiveness may vary from person to person. Food allergies likely are not reversible in most cases, but food intolerances may get better over time for some people.
Disclaimer and Safety Information
This information (and any accompanying material) is not intended to replace the attention or advice of a physician or other qualified health care professional. Anyone who wishes to embark on any dietary, drug, exercise, or other lifestyle change intended to prevent or treat a specific disease or condition should first consult with and seek clearance from a physician or other qualified health care professional. Pregnant women in particular should seek the advice of a physician before using any protocol listed on this website. The protocols described on this website are for adults only, unless otherwise specified. Product labels may contain important safety information and the most recent product information provided by the product manufacturers should be carefully reviewed prior to use to verify the dose, administration, and contraindications. National, state, and local laws may vary regarding the use and application of many of the therapies discussed. The reader assumes the risk of any injuries. The authors and publishers, their affiliates and assigns are not liable for any injury and/or damage to persons arising from this protocol and expressly disclaim responsibility for any adverse effects resulting from the use of the information contained herein.
The protocols raise many issues that are subject to change as new data emerge. None of our suggested protocol regimens can guarantee health benefits. Life Extension has not performed independent verification of the data contained in the referenced materials, and expressly disclaims responsibility for any error in the literature.
- Onyimba F, Crowe SE, Johnson S, Leung J. Food Allergies and Intolerances: A Clinical Approach to the Diagnosis and Management of Adverse Reactions to Food. Clin Gastroenterol Hepatol. Nov 2021;19(11):2230-2240 e1. doi:10.1016/j.cgh.2021.01.025. https://www.ncbi.nlm.nih.gov/pubmed/33493695
- Gaby A, Rakel De. Integrative medicine - Chapter 31 - Food allergy and intolerance. Fourth edition ed. Elsevier Philadelphia, PA; 2018.
- Burks AW, Holgate ST, O'Hehir E, et al. Middleton's Allergy: Principles and Practice . Elsevier; 2020. https://books.google.com/books?id=ZhQrzgEACAAJ
- DeGeeter C, Guandalini S. Food Sensitivities: Fact Versus Fiction. Gastroenterology clinics of North America. Dec 2018;47(4):895-908. doi:10.1016/j.gtc.2018.07.012. https://www.ncbi.nlm.nih.gov/pubmed/30337039
- Anvari S, Miller J, Yeh CY, Davis CM. IgE-Mediated Food Allergy. Clin Rev Allergy Immunol. Oct 2019;57(2):244-260. doi:10.1007/s12016-018-8710-3. https://www.ncbi.nlm.nih.gov/pubmed/30370459
- Gargano D, Appanna R, Santonicola A, et al. Food Allergy and Intolerance: A Narrative Review on Nutritional Concerns. Nutrients. May 13 2021;13(5)doi:10.3390/nu13051638. https://www.ncbi.nlm.nih.gov/pubmed/34068047
- Barshow S, Tirumalasetty J, Sampath V, et al. The Immunobiology and Treatment of Food Allergy. Annu Rev Immunol. Feb 15 2024;doi:10.1146/annurev-immunol-090122-043501. https://www.ncbi.nlm.nih.gov/pubmed/38360544
- Commins S. P. Food intolerance and food allergy in adults: An overview. UpToDate. Updated 6/20/2022. Accessed 4/11/2023, https://www.uptodate.com/contents/food-intolerance-and-food-allergy-in-adults-an-overview?search=food%20sensitivity&source=search_result&selectedTitle=1~150&usage_type=default&display_rank=1
- Daschner A, Gonzalez Fernandez J. Allergy in an Evolutionary Framework. J Mol Evol. Jan 2020;88(1):66-76. doi:10.1007/s00239-019-09895-3. https://www.ncbi.nlm.nih.gov/pubmed/31175388
- Macdougall JD, Kim EH. Macdougall JD, Kim EH. A clinical focus on oral tolerance in the development, prevention, and management of food allergy. Cellular Immunology. 2023:104963.
- Rivas MN, Burton OT, Wise P, Zhang Y-q, A, Suejy H. A microbiota signature associated with experimental food allergy promotes allergic sensitization and anaphylaxis. Journal of Allergy and Clinical Immunology . 2013:201-212.
- Cheng Y, Liu X, Chen F, et al. The Roles and Mechanisms of Gut Microbiota in Food Allergy. Advanced Gut & Microbiome Research. 2023/04/05 2023;2023:9575410. doi:10.1155/2023/9575410. https://doi.org/10.1155/2023/9575410 https://downloads.hindawi.com/journals/agmr/2023/9575410.pdf
- Rezende RM, Weiner HL. Oral tolerance: an updated review. Immunology Letters . 2022/05/01/ 2022;245:29-37. doi:https://doi.org/10.1016/j.imlet.2022.03.007. https://www.sciencedirect.com/science/article/pii/S0165247822000414 https://www.sciencedirect.com/science/article/pii/S0165247822000414?via%3Dihub
- Marrs T, Sim K. Demystifying Dysbiosis: Can the Gut Microbiome Promote Oral Tolerance Over IgE-mediated Food Allergy? Curr Pediatr Rev. 2018;14(3):156-163. doi:10.2174/1573396314666180507120424. https://www.ingentaconnect.com/content/ben/cpr/2018/00000014/00000003/art00006;jsessionid=n0r2idm2ij90.x-ic-live-03
- Zhang S, Sicherer S, Berin CM, Agyemang A. Pathophysiology of non-IgE-mediated food allergy. Immunotargets and Therapy. 2021:431-446.
- Nowak-Węgrzyn A, Chehade M, Groetch M. International consensus guidelines for the diagnosis and management of food protein-induced enterocolitis syndrome: executive summary-Workgroup Report of the Adverse Reactions to Foods Committee, American Academy of Allergy, Asthma & Immunology. The Journal of Allergy and Clinical Immunology. 2017:1111-1126.
- Zhang S, Sicherer S, Berin MC, Agyemang A. Pathophysiology of Non-IgE-Mediated Food Allergy. Immunotargets Ther. 2021;10:431-446. doi:10.2147/ITT.S284821. https://www.ncbi.nlm.nih.gov/pubmed/35004389
- Calvani M, Anania C, Cuomo B, et al. Non-IgE- or Mixed IgE/Non-IgE-Mediated Gastrointestinal Food Allergies in the First Years of Life: Old and New Tools for Diagnosis. Nutrients. Jan 14 2021;13(1):226. doi:10.3390/nu13010226. https://www.ncbi.nlm.nih.gov/pubmed/33466746
- Burks AW, Tang M, Sicherer S, et al. ICON: food allergy. J Allergy Clin Immunol . Apr 2012;129(4):906-20. doi:10.1016/j.jaci.2012.02.001. https://www.ncbi.nlm.nih.gov/pubmed/22365653
- Visaggi P, Mariani L, Pardi V, et al. Dietary Management of Eosinophilic Esophagitis: Tailoring the Approach. Nutrients. May 12 2021;13(5):1630. doi:10.3390/nu13051630. https://www.ncbi.nlm.nih.gov/pubmed/34066243
- Soni M, Peterson KA, Uchida AM. Eosinophilic Gastrointestinal Disease. JAMA. Apr 23 2024;331(16):1407-1408. doi:10.1001/jama.2024.2143. https://www.ncbi.nlm.nih.gov/pubmed/38526463
- Manti S, Pecora G, Patane F, et al. Monoclonal Antibodies in Treating Food Allergy: A New Therapeutic Horizon. Nutrients. Jul 5 2021;13(7):2314. doi:10.3390/nu13072314. https://www.ncbi.nlm.nih.gov/pubmed/34371821
- Tuck CJ, Biesiekierski JR, Schmid-Grendelmeier P, Pohl D. Food Intolerances. Nutrients. Jul 22 2019;11(7)doi:10.3390/nu11071684. https://www.ncbi.nlm.nih.gov/pubmed/31336652
- Staudacher HM, Whelan K. The low FODMAP diet: recent advances in understanding its mechanisms and efficacy in IBS. Gut. Aug 2017;66(8):1517-1527. doi:10.1136/gutjnl-2017-313750. https://www.ncbi.nlm.nih.gov/pubmed/28592442
- Fernandez-Banares F. Carbohydrate Maldigestion and Intolerance. Nutrients . May 4 2022;14(9):1923. doi:10.3390/nu14091923. https://www.ncbi.nlm.nih.gov/pubmed/35565890
- Caminero A, Meisel M, Jabri B, Verdu EF. Mechanisms by which gut microorganisms influence food sensitivities. Nat Rev Gastroenterol Hepatol . Jan 2019;16(1):7-18. doi:10.1038/s41575-018-0064-z. https://www.ncbi.nlm.nih.gov/pubmed/30214038
- Kanikowska A, Janisz S, Mankowska-Wierzbicka D, Gabryel M, Dobrowolska A, Eder P. Management of Adult Patients with Gastrointestinal Symptoms from Food Hypersensitivity-Narrative Review. J Clin Med. Dec 9 2022;11(24)doi:10.3390/jcm11247326. https://www.ncbi.nlm.nih.gov/pubmed/36555942
- Heine RG, AlRefaee F, Bachina P, et al. Lactose intolerance and gastrointestinal cow's milk allergy in infants and children - common misconceptions revisited. The World Allergy Organization journal. 2017;10(1):41. doi:10.1186/s40413-017-0173-0. https://www.ncbi.nlm.nih.gov/pubmed/29270244
- OpenStreetMap. Interactive World Maps of Lactase Persistence. Open Data Commons. Accessed 6/5/2024, https://coblabugr.shinyapps.io/lactasepersistence/
- Storhaug CL, Fosse SK, Fadnes LT. Country, regional, and global estimates for lactose malabsorption in adults: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. Oct 2017;2(10):738-746. doi:10.1016/S2468-1253(17)30154-1. https://www.ncbi.nlm.nih.gov/pubmed/28690131
- Singh SK, Sarma MS. Hereditary fructose intolerance: A comprehensive review. World journal of clinical pediatrics. Jul 9 2022;11(4):321-329. doi:10.5409/wjcp.v11.i4.321. https://www.ncbi.nlm.nih.gov/pubmed/36052111
- Bernardout M, Le Gresley A, ElShaer A, Wren SP. Fructose Malabsorption: Causes, Diagnosis and Treatment. The British Journal of Nutrition . 2022:481-489.
- USDA. U.S. Department of Agriculture. FoodData Central Search Results. Accessed 5/24/2024, https://fdc.nal.usda.gov/fdc-app.html#/?component=1012
- Frissora CL, Rao SSC. Sucrose intolerance in adults with common functional gastrointestinal symptoms. Proceedings (Baylor University Medical Center) . 2022;35(6):790-793. doi:10.1080/08998280.2022.2114070. https://www.ncbi.nlm.nih.gov/pubmed/36304608
- Related Information for Sacrosidase Oral Solution. Accessed May 2. https://www.fda.gov/drugs/drug-shortages/related-information-sacrosidase-oral-solution .
- Treem WR, McAdams L, Stanford L, Kastoff G, Justinich C, Hyams J. Sacrosidase therapy for congenital sucrase-isomaltase deficiency. Journal of Pediatric Gastroenterology and Nutrition . Feb 1999;28(2):137-42. doi:10.1097/00005176-199902000-00008. https://www.ncbi.nlm.nih.gov/pubmed/9932843
- Viswanathan L, Rao SS. Intestinal Disaccharidase Deficiency in Adults: Evaluation and Treatment. Current gastroenterology reports. Jun 2023;25(6):134-139. doi:10.1007/s11894-023-00870-z. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10226910/pdf/11894_2023_Article_870.pdf
- Seth D, Poowutikul P, Pansare M, Kamat D. Food Allergy: A Review. Pediatr Ann. Jan 1 2020;49(1):e50-e58. doi:10.3928/19382359-20191206-01. https://www.ncbi.nlm.nih.gov/pubmed/31930423
- Sulem P, Gudbjartsson DF, Geller F, et al. Sequence variants at CYP1A1-CYP1A2 and AHR associate with coffee consumption. Hum Mol Genet . May 15 2011;20(10):2071-7. doi:10.1093/hmg/ddr086. https://www.ncbi.nlm.nih.gov/pubmed/21357676
- Yang A, Palmer AA, de Wit H. Genetics of caffeine consumption and responses to caffeine. Psychopharmacology (Berl). Aug 2010;211(3):245-57. doi:10.1007/s00213-010-1900-1. https://www.ncbi.nlm.nih.gov/pubmed/20532872
- Lynch T, Price A. The effect of cytochrome P450 metabolism on drug response, interactions, and adverse effects. Am Fam Physician. Aug 1 2007;76(3):391-6. https://www.ncbi.nlm.nih.gov/pubmed/17708140
- Belayneh A, Molla F. The Effect of Coffee on Pharmacokinetic Properties of Drugs : A Review. Biomed Res Int. 2020;2020:7909703. doi:10.1155/2020/7909703. https://www.ncbi.nlm.nih.gov/pubmed/32775441
- Schnedl WJ, Enko D. Histamine Intolerance Originates in the Gut. Nutrients . Apr 12 2021;13(4):1262. doi:10.3390/nu13041262. https://www.ncbi.nlm.nih.gov/pubmed/33921522
- Shulpekova YO, Nechaev VM, Popova IR, et al. Food Intolerance: The Role of Histamine. Nutrients. Sep 15 2021;13(9)doi:10.3390/nu13093207. https://www.ncbi.nlm.nih.gov/pubmed/34579083
- Comas-Basté O, Sánchez-Pérez S, Veciana-Nogués MT, Latorre-Moratalla M, Vidal-Carou MDC. Histamine Intolerance: The Current State of the Art. Biomolecules. 2020;10(8):1181. doi:10.3390/biom10081181. https://dx.doi.org/10.3390/biom10081181
- Comas-Baste O, Sanchez-Perez S, Veciana-Nogues MT, Latorre-Moratalla M, Vidal-Carou MDC. Histamine Intolerance: The Current State of the Art. Biomolecules. Aug 14 2020;10(8)doi:10.3390/biom10081181. https://www.ncbi.nlm.nih.gov/pubmed/32824107
- MN Department of Health. Scombroid Fish Poisoning. Accessed 11/6/2023, https://www.health.state.mn.us/diseases/scombroid/index.html
- Durak-Dados A, Michalski M, Osek J. Histamine and other biogenic amines in food. Journal of Veterinary Research. 2020;64(2):281-288. doi:10.2478/jvetres-2020-0029. https://dx.doi.org/10.2478/jvetres-2020-0029
- Hrubisko M, Danis R, Huorka M, Wawruch M. Histamine Intolerance-The More We Know the Less We Know. A Review. Nutrients. Jun 29 2021;13(7)doi:10.3390/nu13072228. https://www.ncbi.nlm.nih.gov/pubmed/34209583
- Durak-Dados A, Michalski M, Osek J. Histamine and Other Biogenic Amines in Food. J Vet Res. Jun 2020;64(2):281-288. doi:10.2478/jvetres-2020-0029. https://www.ncbi.nlm.nih.gov/pubmed/32587916
- Iversen R, Sollid LM. The Immunobiology and Pathogenesis of Celiac Disease. Annu Rev Pathol. Jan 24 2023;18(1):47-70. doi:10.1146/annurev-pathmechdis-031521-032634. https://www.ncbi.nlm.nih.gov/pubmed/36067801
- Wagh SK, Lammers KM, Padul MV, Rodriguez-Herrera A, Dodero VI. Celiac Disease and Possible Dietary Interventions: From Enzymes and Probiotics to Postbiotics and Viruses. Int J Mol Sci. Oct 4 2022;23(19):11748. doi:10.3390/ijms231911748. https://www.ncbi.nlm.nih.gov/pubmed/36233048
- Odenwald MA, Turner JP. Intestinal Permeability Defects: Is it Time to Treat? Clinical Gastroenterology and Hepatology. 2013:1075-1083.
- Fasano A. All disease begins in the (leaky) gut: role of zonulin-mediated gut permeability in the pathogenesis of some chronic inflammatory diseases. F1000Research. 2020:F1000.
- Mayer EA, Tillisch K, Gupta A. Gut/brain axis and the microbiota. J Clin Invest. Mar 2 2015;125(3):926-38. doi:10.1172/JCI76304. https://www.ncbi.nlm.nih.gov/pubmed/25689247
- Fasano(a) A. Zonulin and its regulation of intestinal barrier function: The Biological Door to Inflammation, Autoimmunity, and Cancer. Physiological Reviews. 2011:151-175.
- Fasano(b) A. Intestional Permeability and its regulation by zonulin: Diagnostic and Therapeutic Implications. The Journal of Clinical Gastroenterology and Hepatology . 2019:1096-1100.
- Vojdani A, Vojdani E, Kharrazian D. Fluctuation of zonulin levels in blood vs stability of antibodies. World J Gastroenterol. Aug 21 2017;23(31):5669-5679. doi:10.3748/wjg.v23.i31.5669. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5569281/pdf/WJG-23-5669.pdf
- Shen X, Li L, Sun Z, et al. Gut Microbiota and Atherosclerosis-Focusing on the Plaque Stability. Front Cardiovasc Med . 2021;8:668532. doi:10.3389/fcvm.2021.668532. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8368126/pdf/fcvm-08-668532.pdf
- Violi F, Cammisotto V, Bartimoccia S, Pignatelli P, Carnevale R, Nocella C. Gut-derived low-grade endotoxaemia, atherothrombosis and cardiovascular disease. Nature Reviews Cardiology. 2023/01/01 2023;20(1):24-37. doi:10.1038/s41569-022-00737-2. https://doi.org/10.1038/s41569-022-00737-2 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9284488/pdf/41569_2022_Article_737.pdf
- Suzuki K, Susaki EA, Nagaoka I. Lipopolysaccharides and Cellular Senescence: Involvement in Atherosclerosis. International Journal of Molecular Sciences . 2022;23(19):11148. https://www.mdpi.com/1422-0067/23/19/11148 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9569556/pdf/ijms-23-11148.pdf
- Cani PD, Bibiloni R, Knauf C, et al. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes. Jun 2008;57(6):1470-81. doi:10.2337/db07-1403. https://www.ncbi.nlm.nih.gov/pubmed/18305141
- Caio G. Non-IgE/Mixed Food Allergies and Functional Gastrointestinal Disorder: A Common Thread between Childhood and Adulthood. Nutrients . Feb 16 2022;14(4):835. doi:10.3390/nu14040835. https://www.ncbi.nlm.nih.gov/pubmed/35215484
- Schroeder BO. Fight them or feed them: how the intestinal mucus layer manages the gut microbiota. Gastroenterology report. Feb 2019;7(1):3-12. doi:10.1093/gastro/goy052. https://www.ncbi.nlm.nih.gov/pubmed/30792861
- Spechler SJ. Eosinophilic esophagitis: novel concepts regarding pathogenesis and clinical manifestations. Journal of Gastroenterology . 2019:837-844.
- Pali-Scholl I, Jensen-Jarolim E. Anti-acid medication as a risk factor for food allergy. Allergy. Apr 2011;66(4):469-77. doi:10.1111/j.1398-9995.2010.02511.x. https://www.ncbi.nlm.nih.gov/pubmed/21121928
- De Petrillo A, Hughes LD, McGuinness S, Roberts D, Godfrey E. A systematic review of psychological, clinical and psychosocial correlates of perceived food intolerance. J Psychosom Res. Feb 2021;141:110344. doi:10.1016/j.jpsychores.2020.110344. https://www.ncbi.nlm.nih.gov/pubmed/33383523
- Preda CM, Istratescu D, Nitescu M, et al. Impact of Dietary Patterns in Inflammatory Bowel Disease Subtypes Versus Healthy Subjects: a Retrospective Cohort Study. Maedica (Bucur). Jun 2023;18(2):174-181. doi:10.26574/maedica.2023.18.2.174. https://www.ncbi.nlm.nih.gov/pubmed/37588829
- Babaei A, Pourmotabbed A, Talebi S, et al. The association of ultra-processed food consumption with adult inflammatory bowel disease risk: a systematic review and dose-response meta-analysis of 4 035 694 participants. Nutr Rev. Jun 10 2024;82(7):861-871. doi:10.1093/nutrit/nuad101. https://www.ncbi.nlm.nih.gov/pubmed/37632227
- Gubatan J, Kulkarni CV, Talamantes SM, Temby M, Fardeen T, Sinha SR. Dietary Exposures and Interventions in Inflammatory Bowel Disease: Current Evidence and Emerging Concepts. Nutrients. Jan 22 2023;15(3)doi:10.3390/nu15030579. https://www.ncbi.nlm.nih.gov/pubmed/36771288
- Celebi Sozener Z, Cevhertas L, Nadeau K, Akdis M, Akdis CA. Environmental factors in epithelial barrier dysfunction. J Allergy Clin Immunol . Jun 2020;145(6):1517-1528. doi:10.1016/j.jaci.2020.04.024. https://www.ncbi.nlm.nih.gov/pubmed/32507229
- Buser MC, Scinicariello F. Perfluoroalkyl substances and food allergies in adolescents. Environ Int. Mar 2016;88:74-79. doi:10.1016/j.envint.2015.12.020. https://www.ncbi.nlm.nih.gov/pubmed/26722671
- Sicherer SH, Sampson HA. Food allergy: A review and update on epidemiology, pathogenesis, diagnosis, prevention, and management. J Allergy Clin Immunol . Jan 2018;141(1):41-58. doi:10.1016/j.jaci.2017.11.003. https://www.ncbi.nlm.nih.gov/pubmed/29157945
- Frozi J, de Carvalho HW, Ottoni GL, Cunha RA, Lara DR. Distinct sensitivity to caffeine-induced insomnia related to age. J Psychopharmacol . Jan 2018;32(1):89-95. doi:10.1177/0269881117722997. https://www.ncbi.nlm.nih.gov/pubmed/28879806
- Stevens T, Conwell DL. Exocrine pancreatic insufficiency. UpToDate. Updated 11/8/2023. Accessed 6/5/2024, https://www.uptodate.com/contents/exocrine-pancreatic-insufficiency?search=exocrine%20pancreatic%20insufficiency&source=search_result&selectedTitle=1%7E69&usage_type=default&display_rank=1
- Baenkler HW. Salicylate intolerance: pathophysiology, clinical spectrum, diagnosis and treatment. Dtsch Arztebl Int. Feb 2008;105(8):137-42. doi:10.3238/arztebl.2008.0137. https://www.ncbi.nlm.nih.gov/pubmed/19633779
- Bold J. Considerations for the diagnosis and management of sulphite sensitivity. Gastroenterol Hepatol Bed Bench. Winter 2012;5(1):3-6. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4017445/pdf/GHFBB-5-003.pdf
- Panacer K, Whorwell PJ. Dietary Lectin exclusion: The next big food trend? World J Gastroenterol. Jun 28 2019;25(24):2973-2976. doi:10.3748/wjg.v25.i24.2973. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6603809/pdf/WJG-25-2973.pdf
- Sicherer SH, Sampson HA. Food allergy: A review and update on epidemiology, pathogenesis, diagnosis, prevention, and management. Journal of Allergy and Clinical Immunology . 2018:41-58.
- Heinzerling L, Mari A, Bergmann KC, et al. The skin prick test - European standards. Clin Transl Allergy. Feb 1 2013;3(1):3. doi:10.1186/2045-7022-3-3. https://www.ncbi.nlm.nih.gov/pubmed/23369181
- Čelakovská J, Krcmova I, Bukac J, Vaneckova J. Sensitivity and specificity of specific IgE, skin prick test and atopy patch test in examination of food allergy. Food and Agricultural Immunology. 11/24 2016;28:1-10. doi:10.1080/09540105.2016.1258548. https://www.tandfonline.com/doi/pdf/10.1080/09540105.2016.1258548
- Burks WB. Diagnostic evaluation of IgE-mediated food allergy. UpToDate. Updated 2/7/2023. Accessed 4/11/2023, https://www.uptodate.com/contents/diagnostic-evaluation-of-ige-mediated-food-allergy?search=food%20sensitivity&source=search_result&selectedTitle=3~150&usage_type=default&display_rank=3
- Al-Ani A., Oakley A. (ed.), Mitchell G. (ed.), McGivern M. (ed.). Atopy patch test. DermNet. Updated Feb. 2018. Accessed Jan. 16, 2024, https://dermnetnz.org/topics/atopy-patch-test
- American Academy of Allergy Asthma & Immunology. What Do Patients and Caregivers Need to Know about Oral Food Challenges? Accessed 11/7/2023, https://www.aaaai.org/tools-for-the-public/conditions-library/allergies/what-do-patients-and-caregivers-need-to-know-about
- Oral food challenges for diagnosis and management of food allergies. Accessed October 6. https://www.uptodate.com/contents/oral-food-challenges-for-diagnosis-and-management-of-food-allergies .
- The Elimination Diet. Accessed November. https://www.fammed.wisc.edu/files/webfm-uploads/documents/outreach/im/handout_elimination_diet_patient.pdf .
- Lessens D, Rindfleisch A. Elimination Diets. US Department of Veterans Affairs. Accessed 03/30/2023, https://www.va.gov/WHOLEHEALTHLIBRARY/tools/elimination-diets.asp
- Kelso JM. Unproven and disproven tests for food allergy. UpToDate. Updated 6/13/2022. Accessed 4/11/2023, https://www.uptodate.com/contents/unproven-and-disproven-tests-for-food-allergy?search=food%20sensitivity&source=search_result&selectedTitle=2~150&usage_type=default&display_rank=2
- van Hage M, Hamsten C, Valenta R. ImmunoCAP assays: Pros and cons in allergology. J Allergy Clin Immunol. Oct 2017;140(4):974-977. doi:10.1016/j.jaci.2017.05.008. https://www.ncbi.nlm.nih.gov/pubmed/28552762
- Bird JA, Burks AW. Food Allergy. Clinical Immunology: Principles and Practices . 2023:623-629:chap 49.
- Yeboa AA, Wang W, Kavitt RT. The Role of Allergy Testing in Eosinophilic Esophagitis. Gastroenterology & Hepatology. 2018:463-469.
- Castro-Dopico T, Clatworthy MR. IgG and Fcgamma Receptors in Intestinal Immunity and Inflammation. Front Immunol. 2019;10:805. doi:10.3389/fimmu.2019.00805. https://www.ncbi.nlm.nih.gov/pubmed/31031776
- Burton OT, Logsdon SL, Zhou JS, et al. Oral immunotherapy induces IgG antibodies that act through FcgammaRIIb to suppress IgE-mediated hypersensitivity. J Allergy Clin Immunol. Dec 2014;134(6):1310-1317 e6. doi:10.1016/j.jaci.2014.05.042. https://www.ncbi.nlm.nih.gov/pubmed/25042981
- Kanagaratham C, El Ansari YS, Lewis OL, Oettgen HC. IgE and IgG Antibodies as Regulators of Mast Cell and Basophil Functions in Food Allergy. Front Immunol. 2020;11:603050. doi:10.3389/fimmu.2020.603050. https://www.ncbi.nlm.nih.gov/pubmed/33362785
- Satitsuksanoa P, Daanje M, Akdis M, Boyd SD, van de Veen W. Biology and dynamics of B cells in the context of IgE-mediated food allergy. Allergy . Jun 2021;76(6):1707-1717. doi:10.1111/all.14684. https://www.ncbi.nlm.nih.gov/pubmed/33274454
- Guo H, Jiang T, Wang J, Chang Y, Guo H, Zhang W. The value of eliminating foods according to food-specific immunoglobulin G antibodies in irritable bowel syndrome with diarrhoea. J Int Med Res. 2012;40(1):204-10. doi:10.1177/147323001204000121. https://www.ncbi.nlm.nih.gov/pubmed/22429360
- Alpay K, Ertas M, Orhan EK, Ustay DK, Lieners C, Baykan B. Diet restriction in migraine, based on IgG against foods: a clinical double-blind, randomised, cross-over trial. Cephalalgia. Jul 2010;30(7):829-37. doi:10.1177/0333102410361404. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2899772/pdf/10.1177_0333102410361404.pdf
- Atkinson W, Sheldon TA, Shaath N, Whorwell PJ. Food elimination based on IgG antibodies in irritable bowel syndrome: a randomised controlled trial. Gut. Oct 2004;53(10):1459-64. doi:10.1136/gut.2003.037697. https://www.ncbi.nlm.nih.gov/pubmed/15361495
- Shakoor Z, AlFaifi A, AlAmro B, AlTawil LN, AlOhaly RY. Prevalence of IgG-mediated food intolerance among patients with allergic symptoms. Ann Saudi Med. Nov-Dec 2016;36(6):386-390. doi:10.5144/0256-4947.2016.386. https://www.ncbi.nlm.nih.gov/pubmed/27920409
- Geiselman JF. The Clinical Use of IgG Food Sensitivity Testing with Migraine Headache Patients: a Literature Review. Current Pain and Headache Reports . 2019;23(11)doi:10.1007/s11916-019-0819-4. https://dx.doi.org/10.1007/s11916-019-0819-4
- Frehn L, Jansen A, Bennek E, et al. Distinct patterns of IgG and IgA against food and microbial antigens in serum and feces of patients with inflammatory bowel diseases. PLoS One. 2014;9(9):e106750. doi:10.1371/journal.pone.0106750. https://www.ncbi.nlm.nih.gov/pubmed/25215528
- Neuendorf R, Corn J, Hanes D, Bradley R. Impact of Food Immunoglobulin G-Based Elimination Diet on Subsequent Food Immunoglobulin G and Quality of Life in Overweight/Obese Adults. J Altern Complement Med . Feb 2019;25(2):241-248. doi:10.1089/acm.2018.0310. https://www.liebertpub.com/doi/10.1089/acm.2018.0310?url_ver=Z39.88-2003&rfr_id=ori%3Arid%3Acrossref.org&rfr_dat=cr_pub++0pubmed
- Mitchell N, Hewitt CE, Jayakody S, et al. Randomised controlled trial of food elimination diet based on IgG antibodies for the prevention of migraine like headaches. Nutr J. Aug 11 2011;10:85. doi:10.1186/1475-2891-10-85. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3199755/pdf/1475-2891-10-85.pdf
- Kelso JM. Unproven Diagnostic Tests for Adverse Reactions to Foods. J Allergy Clin Immunol Pract. Mar-Apr 2018;6(2):362-365. doi:10.1016/j.jaip.2017.08.021. https://www.ncbi.nlm.nih.gov/pubmed/29524991
- Rakel D, Rakel DP, Minichiello VJ. Integrative Medicine . Elsevier; 2022. https://books.google.com/books?id=zFKbzgEACAAJ
- Hashash JG, Elkins J, Lewis JD, Binion DG. AGA Clinical Practice Update on Diet and Nutritional Therapies in Patients With Inflammatory Bowel Disease: Expert Review. Gastroenterology. Mar 2024;166(3):521-532. doi:10.1053/j.gastro.2023.11.303. https://www.ncbi.nlm.nih.gov/pubmed/38276922
- Kliewer KL, Gonsalves N, Dellon ES, et al. One-food versus six-food elimination diet therapy for the treatment of eosinophilic oesophagitis: a multicentre, randomised, open-label trial. Lancet Gastroenterol Hepatol . May 2023;8(5):408-421. doi:10.1016/S2468-1253(23)00012-2. https://www.ncbi.nlm.nih.gov/pubmed/36863390
- D'Adamo CR, Kaplan MB, Campbell PS, et al. Functional medicine health coaching improved elimination diet compliance and patient-reported health outcomes: Results from a randomized controlled trial. Medicine (Baltimore). Feb 23 2024;103(8):e37148. doi:10.1097/md.0000000000037148.
- Jactel SN, Olson JM, Wolin KY, et al. Efficacy of a Digital Personalized Elimination Diet for the Self-Management of Irritable Bowel Syndrome and Comorbid Irritable Bowel Syndrome and Inflammatory Bowel Disease. Clin Transl Gastroenterol. Jan 1 2023;14(1):e00545. doi:10.14309/ctg.0000000000000545. https://www.ncbi.nlm.nih.gov/pubmed/36322404
- Zalewski A, Doerfler B, Krause A, Hirano I, Gonsalves N. Long-Term Outcomes of the Six-Food Elimination Diet and Food Reintroduction in a Large Cohort of Adults With Eosinophilic Esophagitis. Am J Gastroenterol. Dec 1 2022;117(12):1963-1970. doi:10.14309/ajg.0000000000001949.
- Andreae DA, Shreffler WG. Future diagnostic tools for food allergy. UpToDate. Updated 1/7/2022. Accessed 4/11/2023, https://www.uptodate.com/contents/future-diagnostic-tools-for-food-allergy?search=food%20sensitivity&source=search_result&selectedTitle=6~150&usage_type=default&display_rank=6
- Brown NK, Guandalini S, Semrad C, Kupfer SS. A Clinician's Guide to Celiac Disease HLA Genetics. Am J Gastroenterol . Oct 2019;114(10):1587-1592. doi:10.14309/ajg.0000000000000310. https://www.ncbi.nlm.nih.gov/pubmed/31274511
- Johansson E, Mersha TB. Genetics of Food Allergy. Immunology and allergy clinics of North America . May 2021;41(2):301-319. doi:10.1016/j.iac.2021.01.010. https://www.ncbi.nlm.nih.gov/pubmed/33863485
- Cafarotti A, Giovannini M, Begin P, Brough HA, Arasi S. Management of IgE-mediated food allergy in the 21st century. Clin Exp Allergy . Jan 2023;53(1):25-38. doi:10.1111/cea.14241. https://www.ncbi.nlm.nih.gov/pubmed/36200952
- Camilleri M. Leaky gut: mechanisms, measurement and clinical implications in humans. Gut. Aug 2019;68(8):1516-1526. doi:10.1136/gutjnl-2019-318427. https://www.ncbi.nlm.nih.gov/pubmed/31076401
- Matte JJ, Britten M, Girard CL. The importance of milk as a source of vitamin B12 for human nutrition. Animal Frontiers. 2014;4(2):32-37. doi:10.2527/af.2014-0012. https://doi.org/10.2527/af.2014-0012
- Azcarate-Peril MA, Roach J, Marsh A, et al. A double-blind, 377-subject randomized study identifies Ruminococcus, Coprococcus, Christensenella, and Collinsella as long-term potential key players in the modulation of the gut microbiome of lactose intolerant individuals by galacto-oligosaccharides. Gut Microbes. Jan-Dec 2021;13(1):1957536. doi:10.1080/19490976.2021.1957536. https://www.ncbi.nlm.nih.gov/pubmed/34365905
- Masoumi SJ, Mehrabani D, Saberifiroozi M, Fattahi MR, Moradi F, Najafi M. The effect of yogurt fortified with Lactobacillus acidophilus and Bifidobacterium sp. probiotic in patients with lactose intolerance. Food science & nutrition. Mar 2021;9(3):1704-1711. doi:10.1002/fsn3.2145. https://www.ncbi.nlm.nih.gov/pubmed/33747481
- Savaiano DA. Lactose digestion from yogurt: mechanism and relevance. Am J Clin Nutr. May 2014;99(5 Suppl):1251S-5S. doi:10.3945/ajcn.113.073023. https://www.ncbi.nlm.nih.gov/pubmed/24695892
- Barros SEL, Rocha CDS, de Moura MSB, Barcelos MP, da Silva C, Hage-Melim L. Potential beneficial effects of kefir and its postbiotic, kefiran, on child food allergy. Food Funct. May 11 2021;12(9):3770-3786. doi:10.1039/d0fo03182h. https://www.ncbi.nlm.nih.gov/pubmed/33977950
- Schreier HM, Wright RJ. Stress and food allergy: mechanistic considerations. Ann Allergy Asthma Immunol. Apr 2014;112(4):296-301. doi:10.1016/j.anai.2013.08.002. https://www.ncbi.nlm.nih.gov/pubmed/24428964
- Oland AA, Booster GD, Bender BG. Integrated behavioral health care for management of stress in allergic diseases. Ann Allergy Asthma Immunol . Jul 2018;121(1):31-36. doi:10.1016/j.anai.2018.05.002. https://www.ncbi.nlm.nih.gov/pubmed/29751088
- Dua S, Ruiz-Garcia M, Bond S, et al. Effects of Exercise and Sleep Deprivation on Reaction Severity During Oral Peanut Challenge: A Randomized Controlled Trial. J Allergy Clin Immunol Pract . Sep 2022;10(9):2404-2413 e1. doi:10.1016/j.jaip.2022.04.043. https://www.ncbi.nlm.nih.gov/pubmed/35623576
- Shu SA, Yuen AWT, Woo E, et al. Microbiota and Food Allergy. Clin Rev Allergy Immunol. Aug 2019;57(1):83-97. doi:10.1007/s12016-018-8723-y. https://www.ncbi.nlm.nih.gov/pubmed/30564985
- Chernikova DA, Zhao MY, Jacobs JP. Microbiome Therapeutics for Food Allergy. Nutrients. Dec 3 2022;14(23)doi:10.3390/nu14235155. https://www.ncbi.nlm.nih.gov/pubmed/36501184
- Santos SCD, Konstantyner T, Cocco RR. Effects of probiotics in the treatment of food hypersensitivity in children: a systematic review. 10.1016/j.aller.2019.04.009. Allergol Immunopathol (Madr) . Jan-Feb 2020;48(1):95-104. doi:10.1016/j.aller.2019.04.009. https://www.ncbi.nlm.nih.gov/pubmed/31477401
- Qamer S, Deshmukh M, Patole S. Probiotics for cow's milk protein allergy: a systematic review of randomized controlled trials. Eur J Pediatr. Aug 2019;178(8):1139-1149. doi:10.1007/s00431-019-03397-6. https://www.ncbi.nlm.nih.gov/pubmed/31230196
- Tan W, Zhou Z, Li W, Lu H, Qiu Z. Lactobacillus rhamnosus GG for Cow's Milk Allergy in Children: A Systematic Review and Meta-Analysis. Front Pediatr. 2021;9:727127. doi:10.3389/fped.2021.727127. https://www.ncbi.nlm.nih.gov/pubmed/34746056
- Strisciuglio C, Vitale A, Perna F, et al. Bifidobacteria modulate immune response in pediatric patients with cow's milk protein allergy. Pediatric research. Sep 2023;94(3):1111-1118. doi:10.1038/s41390-023-02534-0. https://www.ncbi.nlm.nih.gov/pubmed/36959319
- Yamamoto-Hanada K, Sato M, Toyokuni K, et al. Combination of heat-killed Lactiplantibacillus plantarum YIT 0132 (LP0132) and oral immunotherapy in cow's milk allergy: a randomised controlled trial. Benef Microbes. Mar 14 2023;14(1):17-30. doi:10.3920/BM2022.0064. https://www.ncbi.nlm.nih.gov/pubmed/36815492
- Cukrowska B, Ceregra A, Maciorkowska E, et al. The Effectiveness of Probiotic Lactobacillus rhamnosus and Lactobacillus casei Strains in Children with Atopic Dermatitis and Cow's Milk Protein Allergy: A Multicenter, Randomized, Double Blind, Placebo Controlled Study. Nutrients . Apr 1 2021;13(4)doi:10.3390/nu13041169. https://www.ncbi.nlm.nih.gov/pubmed/33916192
- Nocerino R, Di Costanzo M, Bedogni G, et al. Dietary Treatment with Extensively Hydrolyzed Casein Formula Containing the Probiotic Lactobacillus rhamnosus GG Prevents the Occurrence of Functional Gastrointestinal Disorders in Children with Cow's Milk Allergy. J Pediatr . Oct 2019;213:137-142 e2. doi:10.1016/j.jpeds.2019.06.004. https://www.ncbi.nlm.nih.gov/pubmed/31327562
- Basturk A, Isik I, Atalay A, Yilmaz A. Investigation of the Efficacy of Lactobacillus rhamnosus GG in Infants With Cow's Milk Protein Allergy: a Randomised Double-Blind Placebo-Controlled Trial. Probiotics and antimicrobial proteins . Mar 2020;12(1):138-143. doi:10.1007/s12602-019-9516-1. https://www.ncbi.nlm.nih.gov/pubmed/30656549
- Berni Canani R, Di Costanzo M, Bedogni G, et al. Extensively hydrolyzed casein formula containing Lactobacillus rhamnosus GG reduces the occurrence of other allergic manifestations in children with cow's milk allergy: 3-year randomized controlled trial. J Allergy Clin Immunol . Jun 2017;139(6):1906-1913 e4. doi:10.1016/j.jaci.2016.10.050. https://www.ncbi.nlm.nih.gov/pubmed/28043872
- Ianiro G, Pecere S, Giorgio V, Gasbarrini A, Cammarota G. Digestive Enzyme Supplementation in Gastrointestinal Diseases. Curr Drug Metab . 2016;17(2):187-93. doi:10.2174/138920021702160114150137. https://www.ncbi.nlm.nih.gov/pubmed/26806042
- Ianiro G, Pecere S, Giorgio V, Gasbarrini A, Cammarota G, G. Digestive Enzyme Supplementation in Gastrointestinal Diseases. Current Drug Metabolism . 2016:187-193.
- Wei G, Helmerhorst EJ, Darwish G, Blumenkranz G, Schuppan D. Gluten Degrading Enzymes for Treatment of Celiac Disease. Nutrients . Jul 15 2020;12(7)doi:10.3390/nu12072095.
- Janssen G, Christis C, Kooy-Winkelaar Y, et al. Ineffective degradation of immunogenic gluten epitopes by currently available digestive enzyme supplements. PLoS One. 2015;10(6):e0128065. doi:10.1371/journal.pone.0128065. https://www.ncbi.nlm.nih.gov/pubmed/26030273
- Konig J, Holster S, Bruins MJ, Brummer RJ. Randomized clinical trial: Effective gluten degradation by Aspergillus niger-derived enzyme in a complex meal setting. Sci Rep. Oct 12 2017;7(1):13100. doi:10.1038/s41598-017-13587-7. https://www.ncbi.nlm.nih.gov/pubmed/29026170
- Salden BN, Monserrat V, Troost FJ, et al. Randomised clinical study: Aspergillus niger-derived enzyme digests gluten in the stomach of healthy volunteers. Aliment Pharmacol Ther. Aug 2015;42(3):273-85. doi:10.1111/apt.13266. https://www.ncbi.nlm.nih.gov/pubmed/26040627
- Tack GJ, van de Water JM, Bruins MJ, et al. Consumption of gluten with gluten-degrading enzyme by celiac patients: a pilot-study. World J Gastroenterol. Sep 21 2013;19(35):5837-47. doi:10.3748/wjg.v19.i35.5837. https://www.ncbi.nlm.nih.gov/pubmed/24124328
- Schnedl WJ, Schenk M, Lackner S, Enko D, Mangge H, Forster F. Diamine oxidase supplementation improves symptoms in patients with histamine intolerance. Food Sci Biotechnol. Dec 2019;28(6):1779-1784. doi:10.1007/s10068-019-00627-3. https://www.ncbi.nlm.nih.gov/pubmed/31807350
- Yacoub MR, Ramirez GA, Berti A, et al. Diamine Oxidase Supplementation in Chronic Spontaneous Urticaria: A Randomized, Double-Blind Placebo-Controlled Study. Int Arch Allergy Immunol. 2018;176(3-4):268-271. doi:10.1159/000488142. https://www.ncbi.nlm.nih.gov/pubmed/29698966
- Izquierdo-Casas J, Comas-Baste O, Latorre-Moratalla ML, et al. Diamine oxidase (DAO) supplement reduces headache in episodic migraine patients with DAO deficiency: A randomized double-blind trial. Clin Nutr. Feb 2019;38(1):152-158. doi:10.1016/j.clnu.2018.01.013. https://www.ncbi.nlm.nih.gov/pubmed/29475774
- Jumarie C, Seide M, Marcocci L, Pietrangeli P, Mateescu MA. Diamine Oxidase from White Pea (Lathyrus sativus) Combined with Catalase Protects the Human Intestinal Caco-2 Cell Line from Histamine Damage. Applied biochemistry and biotechnology. Jul 2017;182(3):1171-1181. doi:10.1007/s12010-016-2390-3. https://www.ncbi.nlm.nih.gov/pubmed/28108908
- Calinescu C, Federico R, Mondovi B, Mateescu MA. Zymographic assay of plant diamine oxidase on entrapped peroxidase polyacrylamide gel electrophoresis. A study of stability to proteolysis. Anal Bioanal Chem. Feb 2010;396(3):1281-90. doi:10.1007/s00216-009-3306-7. https://www.ncbi.nlm.nih.gov/pubmed/20091155
- Zheng Y, Zhang Z, Tang P, et al. Probiotics fortify intestinal barrier function: a systematic review and meta-analysis of randomized trials. Front Immunol. 2023;14:1143548. doi:10.3389/fimmu.2023.1143548. https://www.ncbi.nlm.nih.gov/pubmed/37168869
- Rose EC, Odle J, Blikslager AT, Ziegler AL. Probiotics, Prebiotics and Epithelial Tight Junctions: A Promising Approach to Modulate Intestinal Barrier Function. Int J Mol Sci. Jun 23 2021;22(13)doi:10.3390/ijms22136729. https://www.ncbi.nlm.nih.gov/pubmed/34201613
- Ravcheev DA, Thiele I. Systematic genomic analysis reveals the complementary aerobic and anaerobic respiration capacities of the human gut microbiota. Original Research. Frontiers in Microbiology . 2014-December-05 2014;5doi:10.3389/fmicb.2014.00674. https://www.frontiersin.org/journals/microbiology/articles/10.3389/fmicb.2014.00674 https://www.frontiersin.org/journals/microbiology/articles/10.3389/fmicb.2014.00674/pdf
- Leguina-Ruzzi A, Cariqueo M. Glutamine: A Conditionally Essential Amino Acid with Multiple Biological Functions. In: Naofumi S, Viduranga W, eds. Superfood and Functional Food - The Development of Superfoods and Their Roles as Medicine . IntechOpen; 2017:Ch. 10:chap Chapter 10. https://doi.org/10.5772/66488 https://api.intechopen.com/chapter/pdf-download/53125.pdf
- Achamrah N, Dechelotte P, Coeffier M. Glutamine and the regulation of intestinal permeability: from bench to bedside. Curr Opin Clin Nutr Metab Care . Jan 2017;20(1):86-91. doi:10.1097/MCO.0000000000000339. https://www.ncbi.nlm.nih.gov/pubmed/27749689
- Severo JS, da Silva Barros VJ, Alves da Silva AC, et al. Effects of glutamine supplementation on inflammatory bowel disease: A systematic review of clinical trials. Clin Nutr ESPEN. Apr 2021;42:53-60. doi:10.1016/j.clnesp.2020.12.023. https://www.ncbi.nlm.nih.gov/pubmed/33745622
- Zhou Q, Verne ML, Fields JZ, et al. Randomised placebo-controlled trial of dietary glutamine supplements for postinfectious irritable bowel syndrome. Gut. Jun 2019;68(6):996-1002. doi:10.1136/gutjnl-2017-315136. https://www.ncbi.nlm.nih.gov/pubmed/30108163
- Bertrand J, Ghouzali I, Guerin C, et al. Glutamine Restores Tight Junction Protein Claudin-1 Expression in Colonic Mucosa of Patients With Diarrhea-Predominant Irritable Bowel Syndrome. JPEN J Parenter Enteral Nutr . Nov 2016;40(8):1170-1176. doi:10.1177/0148607115587330. https://www.ncbi.nlm.nih.gov/pubmed/25972430
- Zhuang X, Li T, Li M, et al. Systematic Review and Meta-analysis: Short-Chain Fatty Acid Characterization in Patients With Inflammatory Bowel Disease. Inflammatory bowel diseases. Oct 18 2019;25(11):1751-1763. doi:10.1093/ibd/izz188. https://www.ncbi.nlm.nih.gov/pubmed/31498864
- Recharla N, Geesala R, Shi XZ. Gut Microbial Metabolite Butyrate and Its Therapeutic Role in Inflammatory Bowel Disease: A Literature Review. Nutrients. May 11 2023;15(10)doi:10.3390/nu15102275. https://www.ncbi.nlm.nih.gov/pubmed/37242159
- Banasiewicz T, Krokowicz Ł, Stojcev Z, et al. Microencapsulated sodium butyrate reduces the frequency of abdominal pain in patients with irritable bowel syndrome. Colorectal disease : the official journal of the Association of Coloproctology of Great Britain and Ireland . Feb 2013;15(2):204-9. doi:10.1111/j.1463-1318.2012.03152.x. https://onlinelibrary.wiley.com/doi/10.1111/j.1463-1318.2012.03152.x
- Yamagishi M, Akagawa S, Akagawa Y, et al. Decreased butyric acid-producing bacteria in gut microbiota of children with egg allergy. Allergy. Jul 2021;76(7):2279-2282. doi:10.1111/all.14795. https://www.ncbi.nlm.nih.gov/pubmed/33650199
- Vonk MM, Blokhuis BRJ, Diks MAP, et al. Butyrate Enhances Desensitization Induced by Oral Immunotherapy in Cow's Milk Allergic Mice. Mediators Inflamm. 2019;2019:9062537. doi:10.1155/2019/9062537. https://www.ncbi.nlm.nih.gov/pubmed/30800003
- Paparo L, Nocerino R, Ciaglia E, et al. Butyrate as a bioactive human milk protective component against food allergy. Allergy . May 2021;76(5):1398-1415. doi:10.1111/all.14625. https://www.ncbi.nlm.nih.gov/pubmed/33043467
- Burge K, Gunasekaran A, Eckert J, Chaaban H. Curcumin and Intestinal Inflammatory Diseases: Molecular Mechanisms of Protection. Int J Mol Sci. Apr 18 2019;20(8)doi:10.3390/ijms20081912. https://www.ncbi.nlm.nih.gov/pubmed/31003422
- Ghosh SS, He H, Wang J, Gehr TW, Ghosh S. Curcumin-mediated regulation of intestinal barrier function: The mechanism underlying its beneficial effects. Tissue Barriers. Jan 2 2018;6(1):e1425085. doi:10.1080/21688370.2018.1425085. https://www.ncbi.nlm.nih.gov/pubmed/29420166
- Haftcheshmeh SM, Mirhafez SR, Abedi M, et al. Therapeutic potency of curcumin for allergic diseases: A focus on immunomodulatory actions. Biomed Pharmacother. Oct 2022;154:113646. doi:10.1016/j.biopha.2022.113646. https://www.ncbi.nlm.nih.gov/pubmed/36063645
- Kinney SR, Carlson L, Ser-Dolansky J, et al. Curcumin Ingestion Inhibits Mastocytosis and Suppresses Intestinal Anaphylaxis in a Murine Model of Food Allergy. PLoS One. 2015;10(7):e0132467. doi:10.1371/journal.pone.0132467. https://www.ncbi.nlm.nih.gov/pubmed/26147007
- Shin HS, See HJ, Jung SY, et al. Turmeric (Curcuma longa) attenuates food allergy symptoms by regulating type 1/type 2 helper T cells (Th1/Th2) balance in a mouse model of food allergy. J Ethnopharmacol . Dec 4 2015;175:21-9. doi:10.1016/j.jep.2015.08.038. https://www.ncbi.nlm.nih.gov/pubmed/26342520
- Lang A, Salomon N, Wu JC, et al. Curcumin in Combination With Mesalamine Induces Remission in Patients With Mild-to-Moderate Ulcerative Colitis in a Randomized Controlled Trial. Clin Gastroenterol Hepatol . Aug 2015;13(8):1444-9.e1. doi:10.1016/j.cgh.2015.02.019.
- Efthymakis K, Neri M. The role of Zinc L-Carnosine in the prevention and treatment of gastrointestinal mucosal disease in humans: a review. Clin Res Hepatol Gastroenterol. Aug-Sep 2022;46(7):101954. doi:10.1016/j.clinre.2022.101954. https://www.ncbi.nlm.nih.gov/pubmed/35659631
- Shen W, Zhao X, Han Z, et al. Efficacy and safety of polaprezinc in the treatment of gastric ulcer: A multicenter, randomized, double-blind, double-dummy, positive-controlled clinical trial. Med Eng Phys . Dec 2022;110:103860. doi:10.1016/j.medengphy.2022.103860. https://www.ncbi.nlm.nih.gov/pubmed/35999163
- Mahmood A, FitzGerald AJ, Marchbank T, et al. Zinc carnosine, a health food supplement that stabilises small bowel integrity and stimulates gut repair processes. Gut. Feb 2007;56(2):168-75. doi:10.1136/gut.2006.099929. https://www.ncbi.nlm.nih.gov/pubmed/16777920
- Watari I, Oka S, Tanaka S, et al. Effectiveness of polaprezinc for low-dose aspirin-induced small-bowel mucosal injuries as evaluated by capsule endoscopy: a pilot randomized controlled study. BMC gastroenterology . Jul 4 2013;13:108. doi:10.1186/1471-230X-13-108. https://www.ncbi.nlm.nih.gov/pubmed/23826914
- Davison G, Marchbank T, March DS, Thatcher R, Playford RJ. Zinc carnosine works with bovine colostrum in truncating heavy exercise-induced increase in gut permeability in healthy volunteers. The American journal of clinical nutrition . Aug 2016;104(2):526-36. doi:10.3945/ajcn.116.134403. https://www.ncbi.nlm.nih.gov/pubmed/27357095
- Mahmoud A, Abuelazm M, Ahmed AAS, Abdalshafy H, Abdelazeem B, Brasic JR. Efficacy and Safety of Polaprezinc-Based Therapy versus the Standard Triple Therapy for Helicobacter pylori Eradication: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients . Oct 4 2022;14(19)doi:10.3390/nu14194126. https://www.ncbi.nlm.nih.gov/pubmed/36235778
- Sartorio MUA, Pendezza E, Coppola S, et al. Potential Role of Omega-3 Polyunsaturated Fatty Acids in Pediatric Food Allergy. Nutrients. Dec 29 2021;14(1):152. doi:10.3390/nu14010152. https://www.ncbi.nlm.nih.gov/pubmed/35011028
- Lotfi R, Rezaiemanesh A, Mortazavi SH, Karaji AG, Salari F. Immunoresolvents in asthma and allergic diseases: Review and update. J Cell Physiol. Jun 2019;234(6):8579-8596. doi:10.1002/jcp.27836. https://www.ncbi.nlm.nih.gov/pubmed/30488527
- DiNicolantonio JJ, O'Keefe J. The Importance of Maintaining a Low Omega-6/Omega-3 Ratio for Reducing the Risk of Autoimmune Diseases, Asthma, and Allergies. Missouri medicine. Sep-Oct 2021;118(5):453-459. https://www.ncbi.nlm.nih.gov/pubmed/34658440
- Del Duca E, Sansone A, Sgrulletti M, et al. Fatty-Acid-Based Membrane Lipidome Profile of Peanut Allergy Patients: An Exploratory Study of a Lifelong Health Condition. Int J Mol Sci. Dec 21 2022;24(1):120. doi:10.3390/ijms24010120. https://www.ncbi.nlm.nih.gov/pubmed/36613559
- Rakha A, Umar N, Rabail R, et al. Anti-inflammatory and anti-allergic potential of dietary flavonoids: A review. Biomed Pharmacother . Dec 2022;156:113945. doi:10.1016/j.biopha.2022.113945. https://www.ncbi.nlm.nih.gov/pubmed/36411631
- Mannucci C, Casciaro M, Sorbara EE, et al. Nutraceuticals against Oxidative Stress in Autoimmune Disorders. Antioxidants (Basel) . Feb 8 2021;10(2):261. doi:10.3390/antiox10020261. https://www.ncbi.nlm.nih.gov/pubmed/33567628
- Maleki SJ, Crespo JF, Cabanillas B. Anti-inflammatory effects of flavonoids. Food Chem. Nov 30 2019;299:125124. doi:10.1016/j.foodchem.2019.125124. https://www.ncbi.nlm.nih.gov/pubmed/31288163
- Kikut J, Konecka N, Zietek M, Kulpa D, Szczuko M. Diet supporting therapy for inflammatory bowel diseases. Eur J Nutr. Aug 2021;60(5):2275-2291. doi:10.1007/s00394-021-02489-0. https://www.ncbi.nlm.nih.gov/pubmed/33788019
- Ribeiro D, Proenca C, Rocha S, et al. Immunomodulatory Effects of Flavonoids in the Prophylaxis and Treatment of Inflammatory Bowel Diseases: A Comprehensive Review. Curr Med Chem. 2018;25(28):3374-3412. doi:10.2174/0929867325666180214121734. https://www.ncbi.nlm.nih.gov/pubmed/29446723
- Plamada D, Vodnar DC. Polyphenols-Gut Microbiota Interrelationship: A Transition to a New Generation of Prebiotics. Nutrients . Dec 28 2021;14(1):137. doi:10.3390/nu14010137. https://www.ncbi.nlm.nih.gov/pubmed/35011012
- Ribeiro M, Sousa T, Poeta P, Bagulho AS, Igrejas G. Review of Structural Features and Binding Capacity of Polyphenols to Gluten Proteins and Peptides In Vitro: Relevance to Celiac Disease. Antioxidants (Basel) . May 29 2020;9(6):463. doi:10.3390/antiox9060463. https://www.ncbi.nlm.nih.gov/pubmed/32485902
- Van Buiten CB, Elias RJ. Gliadin Sequestration as a Novel Therapy for Celiac Disease: A Prospective Application for Polyphenols. Int J Mol Sci. Jan 8 2021;22(2):595. doi:10.3390/ijms22020595. https://www.ncbi.nlm.nih.gov/pubmed/33435615
- Perez-Gregorio MR, Bessa Pereira C, Dias R, Mateus N, de Freitas V. New-Level Insights into the Effects of Grape Seed Polyphenols on the Intestinal Processing and Transport of a Celiac Disease Immunodominant Peptide. J Agric Food Chem. Nov 17 2021;69(45):13474-13486. doi:10.1021/acs.jafc.1c03713. https://www.ncbi.nlm.nih.gov/pubmed/34727499
- Khoshbin K, Camilleri M. Effects of dietary components on intestinal permeability in health and disease. American journal of physiology Gastrointestinal and liver physiology . Nov 1 2020;319(5):G589-G608. doi:10.1152/ajpgi.00245.2020. https://www.ncbi.nlm.nih.gov/pubmed/32902315
- Dryden GW, Lam A, Beatty K, Qazzaz HH, McClain CJ. A pilot study to evaluate the safety and efficacy of an oral dose of (-)-epigallocatechin-3-gallate-rich polyphenon E in patients with mild to moderate ulcerative colitis. Inflammatory bowel diseases. Aug 2013;19(9):1904-12. doi:10.1097/MIB.0b013e31828f5198. https://www.ncbi.nlm.nih.gov/pubmed/23846486
- Samsami-Kor M, Daryani NE, Asl PR, Hekmatdoost A. Anti-Inflammatory Effects of Resveratrol in Patients with Ulcerative Colitis: A Randomized, Double-Blind, Placebo-controlled Pilot Study. Arch Med Res . May 2015;46(4):280-5. doi:10.1016/j.arcmed.2015.05.005. https://www.ncbi.nlm.nih.gov/pubmed/26002728
- Deepika, Maurya PK. Health Benefits of Quercetin in Age-Related Diseases. Molecules. Apr 13 2022;27(8)doi:10.3390/molecules27082498. https://www.ncbi.nlm.nih.gov/pubmed/35458696
- Jafarinia M, Sadat Hosseini M, Kasiri N, et al. Quercetin with the potential effect on allergic diseases. Allergy, asthma, and clinical immunology : official journal of the Canadian Society of Allergy and Clinical Immunology . 2020;16:36. doi:10.1186/s13223-020-00434-0. https://www.ncbi.nlm.nih.gov/pubmed/32467711
- Suzuki M, Suzuki T, Watanabe M, et al. Role of intracellular zinc in molecular and cellular function in allergic inflammatory diseases. Allergol Int. Apr 2021;70(2):190-200. doi:10.1016/j.alit.2020.09.007. https://www.ncbi.nlm.nih.gov/pubmed/33127267
- Nishida K, Uchida R. Role of Zinc Signaling in the Regulation of Mast Cell-, Basophil-, and T Cell-Mediated Allergic Responses. J Immunol Res. 2018;2018:5749120. doi:10.1155/2018/5749120. https://www.ncbi.nlm.nih.gov/pubmed/30596108
- Peroni DG, Hufnagl K, Comberiati P, Roth-Walter F. Lack of iron, zinc, and vitamins as a contributor to the etiology of atopic diseases. Front Nutr. 2022;9:1032481. doi:10.3389/fnut.2022.1032481. https://www.ncbi.nlm.nih.gov/pubmed/36698466
- Gray NA, Dhana A, Stein DJ, Khumalo NP. Zinc and atopic dermatitis: a systematic review and meta-analysis. J Eur Acad Dermatol Venereol . Jun 2019;33(6):1042-1050. doi:10.1111/jdv.15524. https://www.ncbi.nlm.nih.gov/pubmed/30801794
- Chen M, Sun Y, Wu Y. Lower circulating zinc and selenium levels are associated with an increased risk of asthma: evidence from a meta-analysis. Public Health Nutr. Jun 2020;23(9):1555-1562. doi:10.1017/S1368980019003021. https://www.ncbi.nlm.nih.gov/pubmed/31685060
- Hirahara K, Aoki A, Nakayama T. Pathogenic helper T cells. Allergol Int. Apr 2021;70(2):169-173. doi:10.1016/j.alit.2021.02.001. https://www.ncbi.nlm.nih.gov/pubmed/33637414
- Stiles LI, Ferrao K, Mehta KJ. Role of zinc in health and disease. Clinical and experimental medicine. Feb 17 2024;24(1):38. doi:10.1007/s10238-024-01302-6. https://www.ncbi.nlm.nih.gov/pubmed/38367035
- Peng X, Yang Y, Zhong R, et al. Zinc and Inflammatory Bowel Disease: From Clinical Study to Animal Experiment. Biol Trace Elem Res . May 28 2024;doi:10.1007/s12011-024-04193-6. https://www.ncbi.nlm.nih.gov/pubmed/38805169
- Sturniolo GC, Di Leo V, Ferronato A, D'Odorico A, D'Inca R. Zinc supplementation tightens "leaky gut" in Crohn's disease. Inflammatory bowel diseases. May 2001;7(2):94-8. doi:10.1097/00054725-200105000-00003. https://www.ncbi.nlm.nih.gov/pubmed/11383597
- Wang X, Valenzano MC, Mercado JM, Zurbach EP, Mullin JM. Zinc supplementation modifies tight junctions and alters barrier function of CACO-2 human intestinal epithelial layers. Dig Dis Sci. Jan 2013;58(1):77-87. doi:10.1007/s10620-012-2328-8. https://www.ncbi.nlm.nih.gov/pubmed/22903217
- DiGuilio KM, Rybakovsky E, Abdavies R, et al. Micronutrient Improvement of Epithelial Barrier Function in Various Disease States: A Case for Adjuvant Therapy. Int J Mol Sci. Mar 10 2022;23(6)doi:10.3390/ijms23062995. https://www.ncbi.nlm.nih.gov/pubmed/35328419
- Finamore A, Roselli M, Merendino N, Nobili F, Vignolini F, Mengheri E. Zinc deficiency suppresses the development of oral tolerance in rats. J Nutr. Jan 2003;133(1):191-8. doi:10.1093/jn/133.1.191. https://www.ncbi.nlm.nih.gov/pubmed/12514289
- Hussain S, Khan M, Sheikh TMM, et al. Zinc Essentiality, Toxicity, and Its Bacterial Bioremediation: A Comprehensive Insight. Front Microbiol. 2022;13:900740. doi:10.3389/fmicb.2022.900740. https://www.ncbi.nlm.nih.gov/pubmed/35711754
- Scioli MG, Stasi MA, Passeri D, et al. Propionyl-L-Carnitine is Efficacious in Ulcerative Colitis Through its Action on the Immune Function and Microvasculature. Clin Transl Gastroenterol . Mar 20 2014;5(3):e55. doi:10.1038/ctg.2014.4. https://www.ncbi.nlm.nih.gov/pubmed/24646507
- Mikhailova TL, Sishkova E, Poniewierka E, et al. Randomised clinical trial: the efficacy and safety of propionyl-L-carnitine therapy in patients with ulcerative colitis receiving stable oral treatment. Aliment Pharmacol Ther. Nov 2011;34(9):1088-97. doi:10.1111/j.1365-2036.2011.04844.x. https://www.ncbi.nlm.nih.gov/pubmed/21929562
- Shea KM, Booth SL, Massaro JM, Jacques PF, D'Agostino Sr RB, Dawson-Hughes B. Vitamin K and vitamin D status: associations with inflammatory markers in the Framingham Offspring Study. American Journal of Epidimiology . 2008:313-320.
- Lijima H, Shinzaki S, Takehara T. The importance of vitamins D and K for the bone health and immune function in inflammatory bowel disease. Current Opinion in Clinical Nutrition and Metabolic Care . 2012:635-640.
- Matsui T, Tanaka K, Yamashita H, Saneyasu KI, Tanaka H, Takasato Y. Food allergy is linked to season of birth, sun exposure, and vitamin D deficiency. Allergology International. 2019:172-177.
- Burks WA, Tang M, Sicherer S, Muraro A, Eigenmann PA, Ebisawa M. ICON: Food allergy. The Journal of Allergy and Clinical Immunology . 2012:906-920.
- Hwang DW, Nagler CR, Ciaccio CE. New and emerging concepts and therapies for the treatment of food allergy. Immunother Adv . 2022;2(1):ltac006. doi:10.1093/immadv/ltac006. https://www.ncbi.nlm.nih.gov/pubmed/35434724
- Long A, Borro M, Sampath V, Chinthrajah RS. New Developments in Non-allergen-specific Therapy for the Treatment of Food Allergy. Curr Allergy Asthma Rep. Jan 16 2020;20(1):3. doi:10.1007/s11882-020-0897-8. https://www.ncbi.nlm.nih.gov/pubmed/31950290
- Munoz-Bellido FJ, Moreno E, Davila I. Dupilumab: A Review of Present Indications and Off-Label Uses. Journal of investigational allergology & clinical immunology . Apr 19 2022;32(2):97-115. doi:10.18176/jiaci.0682. https://www.ncbi.nlm.nih.gov/pubmed/33661102
- Lloyd M, Loke P, Mack DP, et al. Varying Approaches to Management of IgE-Mediated Food Allergy in Children Around the World. J Allergy Clin Immunol Pract . Apr 2023;11(4):1010-1027 e6. doi:10.1016/j.jaip.2023.01.049. https://www.ncbi.nlm.nih.gov/pubmed/36805346
- FDA approves first drug for treatment of peanut allergy for children. FDA News Release. U.S. Food and Drug Administration; January 31, 2020, Accessed 4/24/2023. https://www.fda.gov/news-events/press-announcements/fda-approves-first-drug-treatment-peanut-allergy-children
- Clinic C. Food Allergies. Accessed August 25, 2023, https://my.clevelandclinic.org/health/diseases/9196-food-allergies
- Yu W, Freeland DMH, Nadeau KC. Food allergy: immune mechanisms, diagnosis and immunotherapy. Nat Rev Immunol. Dec 2016;16(12):751-765. doi:10.1038/nri.2016.111. https://www.ncbi.nlm.nih.gov/pubmed/27795547
- Nowak-Węgrzyn A. Oral immunotherapy for food allergy. Accessed August 25, 2023, https://www.uptodate.com/contents/oral-immunotherapy-for-food-allergy#H3251371449
- Dantzer JA, Wood RA. Next-Generation Approaches for the Treatment of Food Allergy. Curr Allergy Asthma Rep. Jan 28 2019;19(1):5. doi:10.1007/s11882-019-0839-5. https://www.ncbi.nlm.nih.gov/pubmed/30689123
- FDA. FDA approves first drug for treatment of peanut allergy for children. Accessed August 25, 2023, https://www.fda.gov/news-events/press-announcements/fda-approves-first-drug-treatment-peanut-allergy-children
- Nowak-Węgrzyn A. Experimental therapies for food allergy: Immunotherapy and nonspecific therapies. Accessed August 25, 2023, https://www.uptodate.com/contents/experimental-therapies-for-food-allergy-immunotherapy-and-nonspecific-therapies#topicContent
- Kim EH, Burks AW. Food allergy immunotherapy: Oral immunotherapy and epicutaneous immunotherapy. Allergy. Jun 2020;75(6):1337-1346. doi:10.1111/all.14220. https://www.ncbi.nlm.nih.gov/pubmed/32034781
- FDA. FDA Approves First Treatment for Eosinophilic Esophagitis, a Chronic Immune Disorder. Accessed August 25, 2023, https://www.fda.gov/news-events/press-announcements/fda-approves-first-treatment-eosinophilic-esophagitis-chronic-immune-disorder
- Lucendo AJ, Molina-Infante J. Esophageal dilation in eosinophilic esophagitis: risks, benefits, and when to do it. Current opinion in gastroenterology . Jul 2018;34(4):226-232. doi:10.1097/MOG.0000000000000442. https://www.ncbi.nlm.nih.gov/pubmed/29846259
- Kovacova-Hanuskova E, Buday T, Gavliakova S, Plevkova J. Histamine, histamine intoxication and intolerance. 10.1016/j.aller.2015.05.001. Allergologia et immunopathologia. 2015;43(5):498-506. doi:10.1016/j.aller.2015.05.001. https://www.elsevier.es/en-revista-allergologia-et-immunopathologia-105-articulo-histamine-histamine-intoxication-intolerance-S0301054615000932
- Guntern P, Eggel A. Past, present, and future of anti-IgE biologics. Allergy. Oct 2020;75(10):2491-2502. doi:10.1111/all.14308. https://www.ncbi.nlm.nih.gov/pubmed/32249957
- Zuberbier T, Wood RA, Bindslev-Jensen C, et al. Omalizumab in IgE-Mediated Food Allergy: A Systematic Review and Meta-Analysis. J Allergy Clin Immunol Pract. Apr 2023;11(4):1134-1146. doi:10.1016/j.jaip.2022.11.036. https://www.ncbi.nlm.nih.gov/pubmed/36529441
- Wood RA, Chinthrajah RS, Eggel A, et al. The rationale for development of ligelizumab in food allergy. The World Allergy Organization journal . Sep 2022;15(9):100690. doi:10.1016/j.waojou.2022.100690. https://www.ncbi.nlm.nih.gov/pubmed/36185545
- Dellon ES, Peterson KA, Murray JA, et al. Anti-Siglec-8 Antibody for Eosinophilic Gastritis and Duodenitis. N Engl J Med. Oct 22 2020;383(17):1624-1634. doi:10.1056/NEJMoa2012047. https://www.ncbi.nlm.nih.gov/pubmed/33085861
- Allakos Announces Topline Phase 3 Data from the EoDyssey Study in Patients with Eosinophilic Duodenitis. Allakos. Updated Sept. 9, 2022. Accessed Jan. 5, 2024, https://investor.allakos.com/news-releases/news-release-details/allakos-announces-topline-phase-3-data-eodyssey-study-patients#:~:text=Lirentelimab%20has%20received%20orphan%20drug,expressed%20selectively%20on%20mast%20cells
- Hirano I, Collins MH, Assouline-Dayan Y, et al. RPC4046, a Monoclonal Antibody Against IL13, Reduces Histologic and Endoscopic Activity in Patients With Eosinophilic Esophagitis. Gastroenterology . Feb 2019;156(3):592-603 e10. doi:10.1053/j.gastro.2018.10.051. https://www.ncbi.nlm.nih.gov/pubmed/30395812
- Gann PH, Deaton RJ, McMahon N, et al. An anti-IL-13 antibody reverses epithelial-mesenchymal transition biomarkers in eosinophilic esophagitis: Phase 2 trial results. J Allergy Clin Immunol . Aug 2020;146(2):367-376 e3. doi:10.1016/j.jaci.2020.03.045. https://www.ncbi.nlm.nih.gov/pubmed/32407835
- Dellon ES, Collins MH, Rothenberg ME, et al. Long-term Efficacy and Tolerability of RPC4046 in an Open-Label Extension Trial of Patients With Eosinophilic Esophagitis. Clin Gastroenterol Hepatol . Mar 2021;19(3):473-483 e17. doi:10.1016/j.cgh.2020.03.036. https://www.ncbi.nlm.nih.gov/pubmed/32205221
- Deeks ED, Brusselle G. Reslizumab in Eosinophilic Asthma: A Review. Drugs. May 2017;77(7):777-784. doi:10.1007/s40265-017-0740-2. https://www.ncbi.nlm.nih.gov/pubmed/28421429
- Pitlick MM, Li JT, Pongdee T. Current and emerging biologic therapies targeting eosinophilic disorders. The World Allergy Organization journal . Aug 2022;15(8):100676. doi:10.1016/j.waojou.2022.100676. https://www.ncbi.nlm.nih.gov/pubmed/35983569
- de Rooij WE, Dellon ES, Parker CE, et al. Pharmacotherapies for the Treatment of Eosinophilic Esophagitis: State of the Art Review. Drugs. Sep 2019;79(13):1419-1434. doi:10.1007/s40265-019-01173-2. https://www.ncbi.nlm.nih.gov/pubmed/31352605
- Meek PD, Hemstreet B. Emerging therapies for eosinophilic esophagitis. Pharmacotherapy. Apr 2023;43(4):338-348. doi:10.1002/phar.2783. https://www.ncbi.nlm.nih.gov/pubmed/36840634
- Celgene, Bristol-Myers Squibb. A Study to Evaluate the Efficacy and Safety of CC-93538 in Adult and Adolescent Participants With Eosinophilic Esophagitis. ClinicalTrials.gov. Accessed Jan. 5, 2024, https://clinicaltrials.gov/study/NCT04753697
- Sandborn WJ, Vermeire S, Peyrin-Biroulet L, et al. Etrasimod as induction and maintenance therapy for ulcerative colitis (ELEVATE): two randomised, double-blind, placebo-controlled, phase 3 studies. Lancet. Apr 8 2023;401(10383):1159-1171. doi:10.1016/S0140-6736(23)00061-2. https://www.ncbi.nlm.nih.gov/pubmed/36871574
- Biotherapeutics R. Revolo Biotherapeutics Announces Positive Topline Data from Phase 2a Trial of ‘1104 in Adults with Active Eosinophilic Esophagitis. Accessed August 25, 2023, https://revolobio.com/2023/04/19/revolo-biotherapeutics-announces-positive-topline-data-from-phase-2a-trial-of-1104-in-adults-with-active-eosinophilic-esophagitis/
- Revolo Biotherapeutics. Revolo Biotherapeutics Receives Orphan Drug Designation from the U.S. FDA for its First-in-Class Peptide as a Potential Treatment for Eosinophilic Esophagitis. Updated Jan. 30, 2024. Accessed Apr. 5, 2024, https://revolobio.com/2024/01/30/revolo-biotherapeutics-receives-orphan-drug-designation-from-the-u-s-fda-for-its-first-in-class-peptide-as-a-potential-treatment-for-eosinophilic-esophagitis/
- Liu Z-Q, Zheng P-Y, Yang P-C. Hapten Facilitates Food Allergen-Related Intestinal Hypersensitivity. The American journal of the medical sciences . 2013;345(5):375-379. doi:10.1097/MAJ.0b013e3182571f28. https://doi.org/10.1097/MAJ.0b013e3182571f28
- Ruiz-Hernandez A, Kuo C-C, Rentero-Garrido P, et al. Environmental chemicals and DNA methylation in adults: a systematic review of the epidemiologic evidence. Clinical Epigenetics. 2015/04/29 2015;7(1):55. doi:10.1186/s13148-015-0055-7. https://doi.org/10.1186/s13148-015-0055-7 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4433069/pdf/13148_2015_Article_55.pdf