LIFE EXTENSION MAGAZINE
When we wrote about the dangers of senescent cells in 2015, few had ever heard about this aspect of pathologic aging.
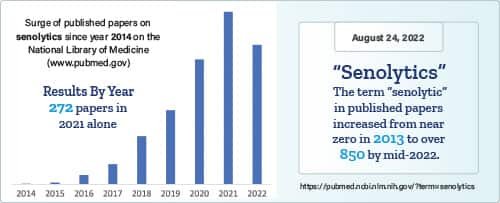
Move forward to 2022 and there are hundreds of published papers in the National Library of Medicine describing compounds that remove these worn-out cells.
Agents that delete senescent cells are called “senolytics.”1,2
In coming years, senolytics will likely become standard care for all individuals over age 40.
The reason is that senescent cells release toxic factors that accelerate degenerative processes throughout our bodies.1,3-8
When senolytics were administered to old mice (the human equivalent of 75-90 years), lifespans increased roughly 36% along with better physical function.9
In 2018 and 2019, mainstream medical journals described the potential ofsenolytics to “transform medical care.”10,11
A huge review article published in August 2022 described many promising animal and human studies. The authors advocated for large, randomized, placebo-controlled clinical trials using senolytics to combat age-related disorders.12
We concur about more extensive research, but this begs the question, what are people over age 40 supposed to do now to reduce their senescent cell burden?
This editorial describes what’s holding back rapid clinical research and what people are practicing today to selectively remove senescent cells.
A major factor in old-age decline is the accumulation of senescent cells that:
- Impede organ function
- Ignite chronic inflammation
- Emit protein-destroying enzymes
- Shorten healthy lifespan
Diabetes, obesity, stroke, vision loss, neurodegenerative disorders, osteoarthritis, and cancer can be connected to the presence of senescent cells.13-17
Senolytic compounds selectively destroy senescent cells.
Numerous studies about senolytics have been published in recent years.18-20
This research provides evidence that senolytics could contribute to better clinical outcomes against age-associated disorders. They show promise in combating heart failure, type II diabetes, Alzheimer’s, vascular insufficiency, and lung impairment.
Senolytic Properties of Fisetin
Fisetin is a flavonoid found in various plants including strawberries, apples, grapes, and onions.
Researchers have found that fisetin is an exceptionally powerful senolytic.
When compared to other plant compounds, fisetin was the most effective at removing senescent cells, both in cell culture and in mice.21
The impact is dramatic. Mice given fisetin lived an average of about 2.5 months longer, an almost 10% extension of lifespan—even when treatment was started at the human equivalent of 75 years of age.21
The Mayo Clinic has been at the forefront in initiating clinical trials to ascertain the ability of fisetin to reduce the senescent cell burden in aging humans.
Researchers, however, ran into an obstacle.
Government Impedes Human Research
Professor James Kirkland is spearheading multiple senolytic studies.
On December 9, 2021, Dr. Kirkland described a clinical trial where he had to complete a 450-page detailed Investigational New Drug application. He submitted this to the FDA for approval to do a human study using fisetin.22
The FDA then mandated that Dr. Kirkland do preclinical (animal and pharmacology) studies before “allowing” a senolytic clinical trial to commence. It took 2.5 years to gather this information, and only then could they begin the human study.
What’s irrational about this delay is that fisetin has long been ingested by people in various fruits and vegetables (albeit at lower potencies). It’s also been used for years as a dietary supplement.
Bureaucratic barriers like this impede rapid testing of senolytic and other compounds that may slow or reverse certain aging processes.
It’s a major reason why most practicing physicians remain in the dark about senolytics, despite favorable reviews published in the New England Journal of Medicine, JAMA, and other respected medical journals.
Senolytic Drug and Nutrient Options
The first clinically tested senolytic approach combined a cancer drug called dasatinib with high-dose quercetin on an intermittent basis (several dosing periods within a year).
Quercetin and dasatinib each have unique senolytic-targeting properties. Taking dasatinib + quercetin together is a validated approach to removing senescent cells.9,23
Obtaining a prescription for dasatinib is challenging, the cost per pill is high, and some people don’t want to take a cancer drug, even though it is only being used several times a year in many cases. Fortunately, an extract from black tea called theaflavins has been shown to have a similar senolytic mechanism (decrease activity of tyrosine kinase receptors) as dasatinib in preclinical research.24
Theaflavins have also been shown to inhibit the BCL-2 family of proteins.25 Compounds that inhibit BCL-2 might help prevent some malignancies in addition to removing certain types of senescent cells.
Fisetin is a broad-spectrum bioactive plant flavonoid with potent senolytic activity.
Fisetin has been shown to:
- Function as a targeted senolytic agent,21
- Protect the brain in various models of neurodegenerative disorders,26-32
- Improve outcomes in people who suffered strokes,33
- Help prevent malignant changes in cells,34-37 and
- Help fight obesity and type II diabetes in animal and experimental models.38-40
The initial challenge was that fisetin is mostly, and rapidly, converted to different metabolites in the liver.
Two years ago, scientists developed a method to increase fisetin bioavailability up to 25 times higher,41 thus enabling it to reach higher concentrations in the blood, and then to stay there longer, compared to ordinary fisetin.
A current nutritional senolytic strategy is to take, just once per week, the following:
Theaflavins + Quercetin + Fisetin
Many readers of this publication already do this, using a formula that combines all three of the above nutrients.
Congress Needs to Amend Regulations
In this instance, I am not criticizing the FDA for intentionally committing wrongdoing.
The problem is an antiquated system that erects so many bureaucratic hurdles that many promising clinical trials never commence.
Congress needs to amend requirements for studying compounds (like repurposed medications) so that clinical trials can be initiated without the strict regulatory requirements for testing a brand-new drug.
Mainstream Recognizes Potential of Senolytics
Here are a few quotes from research published in the Journal of the American Medical Association:
“…many human pathologic conditions are associated with the presence of senescent cells.”42
“Interventions aimed at eliminating those senescent cells, commonly called senolytic, have also been shown to improve health and extend life in various mouse disease models.”42
“If senolytics are shown to be safe and effective in humans, they could transform care of older adults and patients with multiple chronic diseases.”10
In this month’s issue…
In a recent interview, Dr. James Kirkland elaborated on the multiple ways that senolytics can potentially combat a host of degenerative disorders.
The article in this month’s issue provides an update on Dr. Kirkland’s research.
People with higher intake of black tea have lower incidences of cancer. This may be due to a senolytic compound in black tea called theaflavins.
The article describes how theaflavins activate a beneficial cancer-suppressing gene.
Theaflavins may play a dual role by removing senescent cells and protecting normal cells.
We at Life Extension continue to advocate for rapid-fire clinical testing of compounds that may delay and reverse toxic mechanisms that underlie biological aging.
For longer life,
William Faloon
References
- Kirkland JL, Tchkonia T. Senolytic drugs: from discovery to translation. J Intern Med. 2020 Nov;288(5):518-36.
- Wissler Gerdes EO, Zhu Y, Tchkonia T, et al. Discovery, development, and future application of senolytics: theories and predictions. FEBS J. 2020 Jun;287(12):2418-27.
- Calcinotto A, Kohli J, Zagato E, et al. Cellular Senescence: Aging, Cancer, and Injury. Physiol Rev. 2019 Apr 1;99(2):1047-78.
- Childs BG, Li H, van Deursen JM. Senescent cells: a therapeutic target for cardiovascular disease. J Clin Invest. 2018 Apr 2;128(4):1217-28.
- Childs BG, Durik M, Baker DJ, et al. Cellular senescence in aging and age-related disease: from mechanisms to therapy. Nat Med. 2015 Dec;21(12):1424-35.
- Herranz N, Gil J. Mechanisms and functions of cellular senescence. J Clin Invest. 2018 Apr 2;128(4):1238-46.
- Kirkland JL, Tchkonia T. Cellular Senescence: A Translational Perspective. EBioMedicine. 2017 Jul;21:21-8.
- Soto-Gamez A, Quax WJ, Demaria M. Regulation of Survival Networks in Senescent Cells: From Mechanisms to Interventions. J Mol Biol. 2019 Jul 12;431(15):2629-43.
- Xu M, Pirtskhalava T, Farr JN, et al. Senolytics improve physical function and increase lifespan in old age. Nat Med. 2018 Aug;24(8):1246-56.
- Tchkonia T, Kirkland JL. Aging, Cell Senescence, and Chronic Disease: Emerging Therapeutic Strategies. JAMA. 2018 Oct 2;320(13):1319-20.
- Dzau VJ, Inouye SK, Rowe JW, et al. Enabling Healthful Aging for All - The National Academy of Medicine Grand Challenge in Healthy Longevity. N Engl J Med. 2019 Oct 31;381(18):1699-701.
- Chaib S, Tchkonia T, Kirkland JL. Cellular senescence and senolytics: the path to the clinic. Nat Med. 2022 Aug 11.
- Baker DJ, Wijshake T, Tchkonia T, et al. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature. 2011 Nov 2;479(7372):232-6.
- Kirkland JL. Inflammation and cellular senescence: potential contribution to chronic diseases and disabilities with aging. Public Policy and Aging Report. 2013;23:12-5.
- Kirkland JL, Tchkonia T. Clinical strategies and animal models for developing senolytic agents. Exp Gerontol. 2015 Aug;68:19-25.
- Tchkonia T, Zhu Y, van Deursen J, et al. Cellular senescence and the senescent secretory phenotype: therapeutic opportunities. J Clin Invest. 2013 Mar;123(3):966-72.
- Zhu Y, Armstrong JL, Tchkonia T, et al. Cellular senescence and the senescent secretory phenotype in age-related chronic diseases. Curr Opin Clin Nutr Metab Care. 2014 Jul;17(4):324-8.
- Justice JN, Nambiar AM, Tchkonia T, et al. Senolytics in idiopathic pulmonary fibrosis: Results from a first-in-human, open-label, pilot study. EBioMedicine. 2019 Feb;40:554-63.
- Anderson R, Lagnado A, Maggiorani D, et al. Length-independent telomere damage drives post-mitotic cardiomyocyte senescence. EMBO J. 2019 Mar 1;38(5).
- Zhang P, Kishimoto Y, Grammatikakis I, et al. Senolytic therapy alleviates Abeta-associated oligodendrocyte progenitor cell senescence and cognitive deficits in an Alzheimer’s disease model. Nat Neurosci. 2019 May;22(5):719-28.
- Yousefzadeh MJ, Zhu Y, McGowan SJ, et al. Fisetin is a senotherapeutic that extends health and lifespan. EBioMedicine. 2018 Oct;36:18-28.
- Available at: https://www.youtube.com/watch?v=yqjuWdWtJF8. Accessed August 9, 2022.
- Zhu Y, Tchkonia T, Pirtskhalava T, et al. The Achilles’ heel of senescent cells: from transcriptome to senolytic drugs. Aging Cell. 2015 Aug;14(4):644-58.
- Mizuno H, Cho YY, Zhu F, et al. Theaflavin-3, 3’-digallate induces epidermal growth factor receptor downregulation. Mol Carcinog. 2006 Mar;45(3):204-12.
- Leone M, Zhai D, Sareth S, et al. Cancer prevention by tea polyphenols is linked to their direct inhibition of antiapoptotic Bcl-2-family proteins. Cancer Res. 2003 Dec 1;63(23):8118-21.
- Ahmad A, Ali T, Park HY, et al. Neuroprotective Effect of Fisetin Against Amyloid-Beta-Induced Cognitive/Synaptic Dysfunction, Neuroinflammation, and Neurodegeneration in Adult Mice. Mol Neurobiol. 2017 Apr;54(3):2269-85.
- Alikatte K, Palle S, Rajendra Kumar J, et al. Fisetin Improved Rotenone-Induced Behavioral Deficits, Oxidative Changes, and Mitochondrial Dysfunctions in Rat Model of Parkinson’s Disease. J Diet Suppl. 2021 Jan 29;18(1):57-71.
- Chen C, Yao L, Cui J, et al. Fisetin Protects against Intracerebral Hemorrhage-Induced Neuroinflammation in Aged Mice. Cerebrovasc Dis. 2018;45(3-4):154-61.
- Maher P. Modulation of multiple pathways involved in the maintenance of neuronal function during aging by fisetin. Genes Nutr. 2009 Dec;4(4):297-307.
- Maher P, Akaishi T, Abe K. Flavonoid fisetin promotes ERK-dependent long-term potentiation and enhances memory. Proc Natl Acad Sci U S A. 2006 Oct 31;103(44):16568-73.
- Pal HC, Pearlman RL, Afaq F. Fisetin and Its Role in Chronic Diseases. Adv Exp Med Biol. 2016;928:213-44.
- Zhang L, Wang H, Zhou Y, et al. Fisetin alleviates oxidative stress after traumatic brain injury via the Nrf2-ARE pathway. Neurochem Int. 2018 Sep;118:304-13.
- Wang L, Cao D, Wu H, et al. Fisetin Prolongs Therapy Window of Brain Ischemic Stroke Using Tissue Plasminogen Activator: A Double-Blind Randomized Placebo-Controlled Clinical Trial. Clin Appl Thromb Hemost. 2019 Jan-Dec;25:1076029619871359.
- Khan N, Afaq F, Syed DN, et al. Fisetin, a novel dietary flavonoid, causes apoptosis and cell cycle arrest in human prostate cancer LNCaP cells. Carcinogenesis. 2008 May;29(5):1049-56.
- Li J, Cheng Y, Qu W, et al. Fisetin, a dietary flavonoid, induces cell cycle arrest and apoptosis through activation of p53 and inhibition of NF-kappa B pathways in bladder cancer cells. Basic Clin Pharmacol Toxicol. 2011 Feb;108(2):84-93.
- Suh Y, Afaq F, Johnson JJ, et al. A plant flavonoid fisetin induces apoptosis in colon cancer cells by inhibition of COX2 and Wnt/EGFR/NF-kappaB-signaling pathways. Carcinogenesis. 2009 Feb;30(2):300-7.
- Ying TH, Yang SF, Tsai SJ, et al. Fisetin induces apoptosis in human cervical cancer HeLa cells through ERK1/2-mediated activation of caspase-8-/caspase-3-dependent pathway. Arch Toxicol. 2012 Feb;86(2):263-73.
- Jung CH, Kim H, Ahn J, et al. Fisetin regulates obesity by targeting mTORC1 signaling. J Nutr Biochem. 2013 Aug;24(8):1547-54.
- Ge C, Xu M, Qin Y, et al. Fisetin supplementation prevents high fat diet-induced diabetic nephropathy by repressing insulin resistance and RIP3-regulated inflammation. Food Funct. 2019 May 22;10(5):2970-85.
- Vinayagam R, Xu B. Antidiabetic properties of dietary flavonoids: a cellular mechanism review. Nutr Metab (Lond). 2015;12(1):60.
- Akay. A cross over pilot pharmacokinetic study of fisetin 1000mg and formulated fisetin 200mg administered in a single dose to healthy volunteers. Manufacturer’s study (in press for future publication). 2020.
- Barzilai N, Cuervo AM, Austad S. Aging as a Biological Target for Prevention and Therapy. JAMA. 2018 Oct 2;320(13):1321-2.