Life Extension Magazine®
Atherosclerosis is Reversed
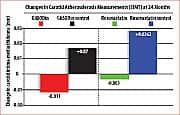
About a year and a half after commencing daily supplementation with GliSODin®, measurable decreases in subjects’ IMT were detected. Approximately two years after starting GliSODin® supplementation, decreases in IMT values became statistically significant. In dramatic contrast, control subjects not receiving GliSODin® experienced increased IMT values over the same period.21 There were no reported side effects in either group. This remarkable study demonstrated that reversal of atherosclerosis in adults with multiple risk factors for future cardiovascular disease is possible through a combination of healthy diet and daily intake of GliSODin® (orally bioavailable superoxide dismutase). These findings were confirmed by monitoring of clinical and biological health parameters, and measurements of carotid IMT. The GliSODin® regimen, “improves, significantly, the anti-oxidant status,” noted investigators, “and diminishes, remarkably, carotid artery IMT.”21 It should be noted that these findings echo those of other researchers, who, in previous and subsequent studies, have convincingly demonstrated GliSODin®’s ability to reduce oxidative damage in human volunteers and animal models.25-27 Pomegranate Fights Oxidative Damage, Reverses AtherosclerosisScientists have recently shown that pomegranate juice offers cardiac health benefits that complement those of GliSODin®. In the past seven years alone, the amount of published research on pomegranate has increased seven-fold over all preceding years in the medical and scientific literature.40 That’s almost certainly because each new study underscores the potential of this fruit to fight cancer and to combat oxidative stress. The latter is of particular importance for atherosclerosis prevention.
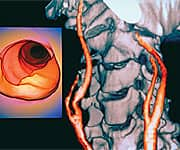
In 2004, researchers published the findings of a three-year study on the daily consumption of pomegranate juice (50 mL, or 1.7 ounces) by patients with advanced atherosclerosis.41 These patients were diagnosed with carotid artery stenosis; a serious condition in which the carotid arteries, responsible for supplying blood to the brain, become noticeably narrowed by buildup of atherosclerotic plaques. Such patients are at increased risk of suffering strokes or other “cerebrovascular accidents”. In this study, common carotid IMT increased by 9% within one year in the non-supplemented control group. Remarkably, patients drinking pomegranate juice experienced a whopping 35% reduction in the IMT score and a 44% improvement in carotid artery blood flow over the same period. Investigators also documented a 21% reduction in systolic blood pressure among the pomegranate juice drinkers. Serum total antioxidant status was increased by an extraordinary 130% after one year of pomegranate supplementation. Additionally, the scientists monitored the status of an enzyme that may protect against the development of atherosclerotic plaque by protecting LDL against oxidative modification. Pomegranate drinkers’ levels of this beneficial enzyme increased by 83% after just one year. “For all studied parameters, the maximal effects were observed after one year of consumption,” wrote the researchers.41
Other researchers from around the globe have obtained similar results when studying pomegranate’s ability to rapidly improve volunteers’ antioxidant status,42 to reduce oxidative stress, and to reverse processes that contribute to the promotion and progression of atherosclerosis, including coronary artery narrowing and LDL oxidation.43-47 ConclusionAtherosclerosis is a serious threat to health. Its progression has been linked to increased risk of heart attack, stroke, atrial fibrillation and dementia, among other potentially fatal conditions. Since it may begin as early as childhood, and aging has been identified as the greatest risk factor for its development, it is vital to combat this arterial-dysfunction disease as early—and as aggressively—as possible. Nature has provided the means to protect ourselves from this insidious threat. By increasing our levels of the natural enzymatic antioxidant, superoxide dismutase (SOD), and by harnessing the potent polyphenol power of pomegranate, scientists have shown that it is now possible to help reverse the course of atherosclerosis—naturally.
| ||||||||
| References | ||||||||
| 1. Osika W, Dangardt F, Gronros J, et al. Increasing peripheral artery intima thickness from childhood to seniority. Arterioscler Thromb Vasc Biol. 2007 Mar;27(3):671-6. 2. Wei EP, Kontos HA, Christman CW, DeWitt DS. Superoxide generation and reversal of acetylcholine-induced cerebral arteriolar dilation after acute hypertension. Circ Res. 1985;57:781–7. 3. Rubanyi GM, Vanhoutte PM. Superoxide anions and hyperoxia inactivate endothelium-derived relaxing factor. Am J Physiol Heart Circ Physiol. 1986; 250: H822–7. 4. Hathaway C, Heistad DD, Piegors DJ, Miller FM. Regression of atherosclerosis in monkeys reduces vascular superoxide levels. Circ Res. 2002;90:277–83. 5. Wilcox CS. Oxidative stress and nitric oxide deficiency in the kidney: A critical link to hypertension? Am J Physiol Reg. 2005; 289: R913–35. 6. Lund DD, Faraci FM, Miller FJ Jr., Heistad DD. Gene transfer of endothelial nitric oxide synthase improves relaxation of carotid arteries from diabetic rabbits. Circulation. 2000;101:1027–33. 7. Bauersachs J, Bouloumie A, Fraccarollo D, Hu K, Busse R, Ertl G. Endothelial dysfunction in chronic myocardial infarction despite increased vascular endothelial nitric oxide synthase and soluble guanylate cyclase expression: role of enhanced vascular superoxide production. Circulation. 1999;100:292–8. 8. Landmesser U, Spiekermann S, Dikalov S, et al. Vascular oxidant stress and endothelial dysfunction in patients with chronic heart failure: role of xanthine-oxidase and extracellular superoxide dismutase. Circulation. 2002;106:3073–8. 9. Kotur-Stevuljevic J, Memon L, Stefanovic A, et al. Correlation of oxidative stress parameters and inflammatory markers in coronary artery disease patients. Clin Biochem. 2007 Feb;40(3-4):181-7. 10. Heistad DD. Oxidative stress and vascular disease: 2005 Duff lecture. Arterioscler Thromb Vasc Biol. 2006 Apr;26(4):689-95. 11. Vicenzini E, Ricciardi MC, Puccinelli F, et al. Common carotid artery intima-media thickness determinants in a population study. J Ultrasound Med. 2007Apr;26(4):427-32. 12. Bruunsgaard H, Pedersen BK. Age-related inflammatory cytokines and disease. Immunol Allergy Clin North Am. 2003 Feb;23(1):15-39. 13. Krabbe KS, Pedersen M, Bruunsgaard H. Inflammatory mediators in the elderly. Exp Gerontol. 2004 May;39(5):687-99. 14. Payne GW. Effect of inflammation on the aging microcirculation: impact on skeletal muscle blood flow control. Microcirculation. 2006 Jun;13(4):343-52. 15. Faloon W. How much fish oil is in your blood? Life Extension. Jun 2007;13(6):6-9. 16. de Groot E, Jukema JW, Montauban van Swijndregt AD, et al. B-mode ultrasound assessment of pravastatin treatment effect on carotid and femoral artery walls and its correlations with coronary arteriographic findings: a report of the Regression Growth Evaluation Statin Study (REGRESS). Am Coll Cardiol. 1998 Jun;31(7):1561-7. 17. Salonen R, Nyyssonen K, Porkkala E, et al. Kuopio Atherosclerosis Prevention Study (KAPS). A population-based primary preventive trial of the effect of LDL lowering on atherosclerotic progression in carotid and femoral arteries. Circulation. 1995 Oct 1;92(7):1758-64. 18. Nissen SE, Nicholls SJ, Sipahi I, et al. Effect of very high-intensity statin therapy on regression of coronary atherosclerosis: the ASTEROID trial. JAMA. 2006 Apr 5;295(13):1556-65. 19. Taylor AJ, Kent SM, Flaherty PJ, Coyle LC, Markwood TT, Vernalis MN. ARBITER: Arterial Biology for the Investigation of the Treatment Effects of Reducing Cholesterol: a randomized trial comparing the effects of atorvastatin and pravastatin on carotid intima medial thickness. Circulation. 2002 Oct 15;106(16):2055-60. 20. Crouse JR, III, Raichlen JS, Riley WA, et al. Effect of rosuvastatin on progression of carotid intima-media thickness in low-risk individuals with subclinical atherosclerosis: the METEOR Trial. JAMA. 2007 Mar 28;297(12):1344-53. 21. Cloarec M, Caillard P, Provost JC, et al. GliSODin, a vegetal sod with gliadin, as preventative agent vs. atherosclerosis, as confirmed with carotid ultrasound-B imaging. Allerg Immunol.(Paris). 2007 Feb;39(2):45-50. 22. Vouldoukis I, Conti M, Krauss P, et al. Supplementation with gliadin-combined plant superoxide dismutase extract promotes antioxidant defences and protects against oxidative stress. Phytother Res. 2004 Dec;18(12):957-62. 23. Dugas B. Glisodin®, a nutraceutical product that promotes the oral delivery of superoxide dismutase. Free Radic Biol Med. 2002;33:S64. 24. Nielsen F, Mikkelsen BB, Nielsen JB, Andersen HR, Grandjean P. Plasma malondialdehyde as biomarker for oxidative stress: reference interval and effects of life-style factors. Clin Chem. 1997 Jul;43(7):1209-14. 25. Kick J, Hauser B, Bracht H, et al. Effects of a cantaloupe melon extract/wheat gliadin biopolymer during aortic cross-clamping. Intensive Care Med. 2007 Apr;33(4):694-702. 26. Muth CM, Glenz Y, Klaus M, et al. Influence of an orally effective SOD on hyperbaric oxygen-related cell damage. Free Radic Res. 2004 Sep;38(9):927-32. 27. Naito Y, Akagiri S, Uchiyama K, et al. Reduction of diabetes-induced renal oxidative stress by a cantaloupe melon extract/gliadin biopolymers, oxykine, in mice. Biofactors. 2005;23(2):85-95. 28. van OM, Jan de JF, Witteman JC, et al. Atherosclerosis and risk for dementia. Ann Neurol. 2007 Feb 27. 29. Muller M, Grobbee DE, Aleman A, Bots M, van der Schouw YT. Cardiovascular disease and cognitive performance in middle-aged and elderly men. Atherosclerosis. 2007 Jan;190(1):143-9. 30. Vaudo G, Marchesi S, Siepi D, et al. Metabolic syndrome and preclinical atherosclerosis: focus on femoral arteries. Metabolism. 2007 Apr;56(4):541-6. 31. Heeringa J, van der Kuip DA, Hofman A, et al. Subclinical atherosclerosis and risk of atrial fibrillation: the rotterdam study. Arch Intern Med. 2007 Feb 26;167(4):382-7 32. Lekakis JP, Papamichael C, Papaioannou TG, et al. Intima-media thickness score from carotid and femoral arteries predicts the extent of coronary artery disease: intima-media thickness and CAD. Int J Cardiovasc Imaging. 2005 Oct;21(5):495-501. 33. Salonen R, Haapanen A, Salonen JT. Measurement of intima-media thickness of common carotid arteries with high-resolution B-mode ultrasonography: inter- and intra-observer variability. Ultrasound Med Biol. 1991;17(3):225-30. 34. Girerd X, Mourad JJ, Acar C, et al. Noninvasive measurement of medium-sized artery intima-media thickness in humans: in vitro validation. J Vasc Res. 1994 Mar;31(2):114-20. 35. Nathan DM, Lachin J, Cleary P, et al. Intensive diabetes therapy and carotid intima-media thickness in type 1 diabetes mellitus. N Engl J Med. 2003 Jun 5;348(23):2294-303. 36. Soroka NN, Ryzhak GA. Ultrasonic diagnostics of mediointimal hyperplasia as a predictor of atherosclerosis in old people. Adv Gerontol. 2006;19:102-6. 37. Abdelghaffar S, El AM, El HA, El MF. Carotid intima-media thickness: an index for subclinical atherosclerosis in type 1 diabetes. J Trop Pediatr. 2006 Feb;52(1):39-45. 38. O’Leary DH, Polak JF, Kronmal RA, et al. Carotid-artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. Cardiovascular Health Study Collaborative Research Group. N Engl J Med. 1999 Jan 7;340(1):14-22. 39. Hodis HN, Mack WJ, LaBree L, et al. The role of carotid arterial intima-media thickness in predicting clinical coronary events. Ann Intern Med. 1998 Feb 15;128(4):262-9. 40. Lansky EP, Newman RA. Punica granatum (pomegranate) and its potential for prevention and treatment of inflammation and cancer. J Ethnopharmacol. 2007 Jan 19;109(2):177-206. 41. Aviram M, Rosenblat M, Gaitini D, et al. Pomegranate juice consumption for 3 years by patients with carotid artery stenosis reduces common carotid intima-media thickness, blood pressure and LDL oxidation. Clin Nutr. 2004 Jun;23(3):423-33. 42. Mertens-Talcott SU, Jilma-Stohlawetz P, Rios J, Hingorani L, Derendorf H. Absorption, metabolism, and antioxidant effects of pomegranate (Punica granatum l.) polyphenols after ingestion of a standardized extract in healthy human volunteers. J Agric Food Chem. 2006 Nov 15;54(23):8956-61. 43. de NF, Williams-Ignarro S, Sica V, et al. Effects of a pomegranate fruit extract rich in punicalagin on oxidation-sensitive genes and eNOS activity at sites of perturbed shear stress and atherogenesis. Cardiovasc Res. 2007 Jan 15;73(2):414-423. 44. Rosenblat M, Hayek T, Aviram M. Anti-oxidative effects of pomegranate juice (PJ) consumption by diabetic patients on serum and on macrophages. Atherosclerosis. 2006 Aug;187(2):363-371. 45. Kaplan M, Hayek T, Raz A, et al. Pomegranate juice supplementation to atherosclerotic mice reduces macrophage lipid peroxidation, cellular cholesterol accumulation and development of atherosclerosis. J Nutr. 2001 Aug;131(8):2082-9. 46. Fuhrman B, Volkova N, Aviram M. Pomegranate juice inhibits oxidized LDL uptake and cholesterol biosynthesis in macrophages. J Nutr Biochem. 2005 Sep;16(9):570-6. 47. Tuttle D. Pomegranate reverses atherosclerosis and slows the progression of prostate cancer. Life Extension. Feb 2007;13(2):72-7. |