Life Extension Magazine®

When a new lifesaving therapy is announced, few people calculate how many died in the waiting room.
Delays in introducing better drugs extend beyond the FDA’s bureaucratic quagmire.
Pharmaceutical companies spend years negotiating their financial “cut” of a medical discovery and patent ownership.
More years slip by as the owners seek funding for clinical trials and negotiate future marketing rights.
As these delays accumulate, a staggering number of Americans needlessly suffer and die.
Even worse are promising compounds that don’t garner financial backing. These therapies fall off the radar screen and are sometimes lost forever.
A recent study on the mortality risk-reducing effects of metformin enabled our scientific team to conduct a startling calculation.1
More than eight million Americans may have died because of the 37-year delay in this one drug becoming available.
Although there is significant uncertainty with this estimate, it’s more than all Americans killed in all wars since the inception of this country!
I’ve written books exposing flaws in today’s drug-development process that was long ago rendered obsolete.
This article summarizes the loss of life that theoretically may have occurred because type II diabetics were denied access to metformin and how these tragedies can be prevented.
I also discuss cancer treatments using “off-label” drugs that are demonstrating remarkable survival improvements.

In 1995 I was given an ultimatum by the FDA.
Either I stop educating Life Extension® readers about lower cost drugs from other countries or I would face criminal charges and years in prison.
I respectfully declined to accept the FDA’s censorship dictate that would deny Life Extension® readers access to lifesaving medications.
Having just defeated the FDA in a nine-year legal battle, I was confident the public would continue to support our efforts to accelerate the availability of more advanced medications.
To put the situation into context, the Internet was in its infancy in the mid-1990s. Americans could not readily find pharmacies in other countries to send them medications.
By censoring Life Extension® magazine, as the FDA attempted, few Americans would know about better ways to treat their disease.
This included drugs like metformin for type II diabetics and ribavirin as an adjunctive hepatitis C medication.
Censored Drugs Now Widely Prescribed

Back in the 1980s-1990s, Life Extension® fought a multi-decade battle with the FDA to force the approval of an antiviral drug called ribavirin.
When ribavirin was combined with interferon-alpha, treatment outcomes in hepatitis C patients markedly improved. Today’s hepatitis C drugs (like Sovaldi®) are curing over 95% of patients.
Yet, when drugs like Sovaldi® were approved in 2013-2014, most still relied on co-administration of ribavirin.
More recent hepatitis C protocols are combining Sovaldi® with newer drugs (in lieu of ribavirin) to better eradicate hepatitis C.
We have no financial interest in ribavirin. We identified its efficacy in the early 1980s and relayed this information to our supporters.
Metformin is often the first drug prescribed to type II diabetics, assuming reasonable cardiac and renal function. Many non-diabetics now take metformin for its potential anti-aging properties. With today’s Internet, ordering lower-cost medications from Canada and other countries has become routine and may soon be formally legalized.
Before the Internet, the FDA bowed to pharmaceutical lobbyists and attacked those seeking to educate Americans about better drugs at lower prices.
The magnitude of this tragedy cannot be overstated. Our efforts to accelerate approval of ribavirin alone may have saved thousands of American lives.
Why Metformin Was Delayed So Long

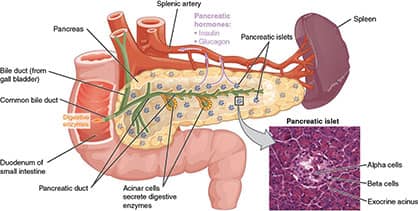
Metformin was first discovered in 1922 but fell by the wayside as doctors saw more immediate results prescribing insulin or insulin-boosting drugs (in the sulfonylurea class).
Sulfonylurea drugs stimulate insulin release from the pancreas. This results in a temporary drop in blood sugar (glucose).
Insulin injections immediately reduce glucose, which is needed in advanced stages of type II diabetes, and by type I diabetics who make little or no insulin themselves.
The problem with this approach is it inadequately addresses the underlying causes of type II diabetes which include beta-cell dysfunction and insulin resistance.
Metformin functions via several mechanisms to combat high blood sugar including activating the AMPK enzyme, favorably altering the gut microbiota, and improving insulin sensitivity in the liver and peripheral muscle cells.2,3
A chemical cousin of metformin called phenformin inflicted a lethal condition called lactic acidosis in some people. This caused doctors to fear drugs in this class, especially with the quick-fix effects of sulfonylurea drugs or insulin injections.
What puzzled us back in the 1990s, however, was that metformin had been approved in England in 1958, Canada in 1972, and much of the world shortly thereafter.
Lactic acidosis was not occurring unless people had significant preexisting kidney, liver, heart, and/or lung failure.
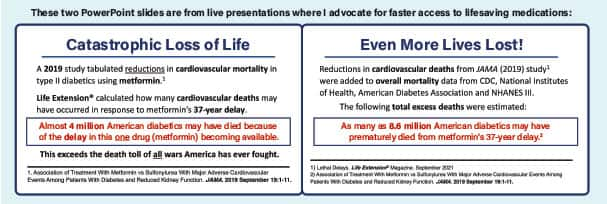
Catastrophic Loss of Life
Metformin became widely available to Americans in late 1995 at relatively high prices (sold under the tradename Glucophage®).
In 2019, a study was published that tabulated the reductions in cardiovascular mortality in type II diabetics who use metformin.4
We at Life Extension® took these data along with data on diabetes prevalence from the CDC and NHANES III, death rates from the National Institutes of Health and the American Diabetes Association, as well as studies assessing the impact of metformin on mortality.
We performed a series of calculations to estimate how many premature deaths may have occurred from cardiovascular causes as a result of metformin’s 37-year delay.
The calculations include a range of possible values, based on the estimate that between 1958 and 1995, there may have been between 1.35 to 28.8 million people with diabetes who died in the United States, of whom 880,000 to 18.7 million died due to diabetes-related cardiovascular disease.
Obviously, there is a large degree of uncertainty with such a series of calculations and estimates from the published literature, but assuming that 100% of people with diabetes had taken metformin beginning in 1958, we can crudely estimate that 405,200 to 8.6 million people might have been spared premature death had metformin been approved in 1958 instead of 1995.
More specifically, we estimate that 175,600 to 3.7 million people might have been spared death from diabetes-related cardiovascular disease.
This is only one example of a broken system that continues to deny Americans rapid access to lifesaving therapies.
One can debate the absolute numbers of total deaths that occurred because of metformin’s delay. But even at the lower end of our calculations, a staggering number of American type II diabetics perished prematurely.
Metformin also Reduces Cancer Risk
When attacking the delays in approval of lifesaving medications as I’ve done for the past 41 years, I use published, peer-reviewed references that provide objective information for accuracy.
As it relates to the delay in approving metformin, however, the death toll (up to 8.6 million Americans) extends beyond classic diabetic complications.
That’s because metformin also reduces cancer risk in type II diabetics.
A study conducted at MD Anderson found that type II diabetics prescribed metformin had a 62% reduced risk of pancreatic cancer.5 This malignancy is more prevalent in diabetics and kills over 48,000 Americans each year.6
Based upon published data, and if all the cancer deaths that metformin could have prevented are assumed, the total mortality resulting over this 37-year delay period means up to 8.6 million American lives might have been spared if metformin was approved sooner.
These needless deaths are intolerable. The solution is introducing aggressive free market reforms to the obsolete drug development process that plagues us today.
Consequences of Denial

Politicians are debating a lot of topics right now, but the most important problem facing Americans is not being discussed.
Once you or a loved one is diagnosed with a serious disease, all other issues become irrelevant. Your overriding concern is whether there is a cure available.
That’s why it’s imperative that free market reforms are enacted that place the FDA in an advisory role that allows rapid medical progress unimpeded by central government bureaucrats.
Back in 2012, the former FDA Commissioner wrote a scathing editorial against the agency (FDA) from which he had resigned.7
Several doctors responded with complimentary letters and emphasized even more deregulation of FDA control is needed to bring about cures for today’s killer diseases.22
Some of these letters exposed how dysfunction and unpredictability at the FDA are precluding vital early-stage scientific research.
Despite these exposés from FDA insiders, most Americans remain in a state of denial about the lethal consequences of today’s antiquated regulatory structure.
This denial turns into harsh reality when one is diagnosed with an illness for which there is no current cure.
Our 41-Year Battle to Reform Drug Approval
Pleas of Former FDA Commissioner Ignored

An increasing number of respected individuals concur that delaying lifesaving therapies can no longer be tolerated. This includes former FDA Commissioner Andrew von Eschenbach.
Dr. von Eschenbach was a director of the National Cancer Institute and later served as FDA Commissioner from 2005 to 2009.
Back in 2012, he authored an editorial published in The Wall Street Journal that was critical of the FDA’s ability to evaluate and approve new lifesaving therapies.7
The editorial began with Dr. von Eschenbach stating:
“We stand on the cusp of a revolution in health care. Advances in molecular medicine will allow us to develop powerful new treatments that can cure or even prevent diseases like Alzheimer’s and cancer.”7
“What’s missing,” according to Dr. von Eschenbach, “is a modernized Food and Drug Administration that can rapidly and efficiently bring new discoveries to patients.”7
Dr. von Eschenbach cited then-current FDA Commissioner Margaret Hamburg’s concession before Congress that, “The FDA is relying on 20th century regulatory science to evaluate 21st century medical products.”7
The most compelling arguments Dr. von Eschenbach made for meaningful reform were:7
“The FDA should approve drugs based on safety and leave efficacy testing for post-market studies. Congress can ensure that the FDA serves as a bridge—not a barrier—to cutting-edge technologies.”
Said differently, once a potentially effective therapy has been cleared for safety, it should be made immediately available to people who may otherwise suffer and die.
While the FDA has expedited some procedures like “fast-tracking” certain drugs to terminal patients, the bureaucratic barriers continue to result in horrific delays.
Pancreatic cancer patients, for example, shouldn’t have to wait for years for FDA-required efficacy studies. They need rapid access to new therapies that offer some hope of saving their lives.
What’s scary about Dr. von Eschenbach’s criticisms are that they were made back in 2012. Yet relatively little has been done to rectify the most lethal threat to the lives of aging Americans, i.e. long delays in introducing lifesaving therapies.

We at Life Extension continue our relentless campaign to alert policy makers and the public about the urgent need to accelerate the introduction of new therapies.
This goes beyond relegating the FDA to an advisory role, away from its current arbitrary power.
The entire drug development process, from start to finish, requires radical change.
Medical innovations need rapid testing on patients who are fully apprised of risks and potential benefits, without costly bureaucratic interference.
Unlike other issues, failure to effect meaningful reforms will continue the needless carnage of human suffering and death.
This is a priority matter that politicians should be debating today!
The box on page 15 reveals marked survival improvements in brain tumor patients treated with combinations of “off-label” drugs that include metformin.
For 41 consecutive years, we at Life Extension® advocated for multipronged treatments for lethal diseases. The scientific literature continues to validate this approach across a spectrum of degenerative disorders.
Why Didn’t John McCain Act Up?

John McCain died from a glioblastoma brain tumor in August 2018.
Nine years prior, Ted Kennedy also died from glioblastoma.
Both senators cordially worked together for decades on Capitol Hill.
John McCain attended Ted Kennedy’s funeral and gave a eulogy.
My question is how could John McCain witness Ted Kennedy in a “box” without Senator McCain using his enormous legislative power to ensure the same did not happen to him?
I know my thinking process differs from most others', but when anyone I know develops a serious medical disorder, I instinctively spend hours searching the medical literature seeking a better way to treat it.
In some cases, I help launch and fund clinical trials to see if a potential treatment yields real-world results.
Effective Brain Cancer Treatments

After John McCain’s death, Scientific American published an insightful essay as to why glioblastoma is such a difficult malignancy to treat.8
Omitted from the Scientific American report, however, were a plethora of potentially effective therapies that have encountered outlandish delays.
These include a roughly 10-year delay in advancing genetically modified poliovirus that has demonstrated impressive responses in some glioblastoma patients.9 We described this therapy in the September 2015 issue of Life Extension® magazine.10
Even more omissions relate to adjuvant therapies about which Life Extension®previously published. Some of these include:
Valganciclovir: Treatment of glioblastoma patients with valganciclovir produced an unheard-of median overall survival of 56.4 months (4.7 years).11
Note: Ted Kennedy lived only 15 months and John McCain about 14 months after their glioblastoma diagnosis. I don’t know if they tried valganciclovir, but there were good data to support it. The clinical trial showed glioblastoma patients treated with valganciclovir lived almost four times longer than Senators Kennedy and McCain.
Not only has valganciclovir been shown to extend survival in glioblastoma patients, but it may be considered along with other complementary therapies that could improve outcomes even more!

Metformin: In glioblastoma patients, survival time without evidence of disease worsening was longer in diabetics receiving metformin (10 months) than in other diabetics (less than five months) and nondiabetics (less than seven months).12
Note: As of mid-2021, there were five clinical trials (two phase II and three phase I) registered with ClinicalTrials.gov that address the potential benefits of metformin in people with glioblastoma (ClinicalTrials.gov 2021). Results of these trials will help establish the value of metformin as an adjuvant therapy for glioblastoma. If I had glioblastoma, however, I would not wait for these clinical trials to complete—I’d initiate metformin immediately. (I’ve been using metformin as a preventative since around year 2000).
Cimetidine: A 2017 study on seven glioblastoma patients found that, when combined with the chemo drug temozolomide, a cocktail of drugs that included cimetidine, lithium, olanzapine, and valproate led to longer-than-expected survival.13
Note: Multi-drug therapy is the most rational approach to inducing complete responses, yet a study like this, using four different off-label medications is uncommon. More of these kinds of multi-drug studies are needed.
Dichloroacetate: An open-label phase I trial on 15 adults with grade III or IV gliomas or brain metastases from other cancers found that dichloroacetate treatment was feasible and well tolerated.14
Antidepressants: Fluoxetine (Prozac), a common antidepressant drug, has been shown to selectively kill glioblastoma cells in laboratory experiments.15 Additionally, fluoxetine may make glioblastoma cells more sensitive to temozolomide.16 Other antidepressant drugs, such as imipramine (Tofranil) and amitriptyline (Elavil), have been shown to stop glioblastoma stem cells from producing more stem cells.17
Natural interventions, such as vitamin D, quercetin, selenium, and melatonin are being explored, with intriguing preliminary results.18-21
Encouraging findings about a new multi-drug (off-label) cocktail treatment for glioblastoma appear at the end of this editorial.
To review Life Extension’s glioblastoma protocol, log on to: https://www.lifeextension.com/glioblastoma.
For longer life,
William Faloon
Life Extension Buyers Club
How I Am Fighting Back

I’ve written hundreds of articles that meticulously describe how misguided FDA policies are the leading causes of disability and death. Many of these articles are compiled into the following three books: Every few years, a publisher chronicles my articles in a book that is widely disseminated.
New Hope for Glioblastoma Patients
Life Extension® has long advocated for combination use of “off-label” drugs that have specific anti-cancer mechanisms. We have suggested these off-label drugs be considered alongside certain conventional treatments. (The FDA did not concur!)
A group called Care Oncology Clinic was established in London, England in 2013. They study the clinical delivery of repurposed (off-label) drugs to target cancer metabolism.
Using a combination-drug approach that included metformin, doxycycline, mebendazole, and atorvastatin, they were able to dramatically improve survival as summarized below:23
Retrospective analysis was done of 95 patients with advanced (stage IV) glioblastoma who were prescribed an adjuvant off-label drug protocol alongside standard care.
Median survival for patients receiving off-label protocol alongside maximal standard care was 27.1 months, compared to 14.8 months for historic controls not receiving the off-label drug combination.
Two-year overall survival for patients receiving the off-label drug protocol alongside maximal care was 55.8%. This is more than double the two-year survival rate (26.3%) for standard care glioblastoma patients.
The protocol of off-label drugs was well-tolerated by most patients.
Recall that Senators John McCain and Ted Kennedy lived only 14-15 months after diagnosis, whereas patients treated with these off-label drugs survived about one year longer on average.
It is important to note that these positive results are preliminary and require further higher quality evidence.
To learn more about the Care Oncology Clinic of London, please visit https://careoncology.com/
In response to today’s healthcare price conundrum, Pharmocracy II documents why conventional medicine costs so much, and provides practical solutions that Congress (not influenced by pharmaceutical lobbyists) can implement to help resolve this nation’s worsening healthcare cost crisis.
Pharmocracy II advocates for a free-market approach that can spare Medicare and other government entitlement programs from insolvency, while improving the health of all Americans.
This book provides a rational basis for removing the compulsory aspect of healthcare regulation and allowing free-market forces to compete against government-sanctioned medicine.
More importantly, Pharmocracy II empowers the citizenry to inundate Congress with a unified demand to tear down corrupt regulations that are bankrupting the United States and suppressing cures for killer diseases.
The cover price for Pharmocracy II is $20. Your price is $15. Please consider buying four or more copies at only $9 each, to send to your representatives and two senators to educate them about misguided and corrupt government policies that are causing needless loss of human life.
Any of these books can be ordered by calling 1-800-544-4440 (24 hours/7 days).
References
- Han Y, Xie H, Liu Y, et al. Effect of metformin on all-cause and cardiovascular mortality in patients with coronary artery diseases: a systematic review and an updated meta-analysis. Cardiovasc Diabetol. 2019 Jul 30;18(1):96.
- Rodriguez J, Hiel S, Delzenne NM. Metformin: old friend, new ways of action-implication of the gut microbiome? Curr Opin Clin Nutr Metab Care. 2018 Jul;21(4):294-301.
- de la Cuesta-Zuluaga J, Mueller NT, Corrales-Agudelo V, et al. Metformin Is Associated With Higher Relative Abundance of Mucin-Degrading Akkermansia muciniphila and Several Short-Chain Fatty Acid-Producing Microbiota in the Gut. Diabetes Care. 2017 Jan;40(1):54-62.
- Roumie CL, Chipman J, Min JY, et al. Association of Treatment With Metformin vs Sulfonylurea With Major Adverse Cardiovascular Events Among Patients With Diabetes and Reduced Kidney Function. JAMA. 2019 Sep 24;322(12):1167-77.
- Li D, Yeung SC, Hassan MM, et al. Antidiabetic therapies affect risk of pancreatic cancer. Gastroenterology. 2009 Aug;137(2):482-8.
- Available at: https://seer.cancer.gov/statfacts/html/pancreas.html. Accessed June 2, 2021.
- Available at: https://online.wsj.com/article/SB10001424052970203646004577215403399350874.html. Accessed June 2, 2021.
- Available at: https://www.scientificamerican.com/article/why-is-glioblastoma-the-cancer-that-killed-john-mccain-so-deadly/. Accessed June 2, 2021.
- Desjardins A, Gromeier M, Herndon JE, 2nd, et al. Recurrent Glioblastoma Treated with Recombinant Poliovirus. The New England journal of medicine. 2018;379(2):150-61.
- Available at: https://www.lifeextension.com/magazine/2015/9/major-advances-in-glioblastoma-treatment. Accessed June 8, 2021.
- Soderberg-Naucler C, Rahbar A, Stragliotto G. Survival in patients with glioblastoma receiving valganciclovir. N Engl J Med. 2013 Sep 5;369(10):985-6.
- Adeberg S, Bernhardt D, Ben Harrabi S, et al. Metformin influences progression in diabetic glioblastoma patients. Strahlenther Onkol. 2015 Dec;191(12):928-35.
- Furuta T, Sabit H, Dong Y, et al. Biological basis and clinical study of glycogen synthase kinase- 3beta-targeted therapy by drug repositioning for glioblastoma. Oncotarget. 2017 Apr 4;8(14):22811-24.
- Dunbar EM, Coats BS, Shroads AL, et al. Phase 1 trial of dichloroacetate (DCA) in adults with recurrent malignant brain tumors. Invest New Drugs. 2014 Jun;32(3):452-64.
- Liu KH, Yang ST, Lin YK, et al. Fluoxetine, an antidepressant, suppresses glioblastoma by evoking AMPAR-mediated calcium-dependent apoptosis. Oncotarget. 2015 Mar 10;6(7):5088-101.
- Song T, Li H, Tian Z, et al. Disruption of NF-kappaB signaling by fluoxetine attenuates MGMT expression in glioma cells. Onco Targets Ther. 2015;8:2199-208.
- Bielecka-Wajdman AM, Lesiak M, Ludyga T, et al. Reversing glioma malignancy: a new look at the role of antidepressant drugs as adjuvant therapy for glioblastoma multiforme. Cancer Chemother Pharmacol. 2017 Jun;79(6):1249-56.
- Norlin M. Effects of vitamin D in the nervous system: Special focus on interaction with steroid hormone signalling and a possible role in the treatment of brain cancer. J Neuroendocrinol. 2020 Jan;32(1):e12799.
- Ertilav K, Naziroglu M, Ataizi ZS, et al. Selenium Enhances the Apoptotic Efficacy of Docetaxel Through Activation of TRPM2 Channel in DBTRG Glioblastoma Cells. Neurotox Res. 2019 May;35(4):797-808.
- Tavana E, Mollazadeh H, Mohtashami E, et al. Quercetin: A promising phytochemical for the treatment of glioblastoma multiforme. Biofactors. 2020 May;46(3):356-66.
- Lissoni P, Meregalli S, Nosetto L, et al. Increased survival time in brain glioblastomas by a radioneuroendocrine strategy with radiotherapy plus melatonin compared to radiotherapy alone. Oncology. 1996 Jan-Feb;53(1):43-6.
- Available at: https://www.wsj.com/articles/SB10001424052970204792404577229641193886650. Accessed June 3, 2021.
- Agrawal S, Vamadevan P, Mazibuko N, et al. A New Method for Ethical and Efficient Evidence Generation for Off-Label Medication Use in Oncology (A Case Study in Glioblastoma). Front Pharmacol. 2019;10:681.

