Life Extension Magazine®
To remain alive, your cells generate a continuous flow of energy.
When energy is produced by burning hydrocarbons such as coal or oil, the result is residual waste pollutants that damage living beings.
A similar event occurs during cellular energy metabolism. Left behind in the energy cycle is toxic debris.
The primary way cells “clean house” is via autophagy.1
Autophagy defines a natural process whereby cells clear damaged proteins and other metabolic waste products.1,2
An emerging body of evidence points to imbalanced autophagy as a driver of premature aging.3,4
Recent discoveries show that almost every intervention proven to extend healthy lifespan involves activation of autophagy (removing toxic cellular waste).3,4
Restoring balanced autophagy is a critical factor in reversing biological aging.4
What you need to know
- Autophagy is a process in which cells “clean house” by removing damaged proteins and other metabolic waste products. Almost every intervention proven to extend healthy lifespan involves the promotion of autophagy.
- Activation of an enzyme in our cells called AMPK is a particularly safe and effective method to optimize autophagy.
- AMPK signals cells to remove internal pollutants via autophagy. This enables cells to function in a more youthful manner, as evidenced by reduced abdominal fat in many who use AMPK-activating compounds.
- Abdominal fat isn’t just unsightly. It can also generate the systemic inflammation and glucose/lipid imbalances at the root of metabolic syndrome and type II diabetes.
- AMPK performs its fat-removing process, in part, by regulating a protein called mTOR. Currently, the best way to sweep out metabolic waste from aging cells is through regulating mTOR by boosting AMPK functionality.
- Laboratory results show that the citrus flavonoid hesperidin markedly activates AMPK.
- As well, an extract from the Gynostemma pentaphyllum plant has demonstrated strong AMPK-activating properties.
- Gynostemma pentaphyllum extract and hesperidin provide a dual boost to cell AMPK activity, which in turn helps reduce fat by burning it for energy and helps mitigate some of the deleterious mechanisms of aging.
- AMPK can be activated with prescription drugs like metformin or by using a new once-daily combination of standardized extracts of Gynostemma pentaphyllum and hesperidin.
How to Restore Autophagy
Rapamycin is a drug that regulates autophagy. Preliminary evidence points to its potential to delay aging.5,6
Although rapamycin has consistently demonstrated lifespan extension in animal models, mainly by delaying cancer onset,5 such a powerful drug needs more human studies to ensure against potential risks.
There are natural methods to improve autophagy that include supplementation with lithium and NAD+ precursors like nicotinamide riboside.7-9
One of the safest and most effective methods to optimize autophagy is by activating an enzyme in our cells called AMPK.10-12
When AMPK is activated, it signals cells to remove internal pollutants via the process of autophagy.13,14
This enables cells to function in a more youthful manner, as evidenced by reduced abdominal fat stores in many people using AMPK-activating compounds.15
AMPK Signals Cells to Devour Internal Fat
AMPK was first identified in 1973 for its cell-regenerative effects.16
When people practice calorie restriction, AMPK activity increases in cells.17,18
Increasing AMPK activity helps protect against degenerative aging.3,19,20
AMPK turns down cell proliferation to conserve energy in the face of restricted food intake.17,18,20,21
AMPK signals cells to devour stored fat in response to perceived energy (food) shortages.20-22
This is how our ancestors survived famines. When confronted with food scarcity, cells react by slowing their replication and utilizing stored fat reserves for essential energy production.
New Longevity Factor: mTOR
AMPK performs its fat-removing process, in part, by regulating a protein called mTOR which stands for “mechanistic target of rapamycin.”14,23-25
The drug called rapamycin is a powerful autophagy-inducer. It is demonstrating significant age-delaying effects in older animals.26 Differing doses of rapamycin are being studied in humans to assess if a once-per-week dosing can provide benefits without the immunosuppression that occurs when rapamycin is taken daily.27
To clean out metabolic waste from aging cells today, the best way of regulating mTOR is to boost AMPK functionality.
Balance mTOR to Burn Fat

When cell mTOR is properly balanced, the initial effect is breakdown of fat stores that are used to fuel cellular energy.14,23-25,28
If mTOR is not balanced, aging individuals often accumulate unwanted fat stores, even when they don’t excessively ingest calories.14,23-25,28
When nutrient signaling pathways are saturated, the result is storage of excess energy in adipose tissue, which manifests outwardly as body fat. This happens because of increased size of adipocytes (fat cells).
Excessive nutrition (calorie intake) causes up-regulation of mTOR, which sets the stage for increased risk of malignancy and atherosclerosis.
In our youth, energy-sensing AMPK delicately balances cellular energy and fat storage.29,30 Young bodies do a wonderful job of adapting to nutrient availability and energy demands.
Many factors contribute to the age-related loss in flexibility of our signaling pathways. Cell culture and animal data suggests that sensitivity of key energy regulators is lost with age. This causes some pathways to become hyperactive, whereas others are underactive.19,31
AMPK is a key energy regulator that seems an ideal target of longevity-enhancing therapeutics that can also help reduce unwanted belly fat.
Optimizing AMPK activity facilitates removal of cellular debris (via autophagy) and suppresses excess cell propagation.10,11,32 When mTOR is inhibited by AMPK activators there are reductions in cancer risks.20,33
As it relates to combatting aging, achieving optimal mTOR activation status is a critical factor that you will learn more about this upcoming year.
Powerful Anti-Aging Mechanism
 |
Optimize AMPK to Balance mTOR signaling
Modern populations consume an abundance of calories throughout the day. The pathologic impact: AMPK is chronically suppressed while mTOR stays elevated. Excess calorie consumption in adults causes pathological mTOR activity that can result in malignant transformation, accelerated aging, and excess fat storage. Those consuming typical modern diets need to enhance AMPK activity, which has the side benefit of normalizing hyperactivated mTOR.
New Way to Activate AMPK
One of the most stubborn aspects of normal aging is accumulation of deep visceral (abdominal) fat.34
Not only can this be unsightly, but it generates systemic inflammation and glucose/lipid imbalances that are root causes of metabolic syndrome and type II diabetes.34-36
Seeking a natural approach to boosting AMPK, researchers studied a citrus flavonoid called hesperidin. Laboratory results show that hesperidin markedly activates AMPK.37
Hesperidin Studied in High-Risk Patients
Metabolic syndrome describes a cluster of factors that markedly increase heart attack and stroke risk.
Patients with metabolic syndrome typically have:38
- High blood pressure
- Elevated blood sugar
- Lipid imbalance (high triglycerides/low HDL)
- Excess fat around the waist
- Low-level systemic inflammation
Metabolic syndrome patients are at increased risk to progress to type II diabetes.
Based on this festering heart attack epidemic, researchers tested standardized hesperidin on a group of metabolic-syndrome patients.37
In this randomized double-blind crossover clinical trial, half the metabolic-syndrome subjects took 500 mg/day of hesperidin or placebo over a three-week period. They were all “crossed over” so that each patient received either hesperidin or placebo at some point during the study period.
Participants were explicitly counseled before initiation of the study to maintain their usual physical activity and dietary habits. Baseline and follow-up tests included blood markers of cardiac risk and an ultrasound measure of arterial function.
C-reactive protein is a marker of inflammation that increases risk of heart attack and stroke. Those with excess belly fat often have elevated C-reactive protein blood levels.39
In this study, metabolic syndrome patients taking the hesperidin supplement had a striking 33% reduction in C-reactive protein levels compared to baseline.37
Flow-mediated dilation is a non-invasive test that utilizes ultrasound to assess endothelial function in humans.40
When these metabolic syndrome study subjects were given hesperidin, there was a 25% improvement in this (ultrasound) assessment of arterial health.37
When the same metabolic syndrome patients were crossed-over and given placebo, endothelial function slightly worsened. (Metabolic syndrome is characterized by declining arterial health along with increased inflammation.)
Metabolic Syndrome Risk Factors
Apolipoprotein B is a protein portion of LDL cholesterol. Those with high apolipoprotein B levels are at significantly greater risk for coronary artery disease.41,42
In this study of metabolic syndrome patients, apolipoprotein B decreased 2.2% in the hesperidin arm but increased 3.3% when the recipients were given placebo.37
Those afflicted with metabolic syndrome have a cluster of conditions that include excess belly fat, high blood pressure, high blood sugar, inflammatory indicators and abnormal lipids. These factors are an underlying cause of many hearts attack and strokes.38
This three-week study demonstrated reductions in measures of cardiovascular risk in metabolic syndrome patients given a standardized hesperidin supplement. We attribute many of these benefits to the increase in AMPK activity that hesperidin was recently found to induce at the cellular level.
Activating AMPK to Reduce Belly Fat

AMPK activation helps remove excess stored fat by telling your cells that energy is needed so they will stop storing fat and begin using it for energy production.
Based on the known fat-reducing effects of AMPK activation, a study evaluated people with moderately high body mass index 24-30 kg/m2.46
Study participants were provided with a daily dose of nearly 400 mg of hesperidin that was administered over a 12-week period.
All participants in this study were advised to maintain their regular activity levels and meal size for the duration of the study. Baseline and follow-up tests included CT-scan imaging of abdominal fat.
At the study conclusion, abdominal fat in the placebo group increased over 5%, whereas the group receiving hesperidin had about a 1.5% abdominal-fat reduction.
This is comparable to average belly fat-loss effects of metformin recorded in a separate open-label study of type II diabetics that were given exercise and diet plans.46,47
Hesperidin demonstrated marked AMPK activation in the laboratory. This discovery has now been corroborated by findings of abdominal fat loss and improved vascular function in humans who supplemented with standardized hesperidin.
Hesperidin Increases AMPK
 |
Preclinical studies have shown that AMPK activity declines with normal aging.19
Specific plant extracts can help reverse this degenerative trend.
One of these AMPK-activating nutrients is the citrus flavonoid hesperidin.
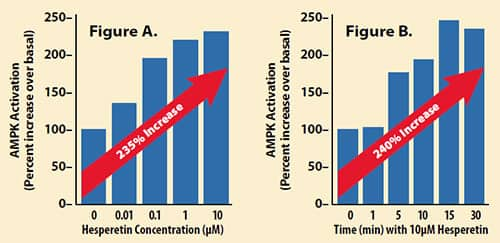
The charts below reveal greater than two-fold increase in AMPK activity in response to the bioactive form of hesperidin:37
Hesperetin stimulated AMPK activation in a concentration- and time-dependent manner.
- Figure A: The amount (10 µM) of hesperetin that resulted in a > two-fold increase in AMPK activity.
- Figure B: Treatment of cells with 10 µM of hesperetin reveals statistically significant activation of AMPK.43
Based on a separate bioavailability clinical study of hesperidin,44 it is expected that after ingesting 500 mg of hesperidin, blood levels of bioactive hesperetin would remain at a concentration greater than 10 µM throughout the day.
Measuring AMPK Activity of Hesperidin
Hesperetin is the bioactive form of hesperidin the body converts to after oral ingestion.37,45 The diagram below shows the conversion of oral hesperidin to bioactive hesperetin:

For cell-based in vitro studies, researchers use hesperetin to measure AMPK activity.37
In vitro cell studies help prove a biological concept, such as the ability of a nutrient or drug to activate AMPK. They do not always correspond to the same in vivo impact.
As it relates to hesperidin, human data indicates beneficial effects that correspond in many ways to AMPK-activating compounds like metformin.37
Impressive Results with Gynostemma Pentaphyllum

An extract from the Gynostemma pentaphyllum plant has previously demonstrated potent AMPK-activating properties.48,49
In a randomized study of 80 obese-but-otherwise healthy people, supplementation with 450 mg a day of Gynostemma— standardized extract resulted in a 6.29% total decline in belly fat compared to a 0.86% drop in the placebo group.15
More impressively, obese individuals receiving the standardized Gynostemma extract had nearly an 11% drop in dangerous visceral fat compared to 2.96% in the placebo arm.15
Visceral fat accumulates around internal organs in the belly. It is the most dangerous kind of fat as it emits inflammatory cytokines that inflict systemic damage.50,51
Reducing visceral fat is a difficult but crucial objective for prevention of cardiovascular disorders, dementia and malignancies.52,53
Gynostemma pentaphyllum extract and hesperidin provide a dual boost to cell AMPK activity, which in turn helps mobilize fat stores by utilizing them as energy sources.
AMPK-Boosting Effects of Gynostemma pentaphyllum
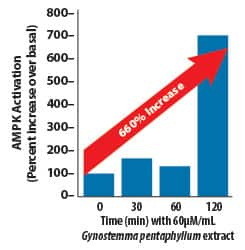 |
In an in vitro study, a standardized extract from the Gynostemma pentaphyllum plant induced a nearly seven-fold increase in AMPK activity compared to baseline.54
When humans take this standardized Gynostemma pentaphyllum,15 study findings reveal some benefits similar to diabetic patients using the drug metformin.47
In particular, standardized Gynostemma pentaphyllum has shown significant reductions in abdominal fat mass compared to placebo in controlled studies.
This chart shows the increase in AMPK activity in response to Gynostemma pentaphyllum.
As noted with the hesperidin data, in vitro studies help prove a biological concept, but do not always correspond to the identical in vivo effect.
As it relates to Gynostemma pentaphyllum, published data reveals significant AMPK activation in the animal (in vivo) model.54
Consistent with these findings, lower doses of standardized Gynostemma pentaphyllum (450 mg/day) used in a human study demonstrate significant reductions in belly fat.15
When analyzing cell culture (in vitro) and animal (in vivo) data showing AMPK activation and then comparing this with human data showing abdominal fat reduction, Gynostemma pentaphyllum demonstrates many beneficial properties of metformin.
Combat Aging While Reducing Belly Fat
The age-related decline in AMPK activity19 is thought to set off a cascade of pathological processes that include:
- Decreased autophagy (removal of cellular debris)
- Abnormal blood lipid profiles (cholesterol/triglycerides)
- Increased C-reactive protein (chronic inflammation)
- Increased abdominal fat storage (especially visceral fat)
Boosting AMPK activity using nutrients like Gynostemma and hesperidin has been demonstrated to mitigate some of these deleterious mechanisms of aging while helping to reduce belly fat.
Comparing AMPK-Boosting Nutrients to Metformin
 |
Metformin is an antidiabetic drug that Life Extension® recommended to combat degenerative aging processes starting in February 1995.
The most studied mechanism of metformin action is its ability to boost AMPK activity.
Research indicates that hesperidin yields significant AMPK-activating properties.
Extracts from the Gynostemma pentaphyllum plant have also demonstrated AMPK-activating effects.48,49
Combining Gynostemma pentaphyllum with hesperidin may promote a greater increase in AMPK activity. This has been suggested by human studies that show reduction in abdominal adiposity in response to supplementation with these nutrients individually.
Do You Need to Suppress mTOR?
Not everyone should consider aggressive suppression of mTOR and turning up autophagy (cellular housecleaning).
If mTOR is excessively and constantly turned down, it could worsen sarcopenia and other frailty-associated conditions.
Western diets are increasingly putting Americans at risk of chronically elevated mTOR activity that contributes to metabolic disorders and unwanted fat accumulation.
There are people today who cycle between aggressively suppressing mTOR via calorie restriction and/or high-dose AMPK activators, and then eating balanced protein-rich diets. This enables mTOR to rebuild muscle mass while suppressing the undesirable impact of pathological mTOR activation.
When it comes to optimal health, balance is key.
Some People Cannot Tolerate Metformin
Metformin causes gastrointestinal upset in some people, along with vitamin B12 deficiency in those who do not supplement with B12.55
People with kidney, lung, cardiac, or significant liver impairment have been historically advised to use metformin with caution.56
The reason is that metformin is safely removed from circulation via the kidneys. Those with pre-existing kidney/liver impairment, or severe circulatory deficit should monitor metformin dosing and blood markers of kidney/liver health.
Metformin cell-culture studies demonstrate that maximal AMPK activation occurs within concentration ranges that metformin is expected to reach in plasma with oral doses of 690 mg to 1,175 mg. This dose is comparable to what many metformin users take today.
This means that those able to tolerate metformin should continue using it, but know there are alternative or additive benefits available with plant extracts that also activate AMPK without metformin’s side effect concerns.
It may not yet be possible to fully restore AMPK to youthful ranges. Promoting optimal AMPK activation with drugs or nutrients provides intriguing potential to partially reverse this aspect of degenerative cell aging.

These charts show metformin-stimulated AMPK activation in cells in a concentration- and time-dependent manner.
Figure A: 100, 500 and 1000 µM of metformin resulted in greater than two-fold to nearly five-fold increase in AMPK activity that was statistically significant.
Figure B: Treatment of cells with 500 µM of metformin revealed that maximal activation of AMPK is reached at 15 minutes and is maintained through at least 60 minutes.57
Summary
Anyone contemplating a weight-loss program that involves reducing calorie intake along with greater physical activity should ensure they get maximum results by increasing their cellular AMPK activity.
AMPK activation can be accomplished with prescription drugs like metformin or by using a once-daily nutrient combination of standardized extracts of Gynostemma pentaphyllum and hesperidin.
Convenient dosing of just one tablet daily will enable more maturing individuals to boost their cellular AMPK.
If you have any questions on the scientific content of this article, please call a Life Extension® Wellness Specialist at 1-866-864-3027.
References
- Mizushima N, Levine B, Cuervo AM, et al. Autophagy fights disease through cellular self-digestion. Nature. 2008;451(7182):1069-75.
- Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132(1):27-42.
- Rubinsztein DC, Marino G, Kroemer G. Autophagy and aging. Cell. 2011;146(5):682-95.
- Madeo F, Zimmermann A, Maiuri MC, et al. Essential role for autophagy in life span extension. J Clin Invest. 2015;125(1):85-93.
- Ehninger D, Neff F, Xie K. Longevity, aging and rapamycin. Cell Mol Life Sci. 2014;71(22):4325-46.
- Sarkar S, Ravikumar B, Floto RA, et al. Rapamycin and mTOR-independent autophagy inducers ameliorate toxicity of polyglutamine-expanded huntingtin and related proteinopathies. Cell Death Differ. 2009;16(1):46-56.
- Motoi Y, Shimada K, Ishiguro K, et al. Lithium and autophagy. ACS Chem Neurosci. 2014;5(6):434-42.
- Sarkar S, Floto RA, Berger Z, et al. Lithium induces autophagy by inhibiting inositol monophosphatase. J Cell Biol. 2005;170(7):1101-11.
- Galluzzi L, Pietrocola F, Levine B, et al. Metabolic control of autophagy. Cell. 2014;159(6):1263-76.
- Pallauf K, Rimbach G. Autophagy, polyphenols and healthy ageing. Ageing Res Rev. 2013;12(1):237-52.
- McCarty MF. AMPK activation--protean potential for boosting healthspan. Age (Dordr). 2014;36(2):641-63.
- Villanueva-Paz M, Cotan D, Garrido-Maraver J, et al. AMPK Regulation of Cell Growth, Apoptosis, Autophagy, and Bioenergetics. Exs. 2016;107:45-71.
- Kroemer G. Autophagy: a druggable process that is deregulated in aging and human disease. J Clin Invest. 2015;125(1):1-4.
- Burkewitz K, Zhang Y, Mair WB. AMPK at the nexus of energetics and aging. Cell Metab. 2014;20(1):10-25.
- Park SH, Huh TL, Kim SY, et al. Antiobesity effect of Gynostemma pentaphyllum extract (actiponin): a randomized, double-blind, placebo-controlled trial. Obesity (Silver Spring). 2014;22(1):63-71.
- Goodman M, Liu Z, Zhu P, et al. AMPK Activators as a Drug for Diabetes, Cancer and Cardiovascular Disease. Pharm Regul Aff. 2014;3(2).
- Lopez-Lluch G, Navas P. Calorie restriction as an intervention in ageing. J Physiol. 2016;594(8):2043-60.
- Ribaric S. Diet and aging. Oxid Med Cell Longev. 2012;2012:741468.
- Salminen A, Kaarniranta K. AMP-activated protein kinase (AMPK) controls the aging process via an integrated signaling network. Ageing Res Rev. 2012;11(2):230-41.
- Luo Z, Zang M, Guo W. AMPK as a metabolic tumor suppressor: control of metabolism and cell growth. Future Oncol. 2010;6(3):457-70.
- Motoshima H, Goldstein BJ, Igata M, et al. AMPK and cell proliferation--AMPK as a therapeutic target for atherosclerosis and cancer. J Physiol. 2006;574(Pt 1):63-71.
- Steinberg GR, Kemp BE. AMPK in Health and Disease. Physiological Reviews. 2009;89(3):1025-78.
- Deblon N, Bourgoin L, Veyrat-Durebex C, et al. Chronic mTOR inhibition by rapamycin induces muscle insulin resistance despite weight loss in rats. Br J Pharmacol. 2012;165(7):2325-40.
- Kim YC, Guan KL. mTOR: a pharmacologic target for autophagy regulation. J Clin Invest. 2015;125(1):25-32.
- Viollet B, Horman S, Leclerc J, et al. AMPK inhibition in health and disease. Crit Rev Biochem Mol Biol. 2010;45(4):276-95.
- Harrison DE, Strong R, Sharp ZD, et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460(7253):392-5.
- Available at: https://clinicaltrials.gov/ct2/show/nct02874924. Accessed November 24, 2017.
- Chakrabarti P, Kandror KV. The role of mTOR in lipid homeostasis and diabetes progression. Curr Opin Endocrinol Diabetes Obes. 2015;22(5):340-6.
- Richter EA, Ruderman NB. AMPK and the biochemistry of exercise: implications for human health and disease. Biochem J. 2009;418(2):261-75.
- Coughlan KA, Valentine RJ, Ruderman NB, et al. AMPK activation: a therapeutic target for type 2 diabetes? Diabetes Metab Syndr Obes. 2014;7:241-53.
- Garza-Lombo C, Gonsebatt ME. Mammalian Target of Rapamycin: Its Role in Early Neural Development and in Adult and Aged Brain Function. Front Cell Neurosci. 2016;10:157.
- Mihaylova MM, Shaw RJ. The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nat Cell Biol. 2011;13(9):1016-23.
- Plews RL, Mohd Yusof A, Wang C, et al. A novel dual AMPK activator/mTOR inhibitor inhibits thyroid cancer cell growth. J Clin Endocrinol Metab. 2015;100(5):E748-56.
- Hunter GR, Gower BA, Kane BL. Age Related Shift in Visceral Fat. Int J Body Compos Res. 2010;8(3):103-8.
- Despres JP. Is visceral obesity the cause of the metabolic syndrome? Ann Med. 2006;38(1):52-63.
- Nomura K, Eto M, Kojima T, et al. Visceral fat accumulation and metabolic risk factor clustering in older adults. J Am Geriatr Soc. 2010;58(9):1658-63.
- Rizza S, Muniyappa R, Iantorno M, et al. Citrus polyphenol hesperidin stimulates production of nitric oxide in endothelial cells while improving endothelial function and reducing inflammatory markers in patients with metabolic syndrome. J Clin Endocrinol Metab. 2011;96(5):E782-92.
- Available at: https://www.nhlbi.nih.gov/health/health-topics/topics/ms. Accessed October 24, 2017.
- Irkulla S, Ujam B, Gaze D, et al. Abdominal adiposity is the main determinant of the C-reactive response to injury in subjects undergoing inguinal hernia repair. J Inflamm (Lond). 2013;10(1):5.
- Bellamkonda K, Williams M, Handa A, et al. Flow Mediated Dilatation as a Biomarker in Vascular Surgery Research. J Atheroscler Thromb. 2017;24(8):779-87.
- Sacks FM, Alaupovic P, Moye LA, et al. VLDL, apolipoproteins B, CIII, and E, and risk of recurrent coronary events in the Cholesterol and Recurrent Events (CARE) trial. Circulation. 2000;102(16):1886-92.
- Jacobson TA. Opening a new lipid “apo-thecary”: incorporating apolipoproteins as potential risk factors and treatment targets to reduce cardiovascular risk. Mayo Clin Proc. 2011;86(8):762-80.
- Internal Data Analysis. 2017.
- Nielsen IL, Chee WS, Poulsen L, et al. Bioavailability is improved by enzymatic modification of the citrus flavonoid hesperidin in humans: a randomized, double-blind, crossover trial. J Nutr. 2006;136(2):404-8.
- Amaretti A, Raimondi S, Leonardi A, et al. Hydrolysis of the rutinose-conjugates flavonoids rutin and hesperidin by the gut microbiota and bifidobacteria. Nutrients. 2015;7(4):2788-800.
- Ohara T, Muroyama K, Yamamoto Y, et al. Oral intake of a combination of glucosyl hesperidin and caffeine elicits an anti-obesity effect in healthy, moderately obese subjects: a randomized double-blind placebo-controlled trial. Nutr J. 2016;15:6.
- Wang H, Ni Y, Yang S, et al. The effects of gliclazide, metformin, and acarbose on body composition in patients with newly diagnosed type 2 diabetes mellitus. Curr Ther Res Clin Exp. 2013;75:88-92.
- Wang M, Wang F, Wang Y, et al. Metabonomics study of the therapeutic mechanism of Gynostemma pentaphyllum and atorvastatin for hyperlipidemia in rats. PLoS One. 2013;8(11):e78731.
- Tan Y, Kamal MA, Wang ZZ, et al. Chinese herbal extracts (SK0506) as a potential candidate for the therapy of the metabolic syndrome. Clin Sci (Lond). 2011;120(7):297-305.
- Ahima RS. Connecting obesity, aging and diabetes. Nat Med. 2009;15(9):996-7.
- Tchernof A, Despres JP. Pathophysiology of human visceral obesity: an update. Physiol Rev. 2013;93(1):359-404.
- Tchernof A, Després JP. Pathophysiology of human visceral obesity: An update. Physiological Reviews. 2013;93(1):359-404.
- Whitmer RA, Gustafson DR, Barrett-Connor E, et al. Central obesity and increased risk of dementia more than three decades later. Neurology. 2008;71(14):1057-64.
- Gauhar R, Hwang SL, Jeong SS, et al. Heat-processed Gynostemma pentaphyllum extract improves obesity in ob/ob mice by activating AMP-activated protein kinase. Biotechnol Lett. 2012;34(9):1607-16.
- Available at: https://reference.medscape.com/drug/glucophage-metformin-342717#4. Accessed October 25, 2017.
- Available at: https://reference.medscape.com/drug/glucophage-metformin-342717#5. Accessed October 25, 2017.
- Zou MH, Kirkpatrick SS, Davis BJ, et al. Activation of the AMP-activated protein kinase by the anti-diabetic drug metformin in vivo. Role of mitochondrial reactive nitrogen species. J Biol Chem. 2004;279(42):43940-51.

